Patient-derived mouse xenografts from pediatric liver cancer predict tumor recurrence and advise clinical management
Potential conflict of interest: Nothing to report.
Supported by: XenTech: internal resources; Macy Easom Cancer Research Foundation grant; Innovative Therapies for Children with Cancer (ITCC) consortium grant; Spanish Plan Nacional de I+D+I, ISCIII grant; Spanish FEDER funds (FIS IP10/08082 and FIS IP13/02340); Ramon y Cajal Program (RYC-2010-07249); Red Temática de Investigación Cooperativa en Cáncer (RTICC, FEDER) (RD12/0036/0044).
Abstract
Identification of new treatments for relapsing pediatric cancer is an unmet clinical need and a societal challenge. Liver cancer occurrence in infancy, 1.5 for million children per year, falls far below the threshold of interest for dedicated drug development programs, and this disease is so rare that it is very difficult to gather enough children into a phase II clinical trial. Here, we present the establishment of an unprecedented preclinical platform of 24 pediatric liver cancer patient-derived xenografts (PLC-PDXs) from 20 hepatoblastomas (HBs), 1 transitional liver cell tumor (TCLT), 1 hepatocellular carcinoma, and 2 malignant rhabdoid tumors. Cytogenetic array and mutational analysis of the parental tumors and the corresponding PLC-PDXs show high conservation of the molecular features of the parental tumors. The histology of PLC-PDXs is strikingly similar to that observed in primary tumors and recapitulates the heterogeneity of recurrent disease observed in the clinic. Tumor growth in the mouse is strongly associated with elevated circulating alpha-fetoprotein (AFP), low rate of necrosis/fibrosis after treatment, and gain of chromosome 20, all indicators of resistance to chemotherapy and poor outcome. Accordingly, the ability of a tumor to generate PLC-PDX is predictive of poor prognosis. Exposure of PLC-PDXs to standards of care or therapeutic options already in use for other pediatric malignancies revealed unique response profiles in these models. Among these, the irinotecan/temozolomide combination induced strong tumor regression in the TCLT and in a model derived from an AFP-negative relapsing HB. Conclusion: These results provide evidence that PLC-PDX preclinical platform can strongly contribute to accelerate the identification and diversification of anticancer treatment for aggressive subtypes of pediatric liver cancer. (Hepatology 2016;64:1121-1135)
Abbreviations
-
- AFP
-
- alpha-fetoprotein
-
- CNVs
-
- copy number variations
-
- D
-
- day
-
- FLC
-
- fibrolamellar carcinoma
-
- gDNA
-
- genomic DNA
-
- HB
-
- hepatoblastoma
-
- HCC
-
- hepatocellular carcinoma
-
- HR
-
- hazard ratio
-
- HS
-
- hepatic sarcoma
-
- MCR
-
- maintained complete response
-
- MET
-
- mesenchymal-epithelial transition
-
- NGS
-
- next-generation sequencing
-
- PCR
-
- polymerase chain reaction
-
- PD
-
- progressive disease
-
- PDXs
-
- patient-derived xenografts
-
- PLC-PDXs
-
- pediatric liver cancer patient-derived xenografts
-
- PPTP
-
- the Pediatric Preclinical Testing Program
-
- RT
-
- rhabdoid tumor
-
- SCUD
-
- small cell undifferentiated
-
- SD
-
- stable disease
-
- SNP
-
- single-nucleotide polymorphism
-
- TCLTs
-
- transitional liver cell tumors
Recent improvement in medical care has raised the percentage of children that survive cancer up to 70%-80%.1 Given that pediatric tumors are rare, for the 20%-30% of patients that will not survive the disease the identification of new therapeutic approaches is hampered by the difficulty to recruit sufficient numbers of patients in clinical trials. As a consequence, very few second-line treatments are available and only few clinical phase I/II trials are currently ongoing in pediatric oncology. In order to overcome this limitation, phase II trials generally include relapsing patients with different tumors, rendering the interpretation of the results a real challenge. Hepatoblastoma (HB) is a rare tumor that constitutes the most frequent form of childhood liver cancer, accounting for 1% of all pediatric malignancies, with a peak of frequency in children less than 3 years of age, whereas in adolescents and young adults the main liver tumor subtypes are fibrolamellar carcinoma (FLC) and hepatocellular carcinoma (HCC).2-4 Very rare transitional forms combining HB and HCC histological features have been described as transitional liver cell tumors (TLCTs) occurring on normal liver typically diagnosed in children and adolescents and associated with poor outcome.5, 6 Additionally, hepatic sarcomas (HSs) and malignant rhabdoid tumors (RTs) of the liver are diagnosed in children, although at very low frequencies.7 Few genetic alterations are known for these tumors. The distinctive genetic hallmark of HB is mutational activation of CTNNB1 gene, which encodes β-catenin, a key player of the WNT pathway.8-10 HB occurrence is associated with premature birth, very low weight at birth, and with genetic syndromes such as familial adenomatous polyposis, caused by germline mutation of APC, also involved in the WNT pathway,11 and Beckwith-Wiedemann syndrome, a disease caused by mutation or deletion of imprinted genes within the chromosome 11p15.5 region.12
