Nomogram for individualized prediction of hepatocellular carcinoma occurrence in hepatitis C virus cirrhosis (ANRS CO12 CirVir)
Potential conflict of interest: Dr. Di Martino consults for Bristol-Myers Squibb, Gilead, AbbVie, and Merck. Dr. Serfaty consults for and is on the speakers' bureau for Gilead, MSD, AbbVie, Janssen, and Bristol-Myers Squibb. Dr. Bourliere consults and advises for Bristol-Myers Squibb, Gilead, MSD, Janssen, and GlaxoSmithKline. Dr. Ganne-Carrie is on the speakers' bureau for and received grants from Bristol-Myers Squibb and Gilead. She received grants from Janssen. Dr. Abergel consults for, is on the speakers' bureau of, and received grants from Gilead and MSD. He consults for and received grants from Janssen and Roche. He consults for AbbVie and Bristol-Myers Squibb. Dr. Dao consults for, is on the speakers' bureau of, and received grants from Gilead. He is on the speakers' bureau and received grants from Janssen. He received grants from AbbVie and Bristol-Myers Squibb. Dr. Zarski consults for and received grants from Gilead, Janssen, Bristol-Myers Squibb, MSD, and AbbVie. Dr. Zoulim consults for and is on the speakers' bureau for AbbVie and Gilead. Dr. Pol consults for, received lecture fees from, and received grants from Bristol-Myers Squibb, Gilead, Roche, and MSD. He consults for and received lecture fees from Boehringer Ingelheim, Janssen, Vertex, Novartis, AbbVie, Sanofi, and GlaxoSmithKline. Dr. Alric consults for and received grants from Bristol-Myers Squibb and Gilead. He is on the speakers' bureau for and received grants from MSD. He consults for AbbVie. He received grants from Janssen. Dr. Bacq has served as a speaker for Roche, Gilead, and Bristol-Myers Squibb. Dr. Nahon received honoraria from Bayer, Bristol-Myers Squibb, and Gilead. Dr Roudot-Thoraval is an advisor for Roche, Gilead, and AbbVie, and served as speaker for Roche, Bristol-Myers Squibb, AbbVie, and Gilead.
This study was sponsored by the ANRS (France REcherche Nord & Sud Sida-hiv Hépatites: FRENSH). The funding sponsor had no role in the design or conducting of the study: collection, management, analysis, interpretation of data, nor preparation, review, or approval of the manuscript.
Abstract
The aim of this work was to develop an individualized score for predicting hepatocellular carcinoma (HCC) in patients with hepatitis C (HCV)-compensated cirrhosis. Among 1,323 patients with HCV cirrhosis enrolled in the French prospective ANRS CO12 CirVir cohort, 720 and 360 were randomly assigned to training and validation sets, respectively. Cox's multivariate model was used to predict HCC, after which a nomogram was computed to assess individualized risk. During follow-up (median, 51.0 months), 103 and 39 patients developed HCC in the training and validation sets, respectively. Five variables were independently associated with occurrence of HCC: age > 50 years (hazard ratio [HR], 1.94; 95% confidence interval [CI], 1.16; 3.25; P = 0.012); past excessive alcohol intake (HR, 1.55; 95% CI, 1.02; 2.36; P = 0.041); low platelet count (<100 Giga/mm3: HR, 2.70; 95% CI, 1.62; 4.51; P < 0.001; [100; 150] Giga/mm3: HR, 1.87; 95% CI, 1.10; 3.18; P = 0.021); gamma-glutamyl transpeptidase above the upper limit of normal (HR, 1.96; 95% CI, 1.11; 3.47; P = 0.021); and absence of a sustained virological response during follow-up (HR, 3.02; 95% CI, 1.67; 5.48; P < 0.001). An 11-point risk score was derived from the training cohort and validated in the validation set. Based on this score, the population was stratified into three groups, in which HCC development gradually increased, from 0% to 30.1% at 5 years for patients with the lowest (≤3) and highest (≥8) scores (P < 0.001). Using this score, a nomogram was built enabling individualized prediction of HCC occurrence at 1, 3, and 5 years. Conclusion: This HCC score can accurately predict HCC at an individual level in French patients with HCV cirrhosis. (Hepatology 2016;64:1136-1147)
Abbreviations
-
- AASLD
-
- American Association for the Study of Liver Diseases
-
- AFP
-
- alpha-fetoprotein
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- AUROC
-
- area under the ROC curve
-
- BMI
-
- body mass index
-
- CI
-
- confidence interval
-
- DAAs
-
- direct antiviral agents
-
- GGT
-
- gamma-glutamyl transpeptidase
-
- HBsAg
-
- hepatitis B surface antigen
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HIV
-
- human immunodeficiency virus
-
- HR
-
- hazard ratio
-
- MetS
-
- metabolic syndrome
-
- IFN
-
- interferon
-
- IQR
-
- interquartile range
-
- Peg-IFN
-
- pegylated interferon
-
- RBV
-
- ribavirin
-
- ROC
-
- receiver operating curve
-
- SH
-
- steatohepatitis
-
- SVR
-
- sustained virological response
-
- ULN
-
- upper limit of normal
-
- US
-
- ultrasonography
Approximately 80% of hepatocellular carcinomas (HCC) worldwide are related to chronic infection with hepatitis B (HBV) or hepatitis C virus (HCV).
