Polymeric immunoglobulin receptor promotes tumor growth in hepatocellular carcinoma
Potential conflict of interest: Nothing to report.
Supported by the National Program on Key Basic Research Project of China (no. 2012CB910704), the National Natural Science Foundation of China (nos. 91229205, 81473243, 81321092, 81572884, and 81372317), the “Personalized Medicines–Molecular Signature-based Drug Discovery and Development”, Strategic Priority Research Program of the Chinese Academy of Sciences, nos. XDA12020000), NSFC-Shandong Joint Fund for Marine Science Research Centers (nos. U1406402), and the Youth Innovation Promotion Association (no. 2013189).
Abstract
Deregulation of the immune system is believed to contribute to cancer malignancy, which has led to recent therapeutic breakthroughs facilitating antitumor immunity. In a malignant setting, immunoglobulin receptors, which are fundamental components of the human immune system, fulfill paradoxical roles in cancer pathogenesis. This study describes a previously unrecognized pro-oncogenic function of polymeric immunoglobulin receptor (pIgR) in the promotion of cell transformation and proliferation. Mechanistically, pIgR overexpression is associated with YES proto-oncogene 1, Src family tyrosine kinase (Yes) activation, which is required for pIgR-induced oncogenic growth. Specifically, pIgR activates the Yes-DNAX-activating protein of 12 kDa-spleen tyrosine kinase-Rac1/CDC42-MEK (extracellular signal-regulated kinase kinase)/ERK (extracellular signal-regulated kinase) cascade in an immunoreceptor tyrosine-based activating motif (ITAM)-dependent manner to promote cell transformation and tumor growth, although pIgR itself does not contain an ITAM sequence. Additionally, the combination of pIgR and phosphorylated Yes (p-Yes) levels serves as a prognostic biomarker for hepatitis B surface antigen–positive and early-stage hepatocellular carcinoma (HCC) patients. Moreover, pharmacological targeting of MEK/ERK or Yes represents a therapeutic option for the subgroup of patients with pIgR/p-Yes–positive HCC based on our results with both cancer cell-line–based xenografts and primary patient-derived xenografts. Conclusion: Our findings reveal the molecular mechanism by which pIgR promotes cancer malignancy, suggest the clinical potential of targeting this pathway in HCC, and provide new insight into the oncogenic role of immunoglobulin receptors. (Hepatology 2017;65:1948-1962).
Abbreviations
-
- CML
-
- chronic myelogenous leukemia
-
- DAP12
-
- DNAX-activating protein of 12 kDa
-
- DFS
-
- disease-free survival
-
- EGFR
-
- epidermal growth factor receptor
-
- EMT
-
- epithelial-mesenchymal transition
-
- ERK
-
- extracellular signal-regulated kinase
-
- FcR
-
- Fc receptor
-
- FcRγ
-
- Fc receptor γ-chain
-
- HBsAg
-
- hepatitis B surface antigen
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
-
- Igs
-
- immunoglobulins
-
- IHC
-
- immunohistochemistry
-
- ITAM
-
- immunoreceptor tyrosine-based activating motif
-
- MAPKs
-
- mitogen-activated protein kinases
-
- MDCK
-
- Madin-Darby canine kidney
-
- MEK
-
- extracellular signal-regulated kinase kinase
-
- OS
-
- overall survival
-
- PDX
-
- patient-derived xenograft
-
- Ph+ ALL
-
- Philadelphia chromosome-positive acute lymphoblastic leukemia
-
- PI3K
-
- phosphatidylinositol 3-kinase
-
- pIgR
-
- polymeric immunoglobulin receptor
-
- PLCγ
-
- phospholipase Cγ
-
- p-Yes
-
- phosphorylated Yes
-
- SCID
-
- severe combined immunodeficiency
-
- shRNAs
-
- small hairpin RNAs
-
- siRNAs
-
- small interfering RNAs
-
- Smad
-
- small mothers against decapentaplegic
-
- Syk
-
- spleen tyrosine kinase
-
- TNM
-
- Tumor-Node-Metastasis
-
- Yes
-
- YES proto-oncogene 1, Src family tyrosine kinase
The Fc receptor (FcR) family of immunoglobulins (Igs), which are widely expressed throughout the immune system, provides antibody-mediated immunity that connects the adaptive and innate immune systems.1, 2 In mammals, several types of classical FcRs with distinct affinities for different Ig isotypes have been identified, including the IgG receptors FcγRI, FcγRII, and FcγRIII, the IgE receptor FcϵRI, the IgA receptors FcαRI and Fcα/μR, the polymeric immunoglobulin receptor (pIgR), and the IgM receptor Fcα/μR.3 By binding to the antibody Fc portion of an Ig, FcRs induce powerful responses that activate, regulate, and modulate immunity. Effector cell activation leads to release of inflammatory mediators or activation of cell-based responses, such as phagocytosis and endocytosis, to eliminate invading pathogens.4, 5 However, FcR family members also promote de novo carcinogenesis through chronic inflammatory programs. For example, FcγR is activated by the stromal accumulation of autoantibodies in premalignant skin lesions; the activation of this receptor promotes neoplastic progression and subsequent carcinoma development through regulation of leukocyte recruitment, composition, and bioeffector functions.6 Similarly, we identified the ability of pIgR overexpression, which is induced by chronic inflammation, to promote cell migration and cancer metastasis.7 Thus, FcRs warrant further study to gain greater insight into the paradoxical roles of the immune system in cancer. Molecular knowledge of these processes may expand therapeutic strategies for cancer by revealing previously unrecognized anticancer targets.