Several clinical studies have provided guidelines for treatment of this disease, including the liver tumor study groups of the American Children's Oncology Group, the German Pediatric Oncology Hematology Group, the Liver Tumor Strategy Group of the International Society of Pediatric Oncology, and the Japanese Pediatric Liver Tumor Group. All groups have adopted a cisplatin-based chemotherapy regimen. Patients with HB are enrolled into two different clinical protocols depending on clinical parameters that define risk. Standard-risk patients present localized tumors free of extrahepatic extensions. These patients undergo cisplatin alone-based chemotherapy before surgery.13 By contrast, high-risk patients display large tumors, vascular invasion, and/or metastasis at diagnosis and/or alpha-fetoprotein (AFP) levels <100 ng/mL.14 These patients are treated with combinations of cisplatin, carboplatin, and doxorubicin with schedules that can differ depending on the treatment protocol that is adopted.15, 16 Unfortunately, no reference second-line treatment is available for patients who relapse or present adverse tumor types, such as TLCT, HCC, or RT. Therefore, children are often enrolled in phase I/II studies available at the time of recurrence. Several recent studies have addressed the genetic basis of pediatric liver tumors using next-generation sequencing (NGS).17-21 Two of these studies showed that the HB mutation rate is very low, and that apart from mutation of the CTNNB1 and NFE2L2 gene in 80 and 10% of samples, respectively, tumors harbor very few and unique mutations or even no other mutation. Co-occurrence of CTNNB1 and NFE2L2 mutations has also been described in a case of pediatric HCC.19 Consistently, β-catenin activation, as a single genetic event, can induce HB formation in the mouse when directed to a well-defined time point during embryonic development,22 whereas CTNNB1 and NFE2L2 mutations have been found in a chemically induced HCC model in rats.23 In FLC, DNAJB1-PRKACA fusion has been found as a distinctive alteration for this type of cancer,20 and in rhabdoid tumors, loss of INI1/SMARCB1 gene is a robust diagnostic marker.21 Unfortunately, none of the mutated genes identified so far are druggable at present. Given the absence of alternative cures and the difficulty to identify new (targeted) treatments, novel experimental approaches are urgently needed. The establishment and use of patient-derived xenografts (PDXs) in preclinical research studies is revolutionizing the experimental oncology landscape.24, 25 PDX is becoming the reference model for functional validation of discoveries in the field of tumor biology and for preclinical evaluation of anticancer therapy. The robustness and reproducibility of the assay, together with the remarkable preservation of the characteristics of the tumor of origin, make the PDX a trustworthy surrogate of patient tumor for many types of cancers. This approach has been extensively validated in pediatric cancers, where collections of PDXs have been established and provide useful strategic indications on patient treatment. A program of reference in this field is the Pediatric Preclinical Testing Program (PPTP) promoted by the National Cancer Institute (http://pptp.nchresearch.org). The PPTP is based on accumulating evidence that appropriate preclinical in vivo models of childhood cancer can recapitulate the antitumor activity of known effective agents and can prospectively identify novel agents against specific cancers. The PPTP includes several types of pediatric cancer PDXs, but none from liver cancer. Previous studies showed successful human HB PDX establishment in mice.26-28 In the present study, we have established PDX models from 20 HBs, one TLCT, one HCC, and 2 RTs. We provide evidence that these models faithfully recapitulate the histology and genomic profile of the tumors of origin and display unique growth properties. Moreover, testing combinations of common and new therapeutic options in these models underscore the importance of PDX as a tool for basic and translational research on these rare malignancies.
Materials and Methods
PDX ESTABLISHMENT
This study was approved by the Assistance Publique-Hôpitaux de Paris ethical committee, and informed written consent was obtained from each patient. Tumor samples were grafted in the interscapular region of 6- to 8-week-old female athymic nude mice. Growing tumors were serially transplanted onto recipient mice and underwent comparative examination to confirm preservation of their histological features and live-frozen to immortalize the model. No impact of tumor growth on liver function of recipient mice was observed (Supporting Table S1). More information is available as Supporting Information.
PDX HISTOLOGICAL AND IMMUNOHISTOCHEMICAL CHARACTERIZATION
Histological and immunohistochemistry studies were performed in the pathology laboratories at Bicêtre Hospital, Gustave Roussy Institute, and Necker Hospital using the Benchmark Autostainer (Roche) and antibodies currently in use in the clinical practice as described in Cairo et al.29 All detailed immunostaining protocols are available upon request.
AFP DETECTION
AFP detection in blood of pediatric liver cancer patient-derived xenograft (PLC-PDX)-carrying mice was performed at Bicêtre Hospital using the AFP diagnostic kit BRAHMS AFP KRYPTOR (Thermo Scientific) and 80-100 μL of blood per animal, according to the manufacturer's instruction.