In Western countries, spread of HCV infection has been partly responsible for a rise in the incidence of cirrhosis and HCC during recent decades.1 In those countries, historical cohort studies reported 5-year cumulative incidences of HCC of nearly 17% in patients with HCV cirrhosis.2 More recently, large, prospective Western cohorts confirmed the high incidence of HCC in HCV cirrhosis: in the subgroup of 410 patients with HCV-cirrhosis enrolled in the HALT-C American cohort, and who did not respond to pegylated interferon (Peg-IFN) and ribavirin (RBV),3 and among 1,323 patients with histologically proven and uncomplicated HCV cirrhosis enrolled from 2006 to 2012 in the ANRS CO12 CirVir French multicenter cohort,4 cumulative HCC incidences were 7% at 5 years and 11.4% at 4 years, respectively. Given that HCC screening is considered cost-effective if the incidence of HCC is over 1.5% per year,5, 6 patients with HCV cirrhosis should thus undergo periodic surveillance for HCC. Moreover, in the ANRS CO12Cirvir French prospective cohort, this policy of periodic surveillance of cirrhosis was successful and resulted in detection of a high proportion of small HCC, fulfilling Milan criteria in 80% of cases and enabling curative treatment in 70% of cases.4
However, although patients with cirrhosis, whatever its etiology, are at risk of HCC and should undergo periodic surveillance, experience shows that not all of them have the same risk of developing HCC, suggesting that HCC surveillance in certain subsets of patients with cirrhosis may not be cost-effective. Several potential factors have been shown to be independently associated with higher risk of HCC occurrence in patients with HCV cirrhosis: (1) host factors, including male sex,7, 8 increasing age,3, 7, 8 black origins,3 and diabetes and overweight8; (2) virological factors, such as detectable serum HCV RNA9 and HCV genotypes 14 or 310; (3) disease factors, including liver failure assessed by albumin serum level4 and portal hypertension assessed either by low platelet count4 or by esophageal varices3, 7; and (4) environmental factors, such as tobacco intake. Conversely, other factors were identified with lower risk of HCC and might be chemoprotective in patients with HCV cirrhosis, such as coffee consumption11 and metformin12 or statin treatment.13
However, at an individual level, it remains difficult to assess the specific risk of HCC in a patient with HCV cirrhosis or advise that person on the need for periodic screening for HCC. Personalized assessment of individual risk of HCC thus represents a challenge.
Therefore, the aims of this study were to build and validate a simple personalized scoring system using baseline independent variables for HCC occurrence and develop a nomogram for predicting HCC risk in patients with HCV-related compensated cirrhosis included in the large French prospective, multicenter ANRS CO12 CirVir cohort.4
Patients and Methods
PATIENT SELECTION
Among patients with biopsy-proven compensated (Child-Pugh A) and uncomplicated virus-related cirrhosis who were enrolled in the French multicenter ANRS CO12 CirVir cohort between March 2006 and July 2012 and prospectively followed up for development of HCC,4 those with chronic infection by HCV (positive HCV serology whatever the level of viral replication) and without HBV coinfection (negative serum hepatitis B surface antigen [HBsAg]) were selected. If data on well-known predictive factors for HCC occurrence in patients with cirrhosis were not available, patients were secondarily excluded.
Preinclusion assessment included usual clinical and biological parameters (sex, age, body mass index [BMI], diabetes, arterial hypertension, dyslipidemia, metabolic syndrome [MetS], prothrombin time, bilirubin serum level, albumin serum level, platelet count, liver enzymes [aspartate aminotransferase {AST}, alanine aminotransferase {ALT}, and gamma-glutamyl transpeptidase {GGT}], alpha-fetoprotein (AFP) serum level, esophageal varices, HCV genotype, HCV viral load, human immunodeficiency virus [HIV] coinfection, and histological lesions of steatohepatitis [SH]) and a Doppler ultrasonography (US) to rule out complications at baseline, including HCC.
Patient information was recorded in a computerized database by a clinical research associate specifically dedicated to the ANRS CO12 CirVir cohort at each center. For all patients, past and ongoing alcohol or tobacco consumption was quantified and recorded at inclusion.
Excessive alcohol consumption was defined according to World Health Organization criteria (more than 2 glasses per day for females and more than 3 glasses per day for males); an overall minimal duration of 5 years was required.
Past medical history was also noted. In particular, the senior hepatologist in charge of a given patient at each center determined whether MetS was present or not, based on clinical guidelines14 and/or histological examination of liver biopsy.15
FOLLOW-UP
Follow-up was scheduled according to French guidelines (Haute Autorité de Santé). Examination by Doppler US was performed every 6 months. For a given patient, it was recommended that US be formed at the same center by an experienced operator. A report was completed by each operator, mentioning the presence or not of focal liver lesions.
All events occurring during follow-up, liver related or not, were recorded based on information obtained from medical files of patients at each center and monitored by a panel of three dedicated clinical research associates. All treatments, including antiviral therapy, were recorded at inclusion, and any modification during follow-up was notified.
HCC DIAGNOSIS AND TREATMENT
In case of focal liver lesions detected by US, the following procedures were carried out: (1) echogenicity, number and diameter of nodules (classified as <10, 11-20, 21-30, 31-50, or >50 mm), and anatomic localization according to the Couinaud classification were reported; (2) portal vasculature (main trunk and branches), hepatic veins, and vena cava were systematically examined; and (3) a diagnostic procedure using contrast-enhanced imaging (computed tomography scan or magnetic resonance imaging), serum AFP assay, and/or guided biopsy was performed according to 2005 American Association for the Study of Liver Diseases (AASLD) guidelines6 updated in 2011.16 HCC diagnosis was thus established either by histological examination by an experienced pathologist or using probabilistic noninvasive criteria (mainly dynamic imaging showing early arterial hypervascularization and portal washout) according to the different periods of time (before and after 2011). When the HCC diagnosis was established, treatment was decided using a multidisciplinary approach according to AASLD or European Association for the Study of the Liver guidelines for HCC.5, 6, 16 Reports of imaging techniques showing liver focal lesions were secondarily reviewed by the two senior hepatologists from institution 1 (authors V.B. and P.N.).
