Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites
Potential conflict of interest: Nothing to report.
Peter Jepsen received funding from the Danish Council for Independent Research under the Danish Agency for Science, Technology and Innovation (10-081838/FSS).
Abstract
Proton pump inhibitors (PPIs) may be a risk factor for hepatic encephalopathy (HE) in patients with cirrhosis, possibly through translocation of gut bacteria, which can also lead to spontaneous bacterial peritonitis (SBP). We examined the associations between PPIs and development of HE or SBP in patients with cirrhosis with ascites. We used data from three 1-year trials of satavaptan for ascites control. We used Cox regression to compare HE and SBP rates between users and nonusers of PPIs. At inclusion, 39% of the 865 patients with cirrhosis with ascites used PPIs, 52% used them at some point during the follow-up, and the proportion of current users was always in the 30%-39% range. There were 189 first-time HE episodes during the follow-up, and the cumulative 1-year risk was 31% for those who used PPIs at baseline versus 25% for those who did not. The confounder-adjusted hazard ratio (HR) of HE for current PPI use versus current nonuse was 1.36 (95% confidence interval [CI], 1.01-1.84). The HR for overt HE was higher (adjusted HR = 1.88; 95% CI, 1.21-1.91). During the follow-up, 86 patients developed SBP. The adjusted HR of SBP for current PPI users versus nonusers was 1.72 (95% CI, 1.10-2.69). Conclusion: PPIs were used by 52% of this international cirrhosis cohort during a 1-year period and was a risk factor for developing HE and SBP. These findings are consistent with the hypothesis that PPIs may increase translocation of gut bacteria. (Hepatology 2016;64:1265-1272)
Abbreviations
-
- CI
-
- confidence interval
-
- HE
-
- hepatic encephalopathy
-
- HR
-
- hazard ratio
-
- MELD
-
- Model for End-Stage Liver Disease
-
- PPI
-
- proton pump inhibitor
-
- RCTs
-
- randomized, controlled trials
-
- SBP
-
- spontaneous bacterial peritonitis
Proton pump inhibitors (PPIs) are used by as many as 46-78% of patients with cirrhosis,1, 2 so it is critical that these drugs' risk profile is clarified. It is therefore disconcerting that they were associated with hepatic encephalopathy (HE) in a small case-control study of Asian patients with hepatitis B-related acute-on-chronic liver failure.3 HE is a devastating complication to cirrhosis associated with a poor quality of life, a high risk of recurrence, and a dismal prognosis.
PPIs' desired effect is to decrease gastric acid production and raise the pH of the stomach, but as a side effect, this elimination of the gastric acid barrier facilitates intestinal bacterial overgrowth. This increases the risk of translocation of gut bacteria to the mesenteric lymph nodes and from there to the blood and lymph.4-6 One possible end result is systemic inflammation, which is an important second hit—after the first hit, hyperammonemia—in HE development among patients with cirrhosis.7 It is consequently plausible that PPI use is a risk factor for HE among patients with cirrhosis.
Apart from HE, the bacteria that translocate from the gut to the blood and lymph can cause spontaneous bacterial peritonitis (SBP) in patients with cirrhosis with ascites. By examining PPIs as a risk factor for SBP, the role of bacterial translocation as the link connecting PPIs with HE can therefore be substantiated or refuted. Several studies have already shown that PPIs do increase the risk of SBP,8-13 whereas others, including a recent multicenter, prospective study, found no such association.14-16
Given this background, we used the complete original data from three randomized, controlled trials (RCTs) of satavaptan treatment in patients with cirrhosis and ascites to examine whether PPIs increase the risk of first-time HE. To corroborate the role of bacterial translocation, we also examined whether PPIs increase the risk of SBP.
Material and Methods
THE SATAVAPTAN TRIALS
Three multinational RCTs were conducted between July 2006 and December 2008 to examine whether satavaptan reduced ascites formation in patients with cirrhosis. The three trials had different target populations: patients with diuretic-manageable ascites (N = 462); patients with ascites managed with diuretics and occasional therapeutic paracentesis (N = 496); and patients with diuretic-resistant ascites managed primarily with therapeutic paracentesis (N = 240). The trials were conducted similarly and included 1,198 patients in total (Fig. 1).