CYTOGENETIC ARRAY
Genomic DNA (gDNA) from eight primary tumours and two intrahepatic recurrences from 9 patients and their corresponding PDXs were profiled by using the cytogenetic array CytoScan HD (Affymetrix). Details are provided as Supporting Information. The statistical environment R (v 3.1.1)30 with the package OmicCircos was used to represent genomic alterations in a Circos plot.31 Data are available at the Gene Expression Omnibus database under accession number GSE76100.
CTNBB1 AND NFE2L2 MUTATION
CTNNB1 mutation analysis was performed on tumor complementary DNA by polymerase chain reaction (PCR) followed by Sanger sequencing as described in Cairo et al.29 PCR conditions for detecting mutations in exon 2 of the NFE2L2 gene are described in Eichenmüller et al.18
FIFTY-EIGHT-GENE PROFILING FOR HB214, HB217, AND RT001 HUMAN AND PDX SAMPLES
Mutation profiling of 58 genes, among the most frequently mutated in cancer according to the COSMIC database, was performed at BGI in Beijing (China) on gDNA by exon trapping with NimbleGen microarray followed by deep sequencing by using Illumina's HiSeq technology, with at least 50× effective mean depth for each sample.
IN VIVO STUDIES
For efficacy studies, mice with subcutaneously growing tumor were allocated to each treatment arm according to their tumor volume A compound inducing a tumor volume reduction of treated versus control group superior to 42% was considered as active according to previously published rules.32 However, for this study we adopted the more stringent PPTP tumor response criteria.33 More details are provided as Supporting Information.
STATISTICAL ANALYSES
All statistical analyses were performed by using the tests described in the article and the GraphPad Prism (version 5) software.
Results
ESTABLISHMENT OF A PRECLINICAL PANEL OF CHILDHOOD LIVER CANCER PDXs
From May 2010 to August 2015, 58 tumor specimens from 51 patients were grafted. These tumors consisted of 48 HBs, one TLCT, one lung metastasis with HCC features, two RTs, two HSs, and four FLCs (Supporting Table S2). The large majority of samples were from primary tumors that received preoperative chemotherapy or at relapse. From these samples, 24 PLC-PDXs were generated from 20 patients, including 20 HB-PDXs, one TLCT-PDX, one HCC-PDX, and two RT-PDXs, whereas no PDXs could be established from HS and FLC samples. Concerning HB samples, successful grafting was observed for the majority of specimens from recurrent or metastatic tumors (9 of 12 [75%]), whereas for primary tumors the take rate was lower (13 of 39 [33.3%]). The characteristics of the PLC-PDXs established recapitulated the heterogeneity observed in the clinic, particularly for the most aggressive forms of pediatric liver cancer. Among the HB-PDXs, one model was derived from an AFP-negative HB, a very aggressive form of the disease observed in less than 1% of cases.14 For 6 patients, we could graft primary tumors and recurrent disease. For patients 13, 19, and 35, PDXs could be established from the recurrent diseases, but not from the primary tumors, whereas for patients 26, 37, and 41, we could generate models from both the primary tumor and at relapse.
HISTOLOGICAL AND MOLECULAR CHARACTERIZATION OF PLC-PDXs
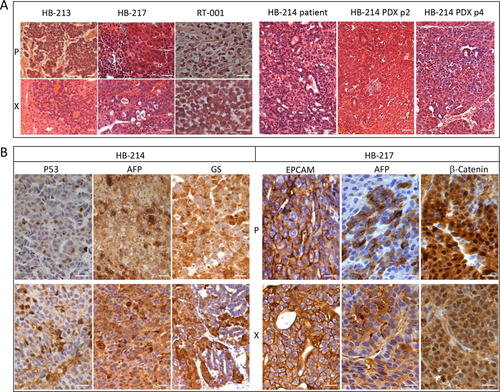
We evaluated the histological, genomic, and genetic relevance of the tumor grafts as compared to the parental patient's tumors. PLC-PDX retained the histological features of the tumors of origin, particularly concerning the tumor cell morphology and tissue organization of the more undifferentiated components (Fig. 1A, left panel). To verify the stability of the histological phenotype over passages, HB-214 PDX histology was checked at passages 2 and 4. Both passages closely resembled the tumor of origin (Fig. 1A, right panel). Immunohistochemical analysis of diagnostic markers currently used to classify liver malignancies showed similar staining of the histological components in the parental tumors and in corresponding PLC-PDXs (Fig. 1B; Supporting Table S3). However, for many PLC-PDXs originating from primary tumors, we noticed over-representation of the more undifferentiated components, namely, embryonal and small cell undifferentiated (SCUD; Table 1). On the other hand, PLC-PDXs generated from intrahepatic recurrences or distant metastases closely reproduced the parental tumor histology, as shown, in particular, for patients 26 and 37, suggesting that the PLC-PDX models could be more representative of the relapsing disease than of the primary pathology.