The Fc receptor family member, pIgR, is a central component of the mucosal immune system. Widely expressed in mucosal epithelial cells, pIgR is commonly up-regulated by proinflammatory cytokines in response to viral or bacterial infections.8, 9 pIgR regulates the transcytosis of polymeric Igs (dimeric IgA and pentameric IgM) from the basolateral plasma membrane to the apical plasma membrane of the mucosal epithelium and facilitates the secretion of IgA and IgM, which are the first line of defense against infection.10, 11 Our previous work revealed a pro-oncogenic role for pIgR in promoting the metastasis of human hepatocellular carcinoma (HCC), which involves a mechanism that includes small mothers against decapentaplegic (Smad) signaling-dependent epithelial-mesenchymal transition (EMT).7 Although the identification of pIgR-associated Smad pathway activation provided mechanistic insight into pIgR-associated cancer metastasis, our parallel observation that pIgR-overexpressing HCC cells exhibited enhanced tumor formation and growth in vivo remained puzzling. This study sought to gain a better understanding of the role of pIgR in tumor malignancy, elucidate the pathogenic mechanisms of immunological regulators in carcinogenesis, and identify new treatment approaches for HCC.
Materials and Methods
Extended materials and methods are provided in the Supporting Methods section.
CELL CULTURE
The Madin-Darby canine kidney (MDCK) cell line was purchased from the American Type Culture Collection (Manassas, VA). The hepatic carcinoma cell line, SMMC-7721, was obtained from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured according to the supplier's instructions. pIgR exogenous overexpression and knockdown cells were developed in our laboratory and cultured as described. All cell lines were authenticated by short tandem repeats analysis by Genesky Biopharma Technology (Shanghai, China).
ANIMAL EXPERIMENTATION
Animal studies were approved by the Institutional Animal Care and Use Committee at Shanghai Institute of Materia Medica (approval no.: 2015-04-DJ-18). The detailed information and procedures are described in the Supporting Materials and Methods.
CLINICAL SPECIMENS
Tissues were collected and tissue microarray analysis were performed as described. Follow-up procedures are described in the Supporting Materials and Methods.
STATISTICAL ANALYSIS
All data (with the exception of the tissue microarray and animal survival analyses) were analyzed using Student t tests. Statistical analysis of tissue microarrays was performed using the statistical software package, SPSS (version 21.0; SPSS, Inc., Chicago, IL), as described. P < 0.05 was considered statistically significant. More details are described in the Supporting Materials and Methods.
Results
pIgR PROMOTES MALIGNANT TRANSFORMATION AND CANCER GROWTH
To examine the impact of pIgR on cancer malignancy, pIgR was overexpressed in MDCK cells, an epithelial cell line that has retained functional basolateral-to-apical Ig transcytosis and is widely utilized to investigate the biological functions of pIgR. Cells that stably overexpressed pIgR, designated MDCK-pIgR cells, exhibited remarkably enhanced colony formation in MDCK cells relative to cells transfected with empty vector (MDCK-mock cells), which failed to form macrocolonies (Fig. 1A). This observation was recapitulated in SMMC-7721 cells, a hepatic carcinoma cell line (Fig. 1B). Moreover, the enhanced clonogenicity induced by pIgR overexpression was largely reversed by small hairpin RNAs (shRNAs) targeting pIgR (Fig. 1A). In addition, the impact of these shRNAs was closely correlated with their gene silencing efficiency, strongly suggesting pIgR-driven cell proliferation. MDCK cells lack clonogenicity, suggesting the involvement of pIgR in promoting cellular transformation. We next examined the ability of MDCK-pIgR cells to form colonies in soft agar, a property that frequently correlates with tumorigenicity. MDCK-mock cells failed to form anchorage-independent colonies in soft agar after 3 weeks of culture. In marked contrast, MDCK-pIgR cells formed large colonies that were eliminated by pIgR shRNA (Fig. 1C), confirming the pIgR-dependent transformation of MDCK cells.