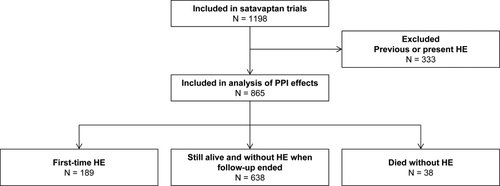
All three trials excluded patients with a functioning transjugular intrahepatic portosystemic shunt and patients with variceal bleeding or SBP in the 10 days before randomization. They also excluded those with a serum creatinine >151 μmol/L, serum potassium ≥5.0 mmol/L, serum sodium >143 mmol/L, serum bilirubin >150 μmol/L, international normalized ratio >3.0, platelets <30,000/mm3, neutrophils <1,000/mm3, hepatocellular carcinoma exceeding the Milan criteria, and those who used a potent modifier of the cytochrome P450 3A pathway or any drug that increased the risk of Q-T interval prolongation.15
The planned treatment duration was 52 weeks, but the second and third trials were stopped early because of a poor risk-benefit ratio.17 All study completers were followed for 1 additional week to assess drug safety. Some patients discontinued treatment prematurely, primarily because of adverse events. These patients were also followed for 1 additional week to assess drug safety. Thus, all patients were followed for 1 week after planned or premature treatment cessation, and in our analysis of the trial data, we stopped follow-up by the end of that 1-week safety period.
Patients were seen every 4 weeks in their hepatology departments. At every visit, all current medications, including their indications and dosages were, recorded, blood tests were taken, patients were examined clinically for HE, and participants in the second and third trials underwent paracentesis of ascites. All clinical events during the follow-up were recorded, including HE and SBP episodes between visits. The analysis of the trial data showed that satavaptan did not affect HE risk (the hazard ratios [HRs] for HE in the trials were 0.96 [95% confidence interval {CI}, 0.63-1.46], 0.89 [95% CI, 0.62 to 1.29], and 0.81 [95% CI, 0.46 to 1.43], respectively) or risk of SBP,17, 18 nor did it have the desired beneficial effect on ascites formation.18 A total of 395 HE episodes were recorded during the follow-up, and they were graded 1-4 according to the West Haven criteria.19 There was no psychometric testing for minimal HE. Factors that precipitated HE episodes were documented. For our analysis of the trial data, we followed this procedure when no precipitant was recorded: Any adverse event during the week before the HE episode was reviewed as a potential precipitant, and the most plausible one was determined. If we were still unable to identify a precipitant, we attributed the HE episode to diuretic overdose if the patient's daily dose of furosemide, spironolactone, or potassium-sparing drug had been increased during the week before the HE episode.
STATISTICAL ANALYSIS
Our primary analysis examined the PPI-HE association (Fig. 2). We excluded patients who had HE grade 1-4 at the time of randomization or had previously had an HE episode. Follow-up began at randomization and ended at the onset of the first HE episode (grade 1, 2, 3, or 4), at death, or in censoring on the date of drug safety assessment after planned or premature treatment discontinuation. SBP episodes were ignored in this analysis.
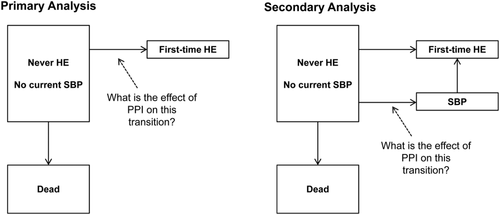
Cumulative risk of HE was computed using the cumulative incidence function, treating death as a competing risk. The cumulative risk is a prediction of a patient's future HE risk based on his or her current PPI use. We did not incorporate data on patients' PPI use during the follow-up because we in the clinic cannot foresee whether our patients will or will not continue using PPIs. Hence, we will not get a clinically meaningful result if our analysis incorporates data on patterns of PPI use during the follow-up. Censoring was likely to depend on cirrhosis severity, because development of cirrhosis complications or other adverse events might result in premature treatment discontinuation. We corrected the estimates of cumulative risk for that dependency using inverse probability of censoring weights.20 With that method, the cumulative risk estimator is reformulated as a step function with a step added whenever a patient develops HE. Step size is determined by that particular cirrhosis patient's probability of not already having been censored (i.e., the patient's inverse probability of censoring). Because censoring occurred late in the study, early HE episodes were unaffected by dependent censoring and gave step sizes identical to what they would have been if censoring had been independent of cirrhosis severity. We derived patients' probability of not having been censored from a Cox regression model that included likely predictors of censoring, that is, predictors of cirrhosis complications that might have caused premature treatment discontinuation. They were PPI use, sex, age, Model for End-Stage Liver Disease (MELD) score, serum sodium, albumin, and platelets.