| Patient ID No. | Donor Tumor |
PDXID |
Origin | Patient Histology | PDX Histology |
|---|---|---|---|---|---|
| 2 | HB213 | HB-213 | Primary tumor | 40% fetal, 40% crowded fetal, 10% embryonal | 45% embryonal, 50% SCUD, 5% fetal |
| 3 | HB214 | HB-214 | Primary tumor | 50% fetal, 40% embryonal, 10% osteoid | Embryonal with calcifications |
| 6 | HB217 | HB-217 | Primary tumor | 60% fetal, 30% embryonal, 10% SCUD | 80% embryonal, 10% fetal, 10% SCUD |
| 8 | RT001 | RT-001 | Primary tumor | Rhabdoid phenotype | Rhabdoid phenotype |
| 13 | HB225 | No PDX | Primary tumor | 90% primary:fetal, 5% embryonal, 5% cholangioblast-like | No PDX |
| HB243 | HB-243-RED-225 | HB225 intrahepatic recurrence | Recurrence: 60% embryonal, 40% crowded fetal, macrotrabecular | 100% embryonal, macrotrabecular | |
| 17 | HB229 | HB-229 | Intrahepatic recurrence | 50% embryonal, 50% SCUD | 50% embryonal, 50% SCUD |
| 19 | HB231 | No PDX | Primary tumor | Primary: 90% fetal, 10% embryonal | No PDX |
| HB238 | HB-238-RED-231 | HB231 intrahepatic recurrence | Recurrence: crowded fetal | Embryonal | |
| 22 | HC001 | HC-001 | Lung metastasis | HCC | HCC |
| 23 | TT001 | TT-001 | Primary tumor | Crowded fetal/HCC | HCC |
| 24 | HB236 | HB-236 | Primary tumor | 95% fetal, 5% embryonal | 100% embryonal, macrotrabecular |
| 26 | HB239 | HB-239 | Primary tumor | 75% embryonal, 15% fetal, 10% SCUD, macrotrabecular foci | 100% embryonal |
| HB244 | HB-244-RED-239 | HB239 intrahepatic recurrence | 100% embryonal | 100% embryonal | |
| 35 | HB250 | No PDX | Primary tumor | 50% fetal, 50% embryonal | No PDX |
| HB262 | HB-262-M-250 | HB250 brain metastasis | Embryonal>>>fetal macrotrabecular | 100% embrional | |
| HB267 | HB-267-M-250 | HB250 lung metastasis | 100% embryonal | 100% embrional | |
| 37 | HB252 | HB-252 | Primary tumor | 60% embryonal, 40% fetal, macrotrabecular foci | 100% embryonal, macrotrabecular |
| HB261 | HB-261-M-252 | HB252 peritoneal metastasis | Embryonal>>>fetal | 100% embryonal, macrotrabecular |
PLC-PDXs REFLECT THE GENOMIC LANDSCAPE OF THE PATIENT POPULATION
Given that the most frequent genetic alterations in HB are mutation of CTNNB1 and NFE2L2, we investigated mutation of these genes in tumors and PDXs by reverse-transcriptase PCR and Sanger sequencing. Identical CTNNB1 mutations were found in patients' tumors and matched PDXs, at a frequency very similar to that found in the patients' population, arguing against a possible growth advantage in mice for the tumors harboring these mutations. CTNNB1 mutation was found in 19 of 22 PLC-PDXs (RT-PDXs are excluded from the analysis), corresponding to 16 of 18 (89%) patients with mutation, with predominant exon 3 deletion, observed in 13 PDXs from 11 patients (69%), and point mutation in six PDXs from 5 patients (31%). Among the three PLC-PDXs with wild-type CTNNB1 are the HCC-PDX and two HB-PDXs established from the same patient. As expected, NFE2L2 showed a much lower mutation rate, we could identify G81S mutation only in two PDXs from the same patient.18 We also checked INI1/SMARCB1 mutation for the two RT-PDXs developed, and found deletion of this gene in the paired donor/PDX samples (Fig. 2A). HB and RT share a very low incidence of mutation as observed by NGS, next-generation sequencing (NGS) analysis.12, 17, 20 To check whether PDX models maintained this distinctive feature, we analyzed the mutational status of 58 genes among the most frequently mutated in solid tumors by exon sequencing in the HB213 parental tumor and PDX model, HB-214 PDX and nontumor sample, HB-217 PDX, and RT001 parental tumor and PDX. Besides CTNNB1, NFE2L2, and INI1/SMARCB1 mutations, no other mutation could be found, and rare single-nucleotide polymorphisms (SNPs), with allele frequency of less than 5% in the Caucasian population, were identified in the paired tumor/PDX or nontumor/PDX samples (Supporting Table S4).