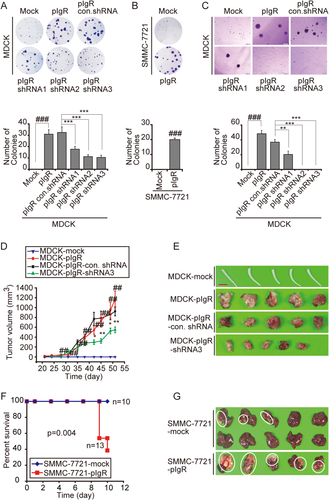
This pIgR-enhanced cell proliferation and malignant transformation led us to examine the potential of pIgR to promote tumorigenesis and tumor growth in vivo. pIgR-overexpressing MDCK cells (MDCK-pIgR) and control cells (MDCK-mock) were implanted subcutaneously into the right flank of severe combined immunodeficiency (SCID) mice, and tumor formation was assessed for a total of 7.5 weeks postimplantation. No tumors were observed in mice injected with MDCK-mock cells. In contrast, pIgR-expressing MDCK cells formed tumors that were first measurable after approximately 3 weeks and continued to grow until the termination of the experiment. For mice injected with MDCK-pIgR-shRNA3 cells, the clone with the best pIgR knockdown efficiency, tumor size was significantly smaller than in the group implanted with MDCK-pIgR cells (Fig. 1D,E). Next, pIgR-overexpressing SMMC-7721 (SMMC-7721-pIgR) cells and control cells (SMMC-7721-mock) were orthotopically injected into the liver parenchyma of nude mice. Ten days postimplantation, approximately 60% of the mice bearing pIgR-expressing cells died of their tumor burden (Fig. 1F). All mice bearing pIgR-expressing cells formed large tumors (Fig. 1G). In contrast, mice implanted with SMMC-7721-mock cells either did not form tumors or displayed a low tendency to form small tumors (3 of 10 mice; Fig. 1F,G). Taken together, these data suggested a previously unappreciated role of pIgR in promoting malignant transformation and tumor growth, which prompted us to investigate the molecular basis underlying this phenomenon.
pIgR-PROMOTED CELL PROLIFERATION REQUIRES THE ACTIVATION OF Yes/EXTRACELLULAR SIGNAL-REGULATED KINASE KINASE/EXTRACELLULAR SIGNAL-REGULATED KINASE SIGNALING
To understand the molecular mechanism responsible for pIgR-induced tumor growth, we first determined whether Smad signaling, which is known to play a major role in pIgR-induced EMT, was involved in pIgR-driven cell proliferation. We found that Smad2/3 knockdown failed to influence the enhanced clonogenicity induced by pIgR (Supporting Fig. S1A,B), indicating that Smad signaling is not involved in pIgR-driven cancer cell proliferation. Then, we investigated the major physiological process that involves pIgR, specifically the transcytosis of polymeric Ig from the basolateral plasma membrane to the apical plasma membrane of mucosal epithelia. This process is known to activate numerous oncogenic proteins, particularly oncogenic kinases, such as the Src family kinase member, Yes, epidermal growth factor receptor (EGFR), mitogen-activated protein kinases (MAPKs), and others.12-15 First, we conducted a small-scale screen using a compound library enriched for inhibitors targeting pIgR transcytosis signaling pathways. Among these inhibitors, AZD6244, an inhibitor of mitogen-activated protein/extracellular signal-regulated kinase (ERK) kinase (MEK), and the Src family kinase inhibitor, dasatinib, inhibited colony formation induced by pIgR overexpression in both MDCK and SMMC-7721 cells (Fig. 2A,B). In parallel, neither of these two inhibitors affected the growth of corresponding parental cells (data not shown). Based on these results, Src family kinases and MAPK signaling may contribute to pIgR-induced cell proliferation. Consistent with these findings, we also observed pIgR-dependent activation of Src family kinases and the MEK/ERK signaling pathway in both MDCK and SMMC-7721 cells (Fig. 2C). Thus, pIgR overexpression may activate Src family kinases and MEK/ERK signaling to promote cell transformation and cancer cell proliferation.