The number of patients needed to treat with PPIs for a full year to cause one extra HE episode was computed using the estimates of cumulative HE risk; it was the reciprocal of the absolute difference in HE risk between those who did versus those who did not use PPIs at inclusion. This method assumes that there is no confounding, an assumption that is rarely fulfilled unless patients are randomized to receive PPI or placebo.
The effect of current PPI use on the hazard rate of first-time HE was estimated with Cox proportional hazards regression. In this analysis, a patient counted as a PPI user when he or she was using PPIs and as a nonuser when he or she was not. Hence, changes in PPI use over time were taken into consideration in the Cox regression analysis, and the HR expresses the risk of a first-time HE episode during the day for a patient who does versus does not use PPI at the beginning of that day. Cirrhosis severity was the most likely confounder, but it cannot be measured by a single variable. Instead, we included several possible correlates of cirrhosis severity as confounders. They were patient sex, age at inclusion, cirrhosis etiology, variceal bleeding, MELD score, serum sodium, albumin, and platelets; and lactulose use (yes or no, regardless of dose and indication), spironolactone dose, furosemide dose, and potassium-sparing diuretic dose. Age, MELD score, biochemistry, and diuretics were included as continuous, linear variables, and all variables except sex, age, cirrhosis etiology, and history of SBP were updated during the follow-up.
The Cox regression analysis was subsequently repeated within patient subgroups defined by nonuse of lactulose and by severity of ascites, that is, separately among patients with diuretic-responsive ascites and among patients with refractory ascites.
Many clinicians find it difficult to distinguish between absence of HE, minimal HE, and grade 1 HE.21 Minimal HE and HE grade 1 are therefore sometimes combined to “covert HE,” whereas HE grades 2, 3, and 4 are combined to “overt HE.” Accordingly, we repeated our primary analysis with overt HE as the outcome (i.e., HE grades 2, 3, or 4).
Our secondary analysis examined the PPI-SBP association (Fig. 2). It included the same patients as the primary analysis because the trial design excluded patients with SBP at inclusion. Follow-up began at randomization and ended at the onset of the first SBP episode, at the onset of the first HE episode (grade 1, 2, 3, or 4), at death, or in censoring on the date of drug safety assessment after planned or premature treatment discontinuation. We used Cox regression to examine the association between current PPI use and hazard rate of SBP (specifically, “SBP before the first HE episode”), adjusting for the same confounders as in the primary analysis, except that here we also adjusted for history of SBP before inclusion.
Finally, we computed the incidence rate of HE for patients with and/or without SBP. In this analysis, a patient counted as a patient with SBP from his or her first SBP episode during the follow-up to the end of follow-up, whether or not the SBP episode resolved.
Results
Of the 865 included patients with cirrhosis with ascites, 340 (39%) used PPIs at inclusion and a further 108 started during the follow-up. Thus, 52% of patients used PPIs at some point during the follow-up, and there was always between 30% and 39% of patients who were currently using PPIs. At the time of inclusion, PPI users and nonusers had similar age, MELD score, and serum sodium, albumin, and platelets (Table 1). We found an acid-related indication for PPI use in 56% of the patients (ulceration, reflux, or esophagitis). In 10%, the indication was bleeding related (bleeding prophylaxis or after banding procedures). The cirrhosis condition as such (portal hypertension or ascites) was the indication in 7%, whereas PPI use in 27% of the patients was compassionate (gastro-protection, heartburn, or nausea).