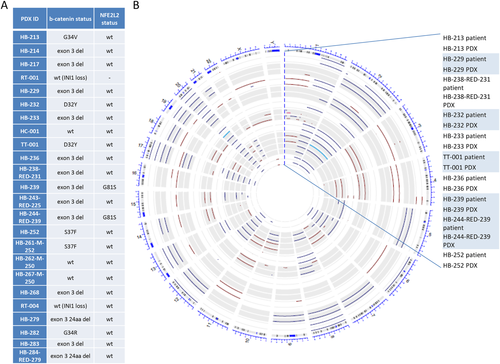
We next evaluated further the relevance of the models by CytoScan HD chip (Affymetrix) for 10 paired donor samples and established PLC-PDXs (nine HB and the one TLCT). First, we evaluated the maintenance of 530,026 SNPs present in the array and found that a mean of 97.7 ± 2.4% of the SNPs were maintained between paired parental tumor and PDX. Second, we studied the copy number variations (CNVs) of the same samples. The CIRCOS plot shown in Fig. 2B illustrates that chromosomal alterations are unique to each tumor and are, overall, well preserved in the corresponding PDXs. In that regard, 78% of the CNVs in the primary tumors are maintained in the PDXs. Only the HB-238-RED-231 tumor/PDX pair showed several discrepancies in the chromosomal CNV, which could be partly attributed to reduced tumor cellularity in the parental tumor. Among frequent alterations, we observed gain of chromosome 1q in 60% of paired cases associated to 1p loss in 2 cases, chromosome 4/4q-ter loss (50%), gain of chromosome 8/8q (50%), gain of chromosome 17 (60%), and gain of chromosome 20 in 90% of paired samples. We also noticed that in HB-239 and in HB-244-RED-239 pairs, which correspond to the primary tumor and its intrahepatic recurrence, some genomic alterations were unique to the primary tumor (i.e., loss of chromosome 1p, 4, 7, 11, and 18) and were well reflected in the corresponding PDX models.
PDXs RECAPITULATE HB BIOLOGY AND PHYSIOPATHOLOGY
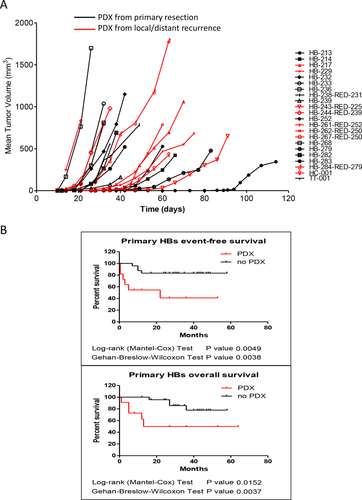
Besides investigating the genetic and histological features of PLC-PDXs, we measured tumor physiopathological properties, such as tumor growth and AFP secretion. These two parameters were well preserved in the PDXs established (Fig. 3A; Supporting Tables S5 and S6). Following primo-implantation, appearance of the tumor burden in animals was observed with a delay varying between 2 weeks and 4 months. Tumorgrafts from the same patient that developed in different mice and with different latency period showed overlapping growth curve and histological traits, suggesting different percentages of living tumor cells in the fragments implanted rather than selection of different tumor clones. Each PLC-PDX showed unique growth curves (Fig. 3A; Supporting Table S5), and AFP secretion in the blood of HB-carrying mice was concordant with the level observed in patients (Supporting Table S6). Mice carrying HB-229 PDX, derived from a nonsecreting tumor, showed very low AFP in the bloodstream. The HCC-PDX showed weaker AFP expression compared to the AFP-secreting HB models, and the PDX with highest relative secretion levels was the TLCT-PDX. Conversely, no AFP was detected in the blood from RT-001 PDX.
HB GROWTH IN MOUSE PREDICTS POOR PROGNOSIS
To evaluate whether the ability to establish a PDX is dependent on specific clinicopathological features, we compared the clinical and histological parameters of all HBs implanted into recipient mice (Table 2). Because HCC-PDX was generated from a recurrence in a patient previously operated of HB and TLCT-PDX shares HB and HCC features, they were included in the analysis, whereas patients with rhabdoid tumors were excluded. Statistical analysis showed that the two parameters most strongly associated with successful tumor growth in mice are the percentage of necrotic/fibrotic tissue in the resected tumor and circulating AFP levels at the end of preoperative chemotherapy (P < 0.0001 and P = 0.0002, respectively; Fisher's exact test). All models were derived from tumors with necrosis/fibrosis not exceeding 50% and high levels of circulating AFP posttreatment measured a few days before surgery. High AFP level at diagnosis (P = 0.0565; Fisher's exact test) and decreased AFP level