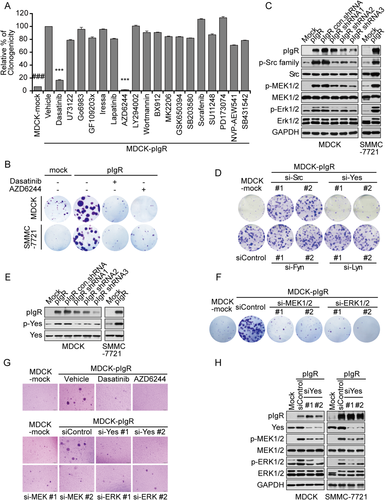
To further confirm these results, the major Src family kinase members, Src, Yes, Fyn, and Lyn, were individually depleted using specific small interfering RNAs (siRNAs) in MDCK-pIgR cells. The down-regulation of Yes, which is known to be involved in the early step of pIgR transcytosis, significantly impaired pIgR-driven colony formation. However, no apparent effects were observed after knocking down other Src family kinase members (Fig. 2D and Supporting Fig. S1C). These findings were in agreement with the elevated Yes phosphorylation observed in pIgR-overexpressing cells (Fig. 2E). Similarly, MEK/ERK signaling was activated in MDCK-pIgR cells, whereas siRNAs targeting either MEK or ERK suppressed pIgR-stimulated cell proliferation (Fig. 2F and Supporting Fig. S1D). The critical roles of Yes and MEK/ERK signaling were also confirmed in pIgR-induced anchorage-independent cell growth (Fig. 2G). We next sought to determine whether pIgR-induced MEK/ERK signaling was dependent on Yes signaling. Indeed, the pIgR-mediated phosphorylation of MEK/ERK was markedly reduced by Yes depletion (Fig. 2H), suggesting that Yes activates MEK/ERK signaling to drive cell growth. Collectively, we demonstrated the ability of pIgR overexpression to promote cell proliferation and anchorage-independent cell growth by a Yes-MEK/ERK–dependent mechanism.
SPLEEN TYROSINE KINASE BRIDGES pIgR-ACTIVATED Yes-MEK/ERK SIGNALING IN AN IMMUNORECEPTOR TYROSINE-BASED ACTIVATING MOTIF–DEPENDENT MECHANISM
The activation of Src family kinases, such as Yes, is a common event associated with classic immunoreceptors, including B-cell and T-cell antigen receptors and Fc receptors.16-19 We next sought to identify the downstream molecules in the Yes signaling cascade that may confer a growth advantage upon pIgR.
Immunoreceptors activate spleen tyrosine kinase (Syk) or the related protein, Zap70, to transmit immunoreceptor-induced signaling.20 Therefore, we tested the possible involvement of Syk or Zap70 in pIgR-induced cell proliferation. We observed elevated Syk phosphorylation in pIgR-overexpressing cells; this effect was reversed by the stable knockdown of pIgR (Fig. 3A). Moreover, the clonogenicity of MDCK-pIgR cells was significantly reduced by Syk, but not Zap70, depletion (Fig. 3B). Consistent with these findings, inhibiting Syk kinase activity with PRT062607, a highly selective Syk kinase inhibitor, almost completely reversed the pIgR-mediated increase in colony formation (Fig. 3C). Similar results were obtained in the anchorage-independent growth assay (Fig. 3D). These results suggested the involvement of Syk kinase in pIgR-stimulated cancer cell proliferation and transformation. Moreover, the pIgR-mediated activation of Syk was markedly reduced by knocking down Yes (Fig. 3E), and inhibiting Syk with siRNAs or a Syk inhibitor blocked pIgR-induced MEK/ERK activation (Fig. 3F,G). Based on these data, Syk precedes the pIgR-associated Yes-mediated activation of MEK/ERK signaling to drive cell proliferation and transformation.