| Characteristic | PPI Users | Nonusers of PPI |
|---|---|---|
| No. of patients | 340 | 525 |
| Total observation time | 148.2 person-years | 186.1 person-years |
| SBP episodes | 43 (12) | 43 (8) |
| First-time HE episodes | 88 | 101 |
| Deaths (%) | 17 | 21 |
| Men (%) | 230 (68) | 364 (69) |
| Age (median, IQR) | 58 (50-64) | 57 (51-64) |
| Cirrhosis etiology | ||
| Alcohol alone (%) | 206 (61) | 309 (59) |
| Hepatitis B alone (%) | 14 (4) | 29 (5) |
| Hepatitis C alone (%) | 37 (11) | 74 (14) |
| NASH or cryptogenic (%) | 24 (7) | 46 (9) |
| Other (%) | 59 (17) | 67 (13) |
| MELD score (median, IQR) | 11.3 (8.2-14) | 10.8 (7.2-14.3) |
| Sodium, mmol/L (median, IQR) | 137 (134-139) | 137 (134-140) |
| Albumin, g/L (median, IQR) | 34 (30-38) | 33 (29-38) |
| Platelets, 109/L (median, IQR) | 130 (87-195) | 135 (97-193) |
| Lactulose, any dose (%) | 113 (33) | 75 (14) |
| Spironolactone, any dose (%) | 219 (64) | 337 (64) |
| Furosemide, any dose (%) | 212 (62) | 321(61) |
| Potassium-sparing diuretic, any dose (%) | 251 (74) | 358 (69) |
- Abbreviations: IQR, interquartile range; NASH, nonalcoholic steatohepatitis.
During follow-up, 38 patients from the study group died without having developed HE, and 189 experienced a first-time HE episode (Table 1). A precipitating factor was identified for 110 (58%). Thirty-nine episodes (21% of 189) were caused by electrolyte disorders, 25 (13%) by infection, eight (4%) by constipation, six (3%) by gastrointestinal bleeding, three (2%) by diuretic overdose, and the remaining 29 (15%) by various other causes. There were no notable differences between PPI users and nonusers in this regard, but the PPI users' HE episodes were more severe: 55% of their episodes were overt (grade 2 or higher) HE compared with 43% of the first-time episodes among nonusers of PPIs (P value for chi-square test of independence between PPI use and peak HE grade = 0.02).
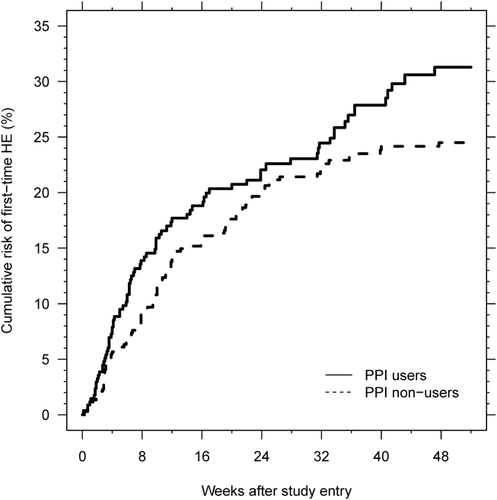
Cumulative 1-year risk of first-time HE was 31% for those who used PPIs at inclusion and 25% for those who did not (Fig. 3). The resulting number of patients needed to treat for 1 year to cause one extra first-time HE episode was 15, assuming that the effect of PPIs on HE development was not confounded by other factors.
Without confounder adjustment, the HR of a first-time HE episode for current PPI users versus current nonusers was 1.50 (95% CI, 1.12-1.99). With adjustment for confounding, it was 1.36 (95% CI, 1.01-1.84). Apart from current PPI use, predictors of HE development included older age, cirrhosis from chronic hepatitis C as opposed to alcohol, variceal bleeding, poor liver function as judged by a high MELD score, low sodium or albumin, and lactulose use (Table 2). Users of lactulose versus nonusers of lactulose were similar with respect to age, cirrhosis etiology, MELD score, sodium, albumin, and platelets (Table 3).