posttreatment measured in log10 scale less than 3-fold (P = 0.0378; Fisher's exact test) are also associated with tumor growth in mouse. Other annotations significantly associated with xenograft growth are the implantation of tumor fragments from relapsing tumors (P = 0.0066; Fisher's exact test), prevalence of an immature epithelial component (P = 0.0011; Fisher's exact test), and, curiously, a preoperatory tumor extent (PRETEXT) stage II or less (P = 0.017; Fisher's exact test). We also observed that primary tumors that allowed PDX establishment relapsed more frequently than those that failed to grow in mice (P = 0.0413; Fisher's exact test). This led us to also investigate whether PDX development can predict patient outcome. PDX growth was associated with shortened disease-free survival (hazard ratio [HR] = 8.494; P = 0.0049; log-rank [Mantel-Cox] test; P = 0.0038; Gehan-Breslow-Wilcoxon test), and with decreased overall survival (HR = 7.224; P = 0.0152; log-rank (Mantel-Cox) test; P = 0.0037; Gehan-Breslow-Wilcoxon test; Fig. 3B). These results indicate that the ability of pediatric liver tumors to grow in immunodeficient mice is predictive of patient outcome, as already shown for other types of cancer.34
| Outcome of HB Engrafted | PDX (n = 22) | no PDX (n = 28) | Fisher's Exact Test | ||
|---|---|---|---|---|---|
| Age ≥24months (Y/N) | 14 | 8 | 17 | 11 | ns |
| Primary surgery (Y/N) versus secondary surgery | 12 | 10 | 26 | 2 | 0.0066 |
| Sex (male/female) | 14 | 8 | 17 | 11 | ns |
| Vascular invasion (Y/N) | 10 | 6 | 12 | 14 | ns |
| Multinodularity (Y/N) | 13 | 6 | 15 | 11 | ns |
| Metastasis at diagnosis (Y/N) | 5 | 11 | 6 | 21 | ns |
| Histology (epithelial/mesenchymal) | 13 | 5 | 13 | 14 | ns |
| MAIN epithelial component (fetal/nonfetal) | 6 | 15 | 22 | 6 | 0.0011 |
| SCUD presence (Y/N) | 5 | 7 | 1 | 10 | ns |
| PRETEXT stage >II (Y/N) | 5 | 7 | 23 | 4 | 0.0170 |
| Chemotherapy (Y/N) | 18 | 2 | 27 | 0 | ns |
| Risk (high/standard) | 4 | 6 | 16 | 9 | ns |
| Necrosis and fibrosis in surgical specimen >50% (Y/N) | 0 | 16 | 15 | 8 | <0.0001 |
| AFP at diagnosis >500,000 ng/mL (Y/N) | 10 | 7 | 7 | 19 | 0.0565 |
| AFP postchemotherapy before surgery >10,000 ng/mL (Y/N) | 8 | 8 | 0 | 24 | 0.0002 |
| Log10 AFP decrease posttreatment >3 (Y/N) | 2 | 13 | 12 | 12 | 0.0378 |
| Recurrence postprimary surgery (Y/N) | 6 | 5 | 4 | 20 | 0.0413 |
- n = number of tumors grafted; Y = yes; N = no.
- Abbreviation: ns = nonsignificant.
PLC-PDXs SHOW DIFFERENT SENSITIVITY TO CISPLATIN
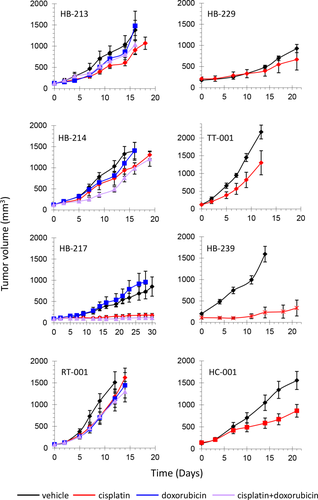
To evaluate PLC-PDX response to standard-of-care treatments, the impact of doxorubicin and cisplatin administration was tested in eight PLC-PDXs. For the in vivo experiments, we used PPTP guidelines33 as read out to interpret tumor response. HB-217 PDX showed impaired tumor growth, classified as stable disease (SD), when treated with cisplatin alone or in combination with doxorubicin, whereas HB-213 and HB-214 were resistant to both treatments, and tumor response was annotated as progressive disease (PD; Fig. 4). Doxorubicin alone had no effect on tumor growth and did not show cooperative effect when administered in combination with cisplatin, except for a slight effect in HB-214 between day 9 (D9) and D14. Response to cisplatin alone was tested in two additional HB-PDXs, the TLCT and the HCC-PDX, with weak or no response classified as PD. Because the RT donor for RT-001 PDX was misdiagnosed as HB, it was initially treated according to the HR protocol, to which it showed no response. When we treated RT-001 PDX with cisplatin and doxorubicin as single drugs or together, the derived xenograft proved completely insensitive, in agreement with what was observed for the patient's tumor.