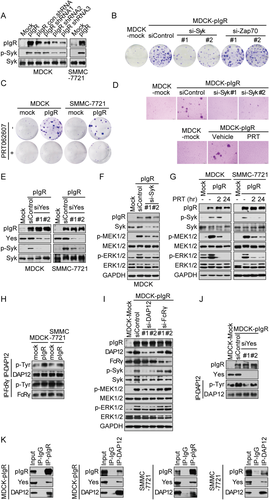
Syk activation by immunoreceptors is known to occur through an immunoreceptor tyrosine-based activating motif (ITAM)-dependent mechanism.21, 22 ITAM is a conserved signaling motif located in the intracellular region of an immunoreceptor or its associated transmembrane adaptor proteins.23 As a member of the Fc receptor family, pIgR does not contain a known ITAM motif within its cytoplasmic domain. We thus speculated on the involvement of ITAM-containing adapters in pIgR-induced Syk activation. DNAX-activating protein of 12 kDa (DAP12) and Fc receptor γ-chain (FcRγ) are ITAM-containing adaptor proteins associated with a wide spectrum of immunoreceptors and participate in the recruitment and activation of Syk.24 As such, we examined the phosphorylation status of DAP12 and FcRγ in pIgR-overexpressing cells. pIgR overexpression led to elevated DAP12 phosphorylation rather than FcRγ phosphorylation, which was determined by immunoprecipitating DAP12 or FcRγ with an anti-phosphorylated-tyrosine antibody (Fig. 3H). Likewise, knockdown of DAP12, but not FcRγ, impaired pIgR-promoted Syk and MEK/ERK phosphorylation (Fig. 3I). Moreover, the pIgR-induced phosphorylation of DAP12 was markedly decreased by Yes knockdown (Fig. 3J). In support of this observation, we detected the presence of pIgR, Yes, and DAP12 in a protein complex precipitated from the lysates of MDCK-pIgR or SMMC-7721-pIgR cells ectopically expressing human DAP12 using either a pIgR antibody or a DAP12 antibody (Fig. 3K).
The results above suggest the coexistence of pIgR, Yes, and DAP12 in a protein complex, which may allow the DAP12 adaptor to recruit and activate Syk. Syk acts as a bridge between activated Yes and downstream MEK/ERK signaling to promote cell proliferation and transformation.
Syk ACTIVATES Rac1/CDC42 TO PRECEDE MEK/ERK ACTIVATION
Syk activates many downstream signaling molecules, including phosphatidylinositol 3-kinase (PI3K), phospholipase Cγ (PLCγ), Ras/Raf, and Rac1/CDC42 GTPase.21 We sought to determine which of these molecules is involved in pIgR overexpression-mediated Syk activation. The involvement of PI3K and PLCγ was largely excluded by the use of pharmacological inhibitors, as shown in Fig. 2A. Thus, we next tested Ras/Raf and Rac1/CDC42 GTPase. A pan-Raf inhibitor (AZ628) had little effect on pIgR-induced colony formation (Supporting Fig. S2A). Similarly, no alterations in MEK/ERK phosphorylation were observed by knocking down A-Raf, B-Raf, or C-Raf in pIgR-expressing cells (Supporting Fig. S2B), ruling out the involvement of Raf in pIgR-initiated MEK/ERK activation.
We thus focused on Rac1/CDC42 GTPases, which are known to play an important role in HCC progression and metastasis.25, 26 Rac1 or CDC42 GTPase were individually knocked down in MDCK-pIgR cells with two specific siRNAs. Both siRNAs efficiently down-regulated Rac1 or CDC42 expression and inhibited the phosphorylation of MEK and ERK (Fig. 4A), indicating both Rac1 and CDC42 are required for MEK/ERK activation. In accord with this observation, pIgR overexpression enhanced the GTPase activity of Rac1 and CDC42, whereas pIgR depletion attenuated GTPase activity (Fig. 4B). In addition, pIgR-induced colony formation was markedly suppressed by the Rac family GTPase inhibitor, EHT1864 (Fig. 4C).
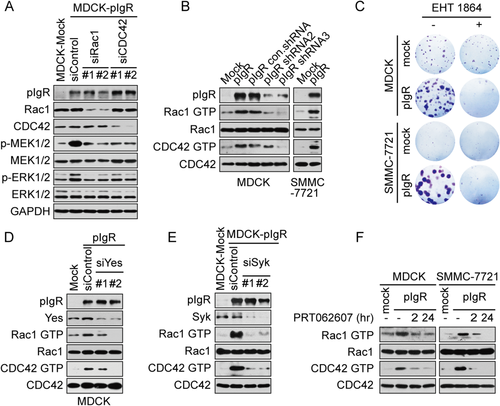
We next examined whether Rac1/CDC42 GTPase activity was controlled by Yes-Syk. Yes knockdown strongly attenuated pIgR-dependent Rac1 or CDC42 GTPase activation (Fig. 4D). Likewise, the depletion of Syk with siRNA or the suppression of Syk kinase activity with PRT062607 markedly decreased Rac1-GTP or CDC42-GTP levels in both pIgR-expressing MDCK cells and pIgR-expressing SMMC-7721 cells (Fig. 4E,F). These observations supported the involvement of the Rac1 and CDC42 GTPases in MEK/ERK activation in the context of pIgR overexpression. Thus, the Yes-DAP12-Syk-Rac1/CDC42-MEK/ERK signaling cascade is responsible for regulating pIgR-mediated cell proliferation.