ANTIVIRAL TREATMENT AND VIRAL REPLICATION
From 2006 to January 2014 (before the introduction of second-generation direct antiviral agents [DAAs] in France), all antiviral therapies conducted were interferon (IFN) based according to international guidelines. Since January 2014, patients could also receive second-generation DAAs with or without Peg-IFN and RBV for 12 to 24 weeks as part of early access programs, or after market approval according to national and/or international guidelines. The primary efficacy outcome was a sustained virological response (SVR), defined as HCV RNA undetectable by qualitative polymerase chain reaction assay (<50 IU/mL) at the end of either a 24-week untreated follow-up period for IFN-based antiviral therapies without second-generation DAAs, or a 12-week untreated follow-up period for antiviral therapy, including second-generation DAAs.
STATISTICAL ANALYSES
To internally validate the final adjusted HCC algorithm, we used a split-sample approach to assess discrimination, calibration, and predictive ability of the model. We randomly divided the studied population of HCV-related Child-Pugh A cirrhosis into a development subset (two thirds, on which model parameter estimates and the corresponding model-based HCC probabilities were derived) and a validation subset (one third, to which model results were applied) and used our final model in these two samples.
Descriptive statistics are presented for the entire cohort and for the two subsets, as median (interquartile range; IQR) for continuous variables and as numbers (percentages) for categorical data. Comparisons of patient subsets were performed using the Student t test or Wilcoxon rank-sum test for continuous variables, and the chi-square or Fisher's exact test for categorical variables.
The cumulative incidence of HCC was estimated in the training cohort in a competing risks framework, where deaths free of HCC were considered as competing events. Because no difference was observed with Kaplan-Meier estimates, univariate and multivariate Cox proportional hazards models were used in the training cohort to assess factors associated with occurrence of HCC. In particular, the effect of an SVR was assessed in Cox regression as a time-dependent covariate, given that patients without SVR at inclusion could be (re)treated and achieve SVR during follow-up. For these patients, end of treatment was defined as time T0, given that patients with undetectable HCV RNA at that time were considered as having an SVR. All variables tested in univariate Cox analysis with a P value less than 0.20 were tested in multivariate Cox regression.
A predictive score was then constructed as a weighted sum of all independent predictive factors for the occurrence of HCC, identified in Cox multivariate analysis. The weight of each factor was given by a linear transformation (multiplied by 3) of the estimated coefficient in Cox regression analysis and rounded off to the nearest integer. Baseline HCC-free probabilities were also estimated from Cox multivariate analysis at 1, 3, and 5 years. Predicted risks for HCC were estimated by the following equation:
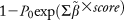 , where
, where
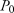 was baseline HCC-free probability and
was baseline HCC-free probability and
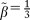 was the inverse multiplicative coefficient used to build the HCC score. Predicted probabilities of HCC at 1, 3, and 5 years are presented for each level of the score as a nomogram.
was the inverse multiplicative coefficient used to build the HCC score. Predicted probabilities of HCC at 1, 3, and 5 years are presented for each level of the score as a nomogram.
Cumulative incidence curves according to the different risk groups of HCC determined from the score were built using a clock reset approach. Patients who switched from non-SVR to SVR status were censored at the time of SVR, which was the date of the end of treatment. The time of SVR was reset at time 0 for patients having SVR status during follow-up.
Comparison of cumulative incidence curves was assessed with univariate Cox regression analyses.
Discrimination was assessed by receiver operating curve (ROC) analysis. Overall predictive accuracy of the derived score for predicting the development of HCC at 1 and 3 years after inclusion was tested by estimating the area under the ROC curve (AUROC).
Calibration of the model was assessed by Harrell's C statistics and the correlation coefficient between predicted and observed probabilities of HCC.
The score was secondarily tested in the validation cohort.
Last, the score was reassessed after 12 months of follow-up.
All statistical analyses were performed using Stata software (version 13.0; StataCorp LP, College Station, TX). A P value of 0.05 or less was considered statistically significant.
Results
Among 1,671 patients consecutively enrolled between March 2006 and July 2012 in the French ANRS CO12 CirVir cohort, 1,323 had HCV cirrhosis and were HBsAg seronegative. Secondarily, 243 of them were excluded from analysis because of missing data regarding known predictive factors for HCC. Finally, 1,080 patients were analyzed and randomly assigned to training (n = 720) and validation (n = 360) sets (Fig. 1).
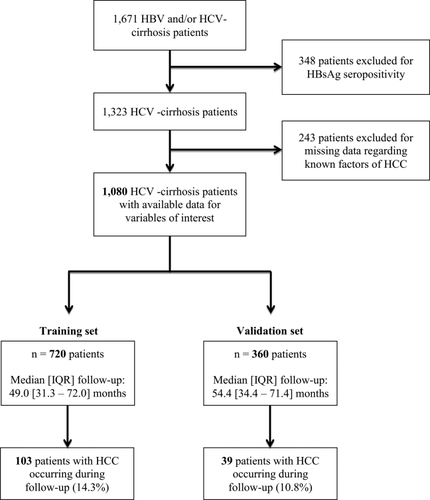
Patient characteristics at inclusion were similar in the two groups, except for prothrombin time, which was slightly higher in the validation group. Patients were mainly male (64.0%); median age was 55.6 years [48.9-64.0]. Median BMI was 25.7 kg/m2. Comorbidities were frequent, including overweight or obesity in 57.8%, diabetes in 18.9%, and past excessive alcohol consumption in 31.3% of cases (median alcohol consumption 80 g/day [IQR, 50-100] for 11.5 years [IQR, 10-20]). Patients were mainly monoinfected (4.6% of patients with HIV coinfection) by genotype 1 (69.1%). Only 212 (19.6%) of them had SVR at baseline, confirmed at endpoint; overall, at endpoint, 427 of the 1,080 selected patients (39.5%) had SVR, including 215 who achieved SVR during follow-up (Table 1).