| Adjusted HR | |
|---|---|
| PPI use | 1.36 (1.01-1.84) |
| Male vs. female | 1.27 (0.91-1.77) |
| Age, per 10-year increase | 1.20 (1.02-1.40) |
| Cirrhosis etiology | |
| Alcohol alone | 1 (reference) |
| Hepatitis B alone | 1.31 (0.74-2.33) |
| Hepatitis C alone | 1.54 (1.00-2.38) |
| NASH or cryptogenic | 1.67 (0.99-2.82) |
| Other | 0.95 (0.61-1.49) |
| Variceal bleeding | 9.97 (4.25-23.4) |
| MELD score, per point | 1.11 (1.08-1.14) |
| Sodium, per 5 mmol/L increase | 0.66 (0.58-0.75) |
| Albumin, per 5 g/L increase | 0.73 (0.64-0.84) |
| Platelets, per 10*109/L increase | 1.05 (0.94-1.16) |
| Lactulose, any dose vs. none | 1.94 (1.42-2.65) |
| Spironolactone dose, per 50-mg daily increase | 1.17 (0.93-1.46) |
| Furosemide dose, per 40-mg daily increase | 1.03 (0.92-1.15) |
| Potassium-sparing diuretic, per-10 mg daily increase | 0.98 (0.93-1.02) |
- Abbreviation: NASH, nonalcoholic steatohepatitis.
| Lactulose Users | Nonusers of Lactulose | |
|---|---|---|
| No. of patients | 188 | 677 |
| Men (%) | 475 (70) | 202 (63) |
| Age (median, IQR) | 59 (52-66) | 57 (50-64) |
| Cirrhosis etiology | ||
| Alcohol alone (%) | 404 (60) | 111 (59) |
| Hepatitis B alone (%) | 36 (5) | 7 (3) |
| Hepatitis C alone (%) | 81 (12) | 30 (16) |
| NASH or cryptogenic (%) | 58 (9) | 12 (6) |
| Other (%) | 98 (14) | 28 (13) |
| MELD score (median, IQR) | 11.7 (7.5-14.2) | 10.7 (7.6-14.2) |
| Sodium, mmol/L (median, IQR) | 136 (133-139) | 137 (134-140) |
| Albumin, g/L (median, IQR) | 33 (30-38) | 33 (29-38) |
| Platelets, 109/L (median, IQR) | 137 (92-195) | 131 (94-193) |
- Abbreviations: IQR, interquartile range; NASH, nonalcoholic steatohepatitis.
When we included only nonusers of lactulose, the HR associated with PPI use was the same as in the primary analysis (i.e., adjusted HR = 1.33; 95% CI, 0.93-1.92). Among patients with diuretic-responsive ascites, the HR of first-time HE of any grade was 1.40 (95% CI, 0.90-2.20). Among patients with refractory ascites, it was 1.31 (95% CI, 0.85-2.02), so the effect of PPIs was essentially the same irrespective of the severity of ascites.
When we analyzed only the 92 episodes of overt HE, PPI use was an even stronger risk factor for HE (adjusted hazard ratio = 1.88; 95% CI, 1.21-1.91).
PPIs AS A RISK FACTOR FOR SBP
There were 86 SBP episodes during the follow-up. The adjusted HR of SBP for current PPI users versus nonusers was 1.72 (95% CI, 1.10-2.69). Other statistically significant predictors of SBP included MELD score (adjusted HR per 1-point increase = 1.07; 95% CI, 1.02-1.11), serum sodium (adjusted HR per 5-mmol/L increase = 0.66; 95% CI, 0.54-0.80), serum albumin (adjusted HR per 5-g/L increase = 0.76; 95% CI, 0.63-0.93), and having a history of one or more SBP episodes before inclusion (adjusted HR = 3.43; 95% CI, 2.05-5.76).
Of the 189 patients who developed HE, 6 were diagnosed with SBP and HE simultaneously, and 22 were diagnosed with SBP first and then later with HE. Excluding the six simultaneous diagnoses, the incidence rate of HE among the 22 patients with SBP was 1.23 episodes per person-year (95% CI, 0.81-1.87). By comparison, the incidence rate of HE for patients without SBP was 0.33 episodes per person-year (95% CI, 0.28-0.38). Thus, SBP was a risk marker for HE development, consistent with the hypothesis that translocation of gut bacteria may lead to both SBP and HE.
Discussion
Our analysis of data from three large, multicenter, randomized trials showed that PPI use was a risk factor for first-time HE and for SBP among patients with cirrhosis with ascites, and that the effect of PPI use on HE risk did not depend on the severity of ascites.