IRINOTECAN/TEMOZOLOMIDE COMBINATION INDUCES STRONG TUMOR REGRESSION IN PDXs FROM RECURRENT HBs WITH AGGRESSIVE CLINICAL FEATURES
To evaluate whether these models could help to identify new therapeutic options for children with aggressive, relapsing HB, we tested several compounds (irinotecan, temozolomide, temsirolimus, sirolimus, nefopam, sorafenib, crizotinib, and paclitaxel) that are currently being tested in children with recurrent HB, alone or in combination. These agents were administered to three PDXs that recapitulate the most aggressive forms of HB: the TLCT-derived TT-001 model, the AFP-negative, SCUD-enriched HB-229 PDX, and the HC-001 model (Table 3). In a first set of experiments using mice with TT-001 PDX, we clearly observed that irinotecan alone or in combination with temozolomide were the only treatments capable of inducing tumor regression (Table 3; Fig. 5). Because the irinotecan/temozolomide combination induced toxicity (Supporting Figure S1A), irinotecan administration was suspended after the first administration, which explains the sudden regrowth observed in the related curve (Fig. 5). Conversely, all other treatments induced only partial or inconsistent growth inhibition in this PDX model (Table 3). In light of the toxicity problems observed, we introduced a reduced dosage of 10 mg/kg of irinotecan every 5 days instead of 40 mg/kg twice a week for the HB-229 PDX model. The reduced irinotecan/temozolomide combination was well tolerated (Supporting Fig. S1B) and very effective on this model, given that all tumors underwent maintained complete response (MCR; Table 3; Fig. 5) with no evidence of disease at the end of the study, 76 days after beginning of treatment (not shown). Irinotecan alone induced transient tumor stabilization, whereas the other treatment groups were classified as progressive disease. In the different models, tumor sensitivity resulted in decreased level of circulating AFP (Supporting Table S7). Histological analysis of residual tumors after chemotherapy was carried out in 2 of the 6 mice bearing HB-229 at the end of the experiment (D76, 7 weeks after the end of treatment). In both animals, treatment efficacy was witnessed by the presence of few residual dysmorphic tumor cells with nuclear vacuoles. In mouse 64, these cells were all Ki67 negative, whereas in mouse 112 two very small tumor cell foci had gained back their original structure and were positive to Ki67 staining (Supporting Fig. S2). This observation strongly correlated with circulating AFP rising above the detectability threshold at this late time point (Supporting Table S7), suggesting tumor relapse. Conversely, nonresponder HC-001 only showed slightly decreased Ki67 staining (not shown). The HCC-PDX study clearly showed that no treatment could induce tumor regression or stabilization, and the best result was attenuation of tumor growth by irinotecan alone or in combination with either temozolomide or sirolimus (Table 3). Sensitivity to the irinotecan/sirolimus combination was witnessed by the decrease of circulating AFP in treated mice (Supporting Table S7). Of note, treatment with irinotecan in combination with temozolomide also showed efficacy on HB-239, but not on its intrahepatic relapse HB-244-RED-239 PDXs (Table 3; Fig. 5). This result perfectly matched the clinical situation, given that no response was observed when the patient was treated with irinotecan/temozolomide at tumor recurrence after liver transplantation. Altogether, these results reinforce the use of irinotecan as a possible new important compound for second-line treatment of patients with HB and the combination with temozolomide as a potentially effective therapeutic option for children with aggressive, recurrent HB.
| Treatment 1 |
Dose (mg/kg) |
Schedule | Treatment 2 | Dose (mg/kg) | Schedule | TT-001 | HB-229 | HC-001 | HB-239 | HB-244-RED-239 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPTP Score | Best T/C % (Day) | PPTP Score | Best T/C % (Day) | PPTP Score | Best T/C % (Day) | PPTP Score | Best T/C % (Day) | PPTP Score | Best T/C % (Day) | ||||||
| Irinotecan | 10 | q5d ×5 | Temozolomide | 68 | qd ×5 | MCR | 3.98 (D28) | PD | 32.9 (D21) | PD | 20.2 (D14) | PD | 55.9 (D7) | ||
| Irinotecan | 40 | 2qwk ×1 | Temozolomide | 68 | qd ×5 | CR | 2.46 (D9) | ||||||||
| Irinotecan | 10 | q5d ×5 | Temsirolimus | 3 | q5d ×5 | PD | 47.6 (D14) | PD | 50.8 (D21) | ||||||
| Irinotecan | 10 | q5d ×5 | Sirolimus | 3 | q5d ×5 | PD | 27.8 (D21) | ||||||||
| Irinotecan | 10 | q5d ×5 | SD | 25.2 (D28) | PD | 41.3 (D17) | PD | 25.8 (D14) | |||||||
| Irinotecan | 40 | 2qwk ×2 | CR | 0.86 (D12) | PD | 30.7 (D21) | |||||||||
| Temozolomide | 68 | qd ×5 | PD | 70.1 (D24) | PD | 41.2 (D4) | |||||||||
| Nefopam | 40 | qd ×21 | PD | 82.4 (D2) | |||||||||||
| Nefopam | 80 | qd ×21 | PD | 78.7 (D4) | |||||||||||
| Sorafenib | 30 | qd ×21 | PD | 34.6 (D30) | PD | 80 (D7) | |||||||||
| Crizotinib | 80 | 5qwk ×3 | PD | 102.3 (D17) | PD | 67.4 (D17) | |||||||||
| Temsirolimus | 10 | qd ×21 | PD | 39 (D12) | |||||||||||
| Paclitaxel | 15 | qd ×7 | PD | 63.7 (D9) | PD | 68.8 (D17) | |||||||||
- Each treatment was administered following the schedules indicated.