| Characteristics | No. of Patients |
Whole HCV CirVir Cohort (n = 1,323) |
Studied Cohort (n = 1,080) |
Training Cohort (n = 720; 66.7%) |
Validation Cohort (n = 360; 33.3%) |
P Valued |
|---|---|---|---|---|---|---|
| Age (years)b | 1,323 | 55.4 [48.9-64.3] | 55.6 [48.9-64.0] | 56.2 [49.4-63.9] | 54.6 [48.2-64.5] | 0.26 |
| Male sexc | 1,323 | 839 (63.4) | 691 (64.0) | 454 (63.1) | 237 (65.8) | 0.37 |
| Prothrombin time (%)b | 1,282 | 89.0 [79.0-98.0] | 89.0 [79.0-98.0] | 88.0 [79.0-98.0] | 90.0 [81.0-99.0] | 0.044 |
| Bilirubin (μmol/L)b | 1,288 | 12.9 [9.4-18.0] | 13.0 [9.4-18.0] | 13.0 [10.0-18.0] | 12.0 [9.0-17.1] | 0.22 |
| Serum albumin (g/L)b | 1,248 | 41.4 [38.0-44.4] | 41.6 [38.0-44.6] | 41.4 [38.0-44.4] | 42.0 [38.3-44.9] | 0.09 |
| Platelet count (103/mm3)b | 1,301 | 136.0 [96.0-182.0] | 138.0 [98.0-187.0] | 135.5 [95.0-183.0] | 148.5 [104.5-192.0] | 0.08 |
| AST (IU/L)b | 1,306 | 58.0 [35.0-92.0] | 58.0 [35.0-93.0] | 58.0 [35.0-92.0] | 57.0 [34.0-96.0] | 0.61 |
| ALT (IU/L)b | 1,311 | 63.0 [35.0-108.0] | 64.0 [35.0-109.0] | 64.0 [35.0-109.0] | 63.0 [36.0-110.0] | 0.57 |
| GGT (IU/L)b | 1,241 | 86.0 [47.0-162.0] | 85.5 [46.0-162.0] | 86.5 [47.0-158.5] | 85.0 [45.0-154.5] | 0.97 |
| AFP (ng/mL)b | 1,097 | 6.0 [3.7-11.2] | 6.0 [3.7-11.6] | 6.0 [3.7-11.3] | 5.6 [3.7-12.0] | 0.65 |
| Esophageal varicesc | 1,070 | 332 (31.0) | 283 (32.0) | 198 (33.3) | 85 (29.2) | 0.22 |
| HCV genotypec | 1,203 | 0.42 | ||||
| 1 | 821 (68.3) | 681 (69.1) | 450 (69.1) | 231 (69.1) | ||
| 2 | 66 (5.5) | 56 (5.7) | 35 (5.4) | 21 (6.3) | ||
| 3 | 188 (15.6) | 146 (14.8) | 91 (14.0) | 55 (16.5) | ||
| 4 | 106 (8.8) | 85 (8.6) | 63 (9.7) | 22 (6.6) | ||
| 5 | 18 (1.5) | 14 (1.4) | 9 (1.4) | 5 (1.5) | ||
| 6 | 4 (0.3) | 3 (0.3) | 3 (0.4) | 0 | ||
| HIV coinfectionc | 1,207 | 56 (4.6) | 46 (4.6) | 30 (4.6) | 16 (4.7) | 0.92 |
| Negative HCV viral loadc | 1,300 | 377 (29.0) | 304 (28.7) | 210 (29.5) | 94 (26.9) | 0.36 |
| BMIb | 1,162 | 25.8 [23.0-28.8] | 25.7 [22.9-28.9] | 25.9 [23.0-29.0] | 25.4 [22.8-28.4] | 0.39 |
| BMI (class)c | 1,162 | 0.49 | ||||
| <25 | 487 (41.9) | 403 (42.2) | 261 (40.9) | 142 (40.9) | ||
| [25; 30] | 457 (39.3) | 374 (39.2) | 255 (40.0) | 119 (37.7) | ||
| ≥30 | 218 (18.8) | 177 (18.6) | 122 (19.1) | 55 (17.4) | ||
| Diabetesc | 1,323 | 253 (19.1) | 204 (18.9) | 139 (19.3) | 65 (18.1) | 0.62 |
| Dyslipidemiac | 1,323 | 69 (5.2) | 53 (4.9) | 41 (5.7) | 12 (3.3) | 0.09 |
| Arterial hypertensionc | 1,323 | 373 (28.2) | 302 (28.0) | 203 (28.2) | 99 (27.5) | 0.81 |
| MetSc, e | 1,323 | 226 (17.1) | 186 (17.2) | 125 (17.4) | 61 (16.9) | 0.86 |
| Past excessive alcohol consumptionc | 1,266 | 406 (32.1) | 338 (31.3) | 215 (29.9) | 123 (34.2) | 0.15 |
| Ongoing alcohol consumption (g/day)c | 1,021 | 0.97 | ||||
| 0 | 918 (74.9) | 770 (75.4) | 509 (75.2) | 261 (75.9) | ||
| <10 | 193 (15.8) | 161 (15.8) | 108 (15.9) | 53 (15.4) | ||
| ≥10 | 114 (9.3) | 90 (8.8) | 60 (8.9) | 30 (8.7) | ||
| Ongoing tobacco consumptionc | 1,229 | 0.21 | ||||
| Never | 491 (40.0) | 406 (39.7) | 279 (41.2) | 127 (36.8) | ||
| Past | 277 (22.5) | 236 (23.1) | 159 (23.5) | 77 (22.3) | ||
| Ongoing | 461 (37.5) | 380 (37.2) | 239 (35.3) | 141 (40.9) |
- a The entire population of patients with HCV-related cirrhosis enrolled in the French ANRSCO12 CirVir cohort (n = 1,323); the entire selected population (n = 1,080); and according to randomly assigned groups (training and validation sets).