In agreement with the in vitro observations above, increased intratumoral phosphorylation of Yes, Syk, and MEK/ERK was observed in both SMMC-7721-pIgR and MDCK-pIgR xenograft tumors relative to that in their vector control counterparts (Supporting Fig. S3A,B). These results indicate the ability of pIgR to promote cancer transformation and proliferation through ITAM-based immunoreceptor signaling.
TUMOR-ASSOCIATED pIgR AND Yes SIGNALING STRATIFIES PATIENTS WITH HCC WITH POOR PROGNOSIS
Our previous work showed the ability of high levels of pIgR to identify a proportion of HCC patients with early recurrence, but there is no association between pIgR levels and overall survival (OS) or disease-free survival (DFS) in patients with HCC.7 The present study expanded our investigation of the role of pIgR in promoting cancer transformation and growth through Yes-MEK/ERK signaling activation. We thus sought to evaluate the clinical significance of this pathway in the same set of patient samples, which comprised 254 HCC tumor specimens (Supporting Table S1). We examined the association between pIgR protein levels and Yes and MEK signaling in these samples. pIgR and p-Yes were stained by performing immunohistochemistry (IHC). Among the pIgR-positive HCC specimens, which accounted for 37% of the total samples, 60% of the samples exhibited high phosphorylated Yes (p-Yes) levels, and 72% exhibited high p-ERK1/2 levels, revealing a positive correlation between pIgR levels and the activation of Yes and ERK1/2 (P < 0.001; Fig. 5A; Supporting Table S2).
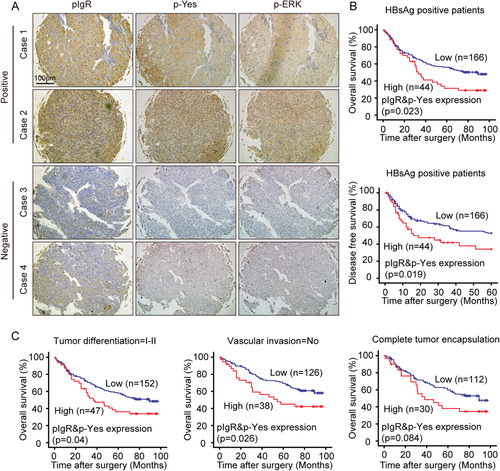
We next examined the combined impact of pIgR and p-Yes levels on patient prognosis. All the samples were subgrouped based on their pIgR total protein levels and p-Yes levels, resulting in a group with high levels of both pIgR and p-Yes (pIgR+ and p-Yes+; n = 56) and a group with low levels of either protein (pIgR– or/and p-Yes–; n = 198). Patients in the pIgR+ and p-Yes+ group had a poor prognosis, with shorter OS and DFS rates than the other patients, although this difference was not statistically significant (P = 0.181 for OS and P = 0.172 for DFS; Supporting Fig. S4A).
Next, we stratified patients according to different clinicopathological parameters. Among the patients positive for hepatitis B surface antigen (HBsAg), which indicates a previous hepatitis B virus (HBV) infection and thus a higher risk of developing HCC, high levels of both pIgR and p-Yes were significantly associated with poor OS and DFS (P = 0.023 for OS and P = 0.019 for DFS; Fig. 5B), which was not the case for HBsAg-negative patients (P = 0.119 for OS and P = 0.137 for DFS; data not shown). The patient samples were also classified by tumor differentiation, vascular invasion, tumor encapsulation, tumor size, and Tumor-Node-Metastasis (TNM) stage. The correlation between high pIgR and p-Yes expression and poor OS was statistically significant in patients with early-stage HCC, particularly in patients with well-differentiated HCC (P = 0.04) or no vascular invasion (P = 0.026; Fig. 5C), whereas a less-significant trend was observed in patients with complete tumor encapsulation, tumor size ≤5 cm, and TNM stage I (P = 0.084, 0.112, and 0.164, respectively; Fig. 5C and Supporting Fig. S4B). Moreover, high pIgR and p-Yes expression also correlated with poor DFS in patients with early-stage HCC, although this association had less statistical significance (Supporting Fig. S4C). In contrast, the combination of pIgR and p-Yes expression was not statistically associated with DFS in patients with late-stage HCC after stratification using these same variables (data not shown). Based on our data, the combination of pIgR and p-Yes expression is closely associated with overall survival and risk of recurrence, particularly in patients with early-stage HCC. When we performed multivariate analysis, the combination of pIgR and p-Yes expression was an independent risk factor for both OS and DFS for patients with HCC (Supporting Table S3) and may be predictive of the prognosis of patients with HCC.