Risk of bias in these analyses is minimal. The follow-up examinations and the collection of drug usage were laid out in the protocol, and there is no reason to suspect that diagnostic criteria for HE and SBP were different for PPI-users and nonusers. The diagnosis of grade 1 HE is difficult, but we showed that our conclusions are not sensitive to the decision to include or exclude grade 1 HE episodes in the definition of HE. Finally, satavaptan itself did not affect the risk of HE or SBP in any of the three trials.17, 18
Patients were not randomized to receive PPIs or not, but we controlled for several markers of cirrhosis severity and for lactulose use and believe that the risk of uncontrolled confounding in the regression analyses is small. By contrast, the estimate of the number needed to treat was not confounder controlled and must therefore be interpreted cautiously. Counterintuitively, current lactulose use was associated with an increased risk of HE, even after adjustment for several correlates of cirrhosis severity. This is likely because lactulose was given to patients perceived to be at a high risk of HE (e.g., those with suspected minimal HE). This assumption could have been substantiated if patients had been examined for minimal HE, or if data on other predictors of minimal HE had been collected during the trials, but they were not. In order to examine the risk of uncontrolled confounding by minimal HE, we repeated our primary analysis within the group of patients who were not on lactulose and therefore presumably free from minimal HE. The effect of PPIs on HE development was the same in this subcohort as in the full cohort, except for reduced precision because of the smaller cohort and fewer HE episodes. This finding provides reassurance that confounding by minimal HE was negligible once we controlled for use of lactulose.
A further limitation is that PPI use varies over time. This gives a possibility of time-dependent confounding.23 Such confounding occurs when a predictor of HE development is affected by PPI use and also predicts future PPI use. However, the predictors of future PPI use are unclear, as evidenced by the variable indications for PPI use in our data. We did conduct two supplementary analyses in which we considered lactulose and lactulose plus SBP as time-dependent confounders.24 In those two more-complicated analyses, the effect of PPI on HE development was 1.45 (95% CI, 1.04-2.03) and 1.49 (95% CI, 1.07-2.07), respectively, compared with 1.36 (95% CI, 1.01-1.84) in our primary analysis. Therefore, we find that time-dependent confounding is more of a theoretical possibility than a threat to our conclusions.
The trials from which our data originate were designed to determine the efficacy of satavaptan treatment, not to determine the mechanisms by which PPIs may or may not cause HE. Nonetheless, the findings that PPI use is a risk factor for SBP and that SBP is a risk factor for HE do provide support for the hypothesis that PPIs contribute to the development of HE by promoting translocation of gut bacteria.
Our findings conflict with data from Brazil published as a letter to the editor.26 In those data, 582 patients with cirrhosis were followed for a median of 5 years, during which time 258 developed ascites. Among those 258 patients, SBP developed in 34 of 151 PPI users (22.5%) and in 23 of 107 nonusers (21.5%). This difference was not significant. Like the Brazilian study, a recently published Argentinian study by Terg et al. found no association between PPI use and SBP.16 They did not adjust the PPI-SBP association for confounding, but nothing in their published data indicates that the null association was attributed to confounding. Thus, the discrepancy between our findings and the two studies remains unexplained. In support of our findings, a recent prospective study collected data from 188 patients hospitalized with cirrhosis and infections. After 6 months of follow-up, a higher proportion of those who used PPI were readmitted with infection.26
PPIs are central in the management of acid-related gastrointestinal disorders. In many countries, they are sold over the counter because of their perceived harmlessness.2 We were astonished that 30% to 39% of cirrhosis patients in our world-wide data set used PPIs at any given time, but that proportion is, in fact, marginally lower than in the Terg et al. study from Argentina (43.5%).16 An operational interpretation of our results is that we might reduce our cirrhosis patients' risk of HE and SBP by insisting on a proper peptic-acid-related indication for PPI use. Such an indication was found in only 56% of our PPI users. Apparently, the remaining 44% of PPI users were put at increased risk of cirrhosis complications for no benefit.
In conclusion, our findings clearly demonstrated that PPIs pose a threat to patients with cirrhosis with ascites by increasing their risk for HE. Future studies designed to answer mechanistic question are needed to draw firm conclusions. However, the parallel increase in SBP risk points to a mechanism that goes through increased translocation of bacteria from the gut to the blood. Our findings suggest that prescription of PPIs to patients with cirrhosis at risk of HE needs an appropriate indication.