- b Median [Q1-Q3].
- c Number (percentage) of patients.
- d Training cohort versus validation cohort
- e The hepatologist in charge of a given patient defined whether MetS was present or not based on clinical guidelines14 and/or histological examination of liver biopsy.15
During a median follow-up of 51.0 months [32.3-71.9], a diagnosis of HCC was established for 142 of the 1,080 selected patients, that is, 103 and 39 in the training and validation sets, respectively (Fig. 1; Table 2).
| Characteristics |
Whole HCV CirVir Cohort (n = 1,323) |
Studied Cohort (n = 1,080) |
Training Cohort (n = 720; 66.7%) |
Validation Cohort (n = 360; 33.3%) |
P Valueb |
|---|---|---|---|---|---|
| No. of HCC patients | 162 (12.2) | 142 (13.2) | 103 (14.3) | 39 (10.8) | 0.08 |
| Tumor type | 0.49 | ||||
| Uninodular | 98 (64.9) | 87 (64.9) | 62 (64.6) | 25 (65.8) | |
| 2 or 3 nodules | 39 (25.8) | 34 (25.4) | 23 (24.0) | 11 (28.9) | |
| >3 nodules | 9 (6.0) | 8 (6.0) | 6 (6.2) | 2 (5.3) | |
| Infiltrating | 5 (3.3) | 5 (3.7) | 5 (5.2) | 0 | |
| MD | 11 | 8 | 7 | 1 | |
| Diameter of largest nodule (mm) | 0.21 | ||||
| ≤30 | 112 (80.0) | 100 (80.0) | 70 (78.6) | 30 (83.3) | |
| 31-50 | 15 (10.7) | 13 (10.4) | 8 (9.0) | 5 (13.9) | |
| >50 | 13 (9.3) | 12 (9.6) | 11 (12.4) | 1 (2.8) | |
| MD | 22 | 17 | 14 | 3 | |
| Portal thrombosis | 12 (8.4) | 10 (7.9) | 10 (10.9) | 0 | 0.061 |
| MD | 19 | 15 | 11 | 4 | |
| Within Milan criteria | 121 (82.0) | 107 (80.5) | 74 (77.1) | 33 (89.2) | 0.12 |
| 1 nodule ≤50 mm | 92 | 81 | 57 | 24 | |
| 2 or 3 nodules ≤30 mm | 29 | 26 | 17 | 9 | |
| Outside Milan criteria | 28 (18.0) | 26 (19.5) | 22 (22.9) | 4 (10.8) | |
| MD | 13 | 9 | 7 | 2 | |
| AFP level at HCC diagnosis (ng/mL) | |||||
| Median [Q1-Q3] | 14.6 [7.0-84.0] | 12.6 [6.0-90.0] | 15.5 [6.4-90.2] | 11.0 [5.4-74.9] | 0.46 |
| >200 ng/mL | 16 (13.2) | 13 (12.3) | 12 (15.6) | 1 (3.5) | 0.11 |
| MD | 41 | 36 | 26 | 10 | |
| HCC treatmenta | 0.08 | ||||
| Curative intent | 97 (67.8) | 83 (66.4) | 57 (62.0) | 26 (78.8) | |
| Palliative intent | 46 (32.2) | 42 (33.6) | 35 (38.0) | 7 (21.2) | |
| MD | 19 | 17 | 11 | 6 |
- a HCC management included one or several associated therapeutic procedures.
- b Training cohort vs validation cohort.
- Abbreviation: MD, missing data.
Most HCCs were uninodular (n = 87; 64.9%) and smaller than 30 mm (n = 100; 80.0%). Portal obstruction and a serum AFP level >200 ng/mL at diagnosis were observed in 7.9% and 12.3%, respectively. Overall, 107 (75%) patients with HCC fell within Milan criteria, and curative treatment as first-line therapy was performed in 83 (66.4%). There were no significant differences in HCC characteristics between the two groups of patients (Table 2).