TARGETING Yes-MEK/ERK SIGNALING REPRESENTS A THERAPEUTIC OPTION FOR CANCERS OVEREXPRESSING BOTH pIgR AND p-Yes
Next, we investigated therapeutic opportunities for the HCC subgroup exhibiting high expression of both pIgR and p-Yes. As described above, the Yes-Dap12-Syk-Rac1/CDC42-MEK/ERK signaling cascade promotes the transformation and proliferation of cancer cells overexpressing pIgR. Dasatinib and the MEK inhibitor, AZD6244, are clinically available and inhibit this signaling cascade. Therefore, we tested the efficacy of dasatinib and AZD6244 in the SMMC-7721-pIgR and MDCK-pIgR tumor models. Mice bearing MDCK-pIgR xenografts were given oral doses of dasatinib (50 mg/kg, once per day) or AZD6244 (25 mg/kg, twice-daily). We observed a significant reduction in tumor volume in both the dasatinib- and AZD6244-treated mice, compared to vehicle-treated mice, after 24 days of dosing (Fig. 6A). Moreover, the therapeutic efficacy of dasatinib and AZD6244 was recapitulated in the SMMC-7721-pIgR orthotopic xenograft mouse models. Mice treated with dasatinib or AZD6244 exhibited significantly prolonged survival compared to those treated with vehicle control (P = 0.003 and P = 0.002, respectively; Fig. 6B). Thus, the Yes-Dap12-Syk-Rac1/CDC42-MEK/ERK signaling cascade is therapeutically significant for HCC tumors with high pIgR/p-Yes expression, and dasatinib and AZD6244 may be therapeutic options for patients with concurrent pIgR/p-Yes–positive HCC.
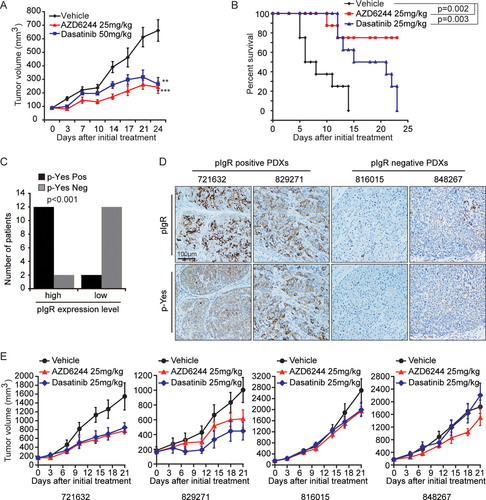
Because the intrinsic properties of artificially cultured cell lines tend to differ from the characteristics of real tumors, we used a patient-derived xenograft (PDX) model to test the potential therapeutic value of MEK or Yes inhibition. The model was established using fresh tumor tissue from cancer patients and recapitulates the heterogeneity and histological characteristics of primary tumors.27 We evaluated a collection of 28 human HCC tumors. pIgR expression and p-Yes levels in these 28 HCC tumors were assessed by performing IHC (data not shown). We then examined the association between pIgR protein levels and p-Yes levels in these samples. Among the pIgR-positive HCC specimens, which accounted for 50% of the samples, 85.7% (12 of 14) exhibited high p-Yes levels, revealing a positive correlation between pIgR levels and p-Yes levels (Fig. 6C). Of the 28 samples, we chose two PDXs (721632 and 829271) that exhibited higher levels of both pIgR and p-Yes (pIgR+ and p-Yes+) and two PDXs (816015 and 848267) that exhibited lower levels of both pIgR and p-Yes (pIgR– and p-Yes–). In agreement with our previous data, the PDXs exhibiting higher levels of pIgR and p-Yes expression were much more sensitive to MEK or Yes inhibition, whereas the PDXs with low levels of pIgR and p-Yes expression remained relatively insensitive to inhibition with the MEK inhibitor, AZD6244, or the Yes inhibitor, dasatinib (Fig. 6D,E). Based on these data, the inhibition of MEK or Yes represents a therapeutic option for patients with HCC that is positive for both pIgR and p-Yes.