TRAINING COHORT
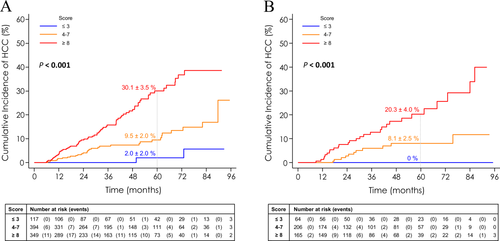
Based on multivariate Cox regression analysis, 5 of 23 variables listed in Table 3 were independently associated with occurrence of HCC in the training cohort: age >50 years (hazard ratio [HR], 1.94; 95% confidence interval [CI], 1.16; 3.25; P = 0.012); past excessive alcohol intake (HR, 1.55; 95% CI, 1.02; 2.36; p = 0.041); low platelet count (<100 Giga/mm3: HR, 2.70; 95% CI, 1.62; 4.51; P < 0.001; [100; 150] Giga/mm3: HR, 1.87; 95% CI, 1.10; 3.18; P = 0.021); GGT above the upper limit of normal (ULN; (HR, 1.96; 95% CI, 1.11; 3.47; P = 0.021); and no occurrence of SVR during the study period (HR, 3.02; 95% CI, 1.67; 5.48; P < 0.001; Table 3). An 11-point risk score was derived from the estimated β-regression coefficients of these five variables, by linear transformation (multiplied by 3) of the coefficients and rounding off (Table 4). The population was stratified into three groups according to this scoring system, with HCC occurrence gradually increasing from 2% to 30.1% at 5 years for patients with the lowest (≤3; n = 117; 13.8%) and highest (≥8; n = 349; 40.6%) HCC risk scores, respectively (P < 0.001; Fig. 2A). AUROC for 1- and 3-year predictions after baseline was 0.678 [0.555-0.801] and 0.717 [0.661-0.773], respectively, in the training group. Implementation of this score after 12 months of follow-up, taking into account increasing age, new values of platelets and GGT, and potential recent SVR, gave an AUROC for the 1- and 3-year predictions of 0.678 [0.574-0.783] and 0.727 [0.655- 0.800], respectively, in this subset of patients still at risk of HCC.
| Univariate Cox | Multivariate Cox | |||||
|---|---|---|---|---|---|---|
| Features | HR | 95% HR CI | P Value | HR | 95% HR CI | P Value |
| Age >50 years | 1.65 | [1.00; 2.72] | 0.049 | 1.94 | [1.16; 3.25] | 0.012 |
| Mal sex | 0.78 | [0.53; 1.15] | 0.21 | |||
| Past excessive alcohol consumption | 1.33 | [0.27; 1.06] | 0.07 | 1.55 | [1.02; 2.36] | 0.041 |
| Ongoing alcohol consumption (g/day) | ||||||
| 0 | Ref | |||||
| <10 | 0.53 | [0.27; 1.06] | 0.46 | |||
| ≥10 | 0.75 | [0.34; 1.62] | 0.40 | |||
| Tobacco consumption | ||||||
| Never | Ref | |||||
| Past | 1.25 | [0.76; 1.99] | 0.40 | |||
| Ongoing | 0.85 | [0.53; 1.39] | 0.52 | |||
| Arterial hypertension | 1.32 | [0.87; 1.98] | 0.19 | |||
| Dyslipidemia | 0.88 | [0.38; 2.01] | 0.76 | |||
| Platelet count (103/mm3) | <0.001 | <0.001 | ||||
| <100 | 3.32 | [2.01; 5.50] | <0.001 | 2.70 | [1.62; 4.51] | <0.001 |
| [100; 150] | 2.21 | [1.30; 3.74] | 0.003 | 1.87 | [1.10; 3.18] | 0.021 |
| >150 | Ref | Ref | ||||
| Prothrombin time (%) | 0.10 | |||||
| <60 | 1.18 | [0.37; 3.74] | 0.79 | |||
| [60; 80] | 1.57 | [1.04; 2.38] | 0.033 | |||
| >80 | Ref | |||||
| Serum albumin (g/L) | ||||||
| ≤35 | 1.65 | [0.92; 2.97] | 0.09 | |||
| >35 | Ref | |||||
| Bilirubin (μmol/L) | . | |||||
| ≤ 17 | Ref | |||||
| >17 | 1.34 | [0.89; 2.04] | 0.17 | |||
| AST (IU/L) | <0.001 | |||||
| <ULN | Ref | |||||
| [ULN; 2 ULN] | 2.25 | [1.23; 4.12] | 0.009 | |||
| >2 ULN | 3.03 | [1.70; 5.38] | <0.001 | |||
| ALT (IU/L) | 0.038 | |||||
| < ULN | Ref | |||||
| [ULN; 2 ULN] | 1.52 | [0.89; 2.59] | 0.13 | |||
| >2 ULN | 1.90 | [1.16; 3.10] | 0.011 | |||
| GGT (IU/L) | ||||||
| <ULN | Ref | |||||
| ≥ULN | 3.39 | [2.02; 5.70] | <0.001 | 1.96 | [1.11; 3.47] | 0.021 |
| Presence of esophageal varices | 1.40 | [0.93; 2.09] | 0.11 | |||
| AFP (ng/mL) | 1.01 | [1.00; 1.01] | 0.002 | |||
| BMI | ||||||
| <25 | Ref | |||||
| 25-30 | 0.89 | [0.56; 1.43] | 0.21 | |||
| >30 | 1.11 | [0.64; 1.93] | 0.71 | |||
| Diabetes | 1.07 | [0.67; 1.71] | 0.78 | |||
| MetS (overweight, diabetes, and dyslipidemia) | 1.16 | [0.74; 1.83] | 0.51 | |||
| MetS (histological NASH)a | 0.82 | [0.46; 1.46] | 0.50 | |||
| HIV coinfection | 0.29 | [0.04; 2.06] | 0.21 | |||
| HCV genotype | ||||||
| Non-1 | Ref | |||||
| 1 | 2.53 | [1.45; 4.39] | 0.001 | |||
| Non-SVR during the study periodb | 3.70 | [2.04; 6.25] | <0.001 | 3.02 | [1.67; 5.48] | <0.001 |
| Features | Coefficient | HR | 95% HR CI | P Value | Risk Score |
|---|---|---|---|---|---|
| Age >50 years | 0.664 | 1.94 | [1.16; 3.25] | 0.012 | 2 |
| Past excessive alcohol intake | 0.440 | 1.55 | [1.02; 2.36] | 0.041 | 1 |
| Platelet count (103/mm3) | |||||
| <100 | 0.995 | 2.70 | [1.62; 4.51] | <0.001 | 3 |
| [100; 150] | 0.624 | 1.87 | [1.10; 3.18] | 0.021 | 2 |
| >150 | Ref | ||||
| GGT (UI/L) > ULN | 0.672 | 1.96 | [1.11; 3.47] | 0.021 | 2 |
| Nonsustained virological response during the study perioda | 1.105 | 3.02 | [1.67; 5.48] | <0.001 | 3 |
- a Included as a time-dependent covariate.