Discussion
The interactions between tumor cells and the immune system have long been recognized, triggering a decade-long effort to identify opportunities for cancer immunotherapy. These efforts have been rewarded by the recent remarkable clinical success of approved therapies targeting immune checkpoint machinery.28, 29 Although an increasing number of immunological regulators have been considered potential anticancer targets, our current overall understanding favors the single therapeutic strategy of boosting anticancer immunity to impede cancer progression. Recent studies investigating FcRs, both by our group and others,6 have provided the first evidence suggesting that FcR activation may promote cancer progression. These observations provide insight into the paradoxical role of the immune system in cancer, which is in apparent conflict with immune surveillance. The current study, in which pIgR overexpression promotes cell transformation and proliferation, advances and complements our previous study. Mechanistically, pIgR utilizes an ITAM-based pattern of immunoreceptor signaling to initiate a Yes-Dap12-Syk-Rac1/CDC42-MEK/ERK signaling cascade to promote transformation and cancer cell growth.
Our findings may also provide mechanistic insight into pIgR-associated signal transduction in transcytosis. pIgR-mediated transcytosis is associated with the activation of kinase signaling cascades, but it remains unclear how pIgR activates downstream signaling. Although pIgR itself lacks an ITAM sequence, we suggest that pIgR employs an ITAM-dependent, immunoreceptor-like signaling mechanism by noncovalently associating with DAP12, an ITAM-containing adaptor. The presence of pIgR-DAP12-Yes in a protein complex permits the tyrosine phosphorylation of DAP12. DAP12 phosphorylation further recruits and activates Syk, thereafter initiating the Rac1/CDC42-MEK/ERK signaling cascade. This signaling model, analogous to the ITAM-based signaling of classical immunoreceptors, provides a foundation to further explore the molecular basis of transcytosis and understand the stimulatory role of pIgR in cancer. It remains unclear how pIgR fulfills this role in carcinogenesis. A conceivable link is chronic inflammation, which occurs when the interactions between innate and adaptive immune cells are disturbed. In accord with this model, previous work by Andreu et al. has demonstrated the ability of FcγR members of the FcR family to promote de novo carcinogenesis through chronic inflammatory programs. We have also shown the induction of pIgR expression by cytokines such as tumor necrosis factor alpha, interferon-gamma, and interleukin-4.7 Nevertheless, it is important to provide direct evidence of pIgR overexpression under physiological or pathological conditions in vivo.
This study has important clinical implications and therapeutic significance. In our previous work, we identified pIgR as a potential link between HBV-derived hepatitis and HCC metastasis and as a prognostic biomarker for HCC.7 However, pIgR failed to predict OS in patients with HCC. In this study, we observed a statistically significant association between high pIgR expression and high phosphorylation levels of Yes and ERK, indicative of the clinical significance of pIgR in the activation of Yes and ERK signaling. Of note, clinical follow-up studies further showed a strong correlation between the combination of pIgR expression with p-Yes levels and clinical outcomes in HBsAg-positive patients with HCC and those with early-stage HCC. The combination of pIgR and p-Yes expression may represent a prospective prognostic biomarker for patients with HCC, particularly those in the early stages of disease or those with HBsAg infection. More importantly, targeting the Yes or MEK/ERK signaling pathways may confer therapeutic benefits to patients with HCC that is positive for pIgR and p-Yes. Our study tested this therapeutic strategy both in cell-line–based xenografts and in a PDX model, and the double-positive or -negative expression of pIgR/p-Yes correlated well with HCC sensitivity or resistance to Yes or MEK/ERK inhibition. Given that dasatinib and MEK inhibitors are clinically available, our findings may be readily translated into therapeutic options and rapidly evaluated in clinical trials. Moreover, our results will help expand new indications for either MEK inhibitors or dasatinib, which are currently limited to B-RAF mutant melanoma30-32 or chronic myelogenous leukemia (CML)33, 34 and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL),35-37 respectively.
In summary, our findings have identified a pathological role for pIgR in promoting transformation and tumor growth as well as a pIgR signaling pathway involved in HCC development and progression. Our study also provides a foundation for the use of pIgR- and p-Yes-based biomarkers as prognostic indicators and to inform treatment strategies for HCC.
REFERENCES
Author names in bold designate shared co-first authorship.