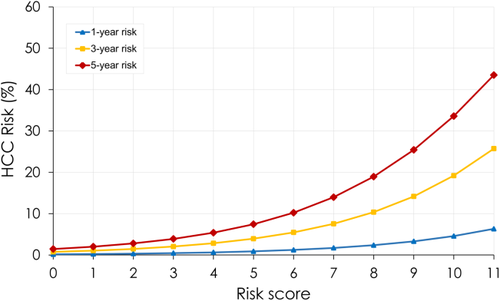
In order to develop an individualized prediction of HCC, a nomogram derived from this score was also built, given the 1-, 3-, and 5-year probabilities of HCC for each level of the score (Fig. 3). The fit of the model was assessed by Harrell's C statistics at 0.72.
Calibration was also assessed for predicted HCC risk and observed risk, and showed a good correlation (r = 0.0.91; P < 0.001).
VALIDATION SET
The cumulative incidence of HCC risk was estimated by the Kaplan-Meier method according to the three groups of the score built from the training cohort. HCC occurrence gradually increased from 0% to 20.3% at 5 years, for patients with the lowest (≤3; n = 64; 14.7%) and highest (≥8; n = 165; 37.9%) HCC risk scores (P < 0.001; Fig. 2B).
In this group, the AUROC for the 3-year prediction of HCC was 0.736 [0.639-0.833], similar to that of the training group (P = 0.75 between AUCs). Correlation between observed and predicted HCC risk was estimated with a coefficient at 0.86 (P < 0.001).
Discussion
Our results show that personalized prediction of HCC using easily available variables is feasible in patients with HCV-related cirrhosis. Indeed, a simple score combining four baseline variables and one time-dependent variable was accurate in predicting HCC at the individual level in a large, prospective cohort of French patients with HCV cirrhosis. Relevant baseline independent variables used in this score were in accord with the literature; indeed, increasing age,3, 7, 8 low platelet counts,4 and detectable serum HCV RNA9 are the usual predictive factors for occurrence of HCC in patients with cirrhosis.
Conversely, it takes in account an original variable—the serum level of GGT—that was less frequently reported as an independent predictive factor for HCC17-19 and not previously used by another scoring system. This simple test mainly summarizes the weight of comorbidities, given that patients with GGT above ULN (76%) had a higher BMI (25.9 [23.1-29.1] vs. 25.4 [22.7-28.1]; P = 0.038), more frequent dyslipidemia (6% vs. 3.2%; P = 0.042) and more-frequent histological evidence of SH (44.8% vs. 25.5 %; P = 0.01). Conversely, patients with GGT above the ULN did not have an increased proportion of diabetes (20.1% vs. 16.4%; P = 0.13), suggesting that increased serum GGT could be a very early surrogate marker of insulin resistance. Furthermore, an increased serum level GGT (>ULN) is usually associated with more-severe liver disease.
This model, which can be easily applied to clinical practice, performed well in terms of discrimination (AUROC for 1- and 3-year predictions was 0.678 [0.555-0.801] and 0.717 [0.661-0.773], respectively, in the training group) in a split derivation sample of patients with HCV-related cirrhosis. Internal validation in the split sample confirmed the accuracy of this score in predicting HCC; the derived nomogram had good calibration, with an accurate correlation between predicted and observed HCC risk (r = 0.91). Moreover, updating of the score value 12 months after inclusion for patients still at risk of HCC was able to predict, with the same discriminatory accuracy, the risk of HCC, demonstrating the evolutive capacity of the score.
Numerous personalized risk-scoring systems constructed using baseline-independent variables for HCC occurrence had been previously developed and validated in patients with cirrhosis attributed to various causes,8, 20, 21 and more recent ones are targeting patients with hepatitis B.22-24 In the last few years, Asian groups have derived and validated several HCC risk scores based on well-known risk factors, such as cirrhosis, age, male sex, and high HBV viral load (REACH-B, GAG-HCC, CU-HCC).22-24 Overall, these recent scores have shown a high negative predictive value of over 95% for excluding HCC development in 3-10 years. These HCC risk scores were tested in several Western cohorts25, 26 and appeared less effective in Caucasians.
In patients infected by HCV, a risk score for HCC was built whatever the stage of fibrosis27, 28 and only one algorithm for HCC detection was developed and validated in American veterans with HCV-related cirrhosis29 and in broader populations. Whereas all risk scores for HCC in patients with HBV included viral load,22-24 no risk score for HCC in patients with HCV took into account an SVR.
The enhanced HCC risk assessment in our model would enable personalized screening practices. The algorithm could optimize HCC surveillance by reducing false positives, thus having considerable impact upon subsequent testing, reducing surveillance costs, and avoiding unnecessary patient/provider anxiety, whereas optimizing approximations of individualized patient risk. HCC risk assessments yielded by our model-based instrument can be used to create a binary decision rule for clinical action; for example, if HCC probability exceeds 20% at 5 years, then additional testing (e.g., cross-sectional imaging) could be performed. In contrast, in a very select group of patients (around 15%) with very low HCC risk (score ≤3; 5 years of HCC cumulative incidence around 2%), the need for HCC surveillance is questionable. However it is premature to adopt fixed cutoffs at this time.
The strengths of our study lie in the following: (1) the homogeneity of patients selected using stringent criteria, particularly biopsy-proven cirrhosis; (2) its prospective design, conducted at 35 centers, with systematic periodic surveillance by liver imaging for HCC screening according to international guidelines; (3) stringent monitoring of all events during follow-up, in particular HCC; (4) internal validation of results in the split sample; and (5) the fact that SVR was taken into account. Conversely, our study had several limitations, mainly linked to the small number of patients with SVR at endpoint (39.5%), attributable to an inclusion period preceding introduction of second-generation DAAs in clinical practice.30, 31 However, because of the high financial cost of these new antiviral treatments, only a minority of patients with HCV cirrhosis worldwide had received them, even in Western countries, mainly in Europe. In addition, this HCC score could be used in countries with limited resources. Finally, our score could be applied to SVR patients whose risk scores varied from 0 to 8, with a cumulative risk of HCC at 5 years ranging from 0 (for a score ≤3) to 30.1% (for a score ≥8). In addition, external validation outside of France is warranted. Interestingly, we showed that the score could be accurately updated with increasing age, but also with alterations in biochemical values and modification of viral status.
In conclusion, an HCC score constructed using four baseline variables (age, past excessive alcohol consumption, platelet count, and GGT serum level) and SVR considered as a time-dependent covariate can accurately predict HCC occurrence at an individual level in a prospective French cohort of patients with HCV cirrhosis. It can provide individualized estimation of HCC risk of occurrence in patients with HCV cirrhosis whatever their viral status and can be periodically reassessed in order to decide whether or not they should be included in a surveillance program. External validation in non-French populations is warranted.
Acknowledgment
This work is dedicated to the memory of Prof. Jean-Claude Trinchet.
Appendix: ANRS CO12 CirVir Group:
Pierre Nahon,1 Patrick Marcellin,2 Dominique Guyader,3 Stanislas Pol,4 Hélène Fontaine,4 Dominique Larrey,5 Victor De Lédinghen,6 Denis Ouzan,7 Fabien Zoulim,8 Dominique Roulot,9 Albert Tran,10 Jean-Pierre Bronowicki,11 Jean-Pierre Zarski,12 Vincent Leroy,12 Ghassan Riachi,13 Paul Calès,14 Jean-Marie Péron,15 Laurent Alric,16 Marc Bourlière,17 Philippe Mathurin,18 Sebastien Dharancy,18 Jean-Frédéric Blanc,19 Armand Abergel,20 Lawrence Serfaty,21 Ariane Mallat,22 Jean-Didier Grangé,23 Pierre Attali,24 Yannick Bacq,25 Claire Wartelle,26 Thông Dao,27 Yves Benhamou,28 Christophe Pilette,29 Christine Silvain,30 Christos Christidis,31 Dominique Capron,32 Gérard Thiefin,33 Sophie Hillaire,34 and Vincent Di Martino.35
1AP-HP, Hospital Jean Verdier, Liver Unit, Bondy, France; 2AP-HP, Hospital Beaujon, Liver Unit, Clichy, France; 3University Hospital Pontchaillou, Liver Unit, Rennes, France; 4APHP, Liver Unit, Hospital Cochin; INSERM USM20, Institut Pasteur, Paris, France; 5Hospital Saint Eloi, Liver Unit, Montpellier, France; 6Hospital Haut-Lévêque, Liver Unit, Bordeaux, France; 7Institut Arnaud Tzanck, Liver Unit, St. Laurent du Var, France; 8Hospital Hôtel Dieu, Liver Unit, Clermont-Ferrand, France; 9AP-HP, Hospital Avicenne, Liver Unit, Bobigny, France; 10University Hospital of Nice, Liver Unit, and INSERM U1065, C3M, Team 8, “Hepatic Complications in Obesity,” Nice, France; 11Hospital Brabois, Liver Unit, Vandoeuvre-les-Nancy, France; 12Hospital Michallon, Liver Unit, Grenoble, France; 13Hospital Charles-Nicolle, Liver Unit, Rouen, France; 14University Hospital of Angers, Liver Unit, Angers, France; 15Hospital Purpan, Liver Unit, Toulouse, France; 16University Hospital of Toulouse, Internal Medicine Unit – Digestive pole UMR 152, Toulouse, France; 17Hospital Saint Joseph, Liver Unit, Marseille, France; 18Hospital Claude Huriez, Liver Unit, Lille, France; 19Hospital St André, Liver Unit, Bordeaux, France; 20Hospital Hôtel Dieu, Liver Unit, Clermont-Ferrand, France; 21AP-HP, Hospital Saint-Antoine, Liver Unit, Paris, France; 22AP-HP, Hospital Henri Mondor, Liver Unit, Créteil, France; 23AP-HP, Hospital Tenon, Liver Unit, Paris, France; 24AP-HP, Hospital Paul Brousse, Liver Unit, Villejuif, France; 25Hospital Trousseau, Liver Unit, Tours, France; 26Hospital of Aix-En-Provence, Liver Unit, Aix-En-Provence, France; 27Hospital de la Côte de Nacre, Liver Unit, Caen, France; 28AP-HP, Pitié-Salpêtrière Hospital, Liver Unit, Paris, France; 29General Hospital, Liver Unit, Le Mans, France; 30University Hospital of Poitiers, Liver Unit, Poitiers, France; 31Institut Mutualiste Montsouris, Liver Unit, Paris, France; 32Hospital Amiens Nord, Liver Unit, Amiens, France; 33Hospital Robert Debré, Liver Unit, Reims, France; 34Hospital Foch, Liver Unit, Suresnes, France; 35Hospital Jean Minjoz, Liver Unit, Besançon, France.