- Abbreviations: qd, once a day; q5d, once every 5 days; 2qwk, twice a week; 5qwk, 5 times a week; the schedule is renewed X times as indicated; T, treated group; C, control group; CR, complete response.

Discussion
In this article, we report on the development of a large panel of pediatric liver cancer xenografts that provide clinically relevant models for evaluating the performance of chemotherapeutic agents in tumors resistant to current treatment regimen. The PLC-PDX program initiated in May 2010 aims to be a breakthrough in liver pediatric preclinical oncology. The results provided show that PLC-PDXs are robust preclinical surrogates of pediatric liver cancers. All these models have been live-frozen, thus constituting a bank of living tumor tissue that can be used to boost research on pediatric liver cancer. Because the large majority of PLC-PDXs were developed from tumors that received first- or second-line chemotherapy, these models can be considered as surrogates of relapsing disease. Moreover, because all tumors were grafted in the mouse interscapular region, they are physiologically more similar to distant metastasis than to intrahepatic recurrence. The impact of the environmental context on response to treatment is an open question that might be clarified by performing in vivo studies upon orthotopic implantation of the PDX models established. Recurrent HBs showed a much higher engraftment rate than primary tumors (75% vs. 33.3%). This observation indicates that tumor growth in mice is strongly correlated with aggressive phenotype. Insufficient tumor necrosis/fibrosis and high residual AFP levels postchemotherapy are two clinical parameters strongly predictive of PDX growth. It will be interesting to evaluate these two parameters as predictors of tumor recurrence in HB patients. Most chromosomal alterations found by cytogenetic array in parental tumors and PDX samples have been described.35 Particularly, gain of chromosomes 8, 17, and, above all, 20 are more frequent than in previous reports, where the incidence of gains of chromosomes 8/8q and 20 was 15% and 24% in HBs, respectively.36 Given that chromosome 20 gain has been associated with poor prognosis, the high frequency observed in tumors that are able to be grown in mice further supports the notion that PDX are mainly developed from aggressive tumors or tumor components. It will be interesting to evaluate whether this specific alteration is associated with increased tumor ability to grow in mice.
Response to cisplatin alone tested in our PDX models showed an overall weak or no response to treatment. The clinical history of the parental tumors showed instead an overall reduction of tumor size and AFP levels upon treatment. This discrepancy could be attributed to the eradication of cisplatin-sensitive cells by chemotherapy during first-line chemotherapy and therefore the enrichment of cisplatin-resistant residual cells when PDXs were established. In agreement with SIOPEL's oncologists, we evaluated PDX response to treatments that could be proposed to children with recurrent disease, including irinotecan alone or in combination with temsirolimus or sirolimus, two mammalian target of rapamycin inhibitors, sorafenib, which is currently used in HCC in adults,37 crizotinib, given that its activity as a epithelial-mesenchymal transition (EMT) inhibitor might counteract increased MET signaling previously described in HB,38 and paclitaxel, also extensively evaluated in pediatric solid tumors.39 In addition to these clinically well-known compounds, we tested tumor response to Nefopam, a nonopioid analgesic drug, because it was shown to decrease CTNNB1 product β-catenin expression in cancer cells.40 For all these targeted therapies, tumor response in the PDXs tested was highly insufficient. Irinotecan alone has been tested with promising results in a phase II trial of the SIOPEL group,41 whereas irinotecan in combination with rapamycin (sirolimus) is currently administered in a phase I protocol for pediatric patients with refractory solid tumors (ClinicalTrials identifier: NCT01282697). Another compound, temozolomide, is currently associated with irinotecan in several protocols to evaluate its efficacy in different forms of pediatric cancers.42, 43 The use of irinotecan in pediatric oncology, alone or in combination with different drugs, has shown promising results, which encourage its use as a new therapeutic indication. Given that irinotecan conversion into its active form, SN38, occurs more efficiently in mice than in humans,44 it is very important to ensure that the dose used when testing irinotecan in mice is adequate. In our studies, we observed heterogeneous response of the PDXs tested to irinotecan alone or in combination with temozolomide. Also, when we compared two different dosages (10 mg/kg and 40 mg/kg) in the HCC-PDX, both treatments were unsuccessful and classified as PD. Overall, these results intrinsically validate that irinotecan dosage allows objective evaluation of tumor response rate. The combination irinotecan/temozolomide showed very promising results in PDXs derived from two very aggressive forms of HB. However, when tested in HB-239 and HB-244-RED-239, the two models showed partial and no response to the combination, respectively. HB-239 and HB-244-RED-239 are the only models that carry NFE2L2 mutation in addition to CTNNB1. Given that NFE2L2 mutation is predictive of poor outcome,13 it would be interesting to understand whether the mutation is functionally involved in tumor resistance to treatment. From a more general perspective, translation of these results into the clinic will require careful evaluation of the efficacy of this combination in the human background. As the enzymes that participate in irinotecan-SN38 conversion, such as carboxylesterases expression levels (conversion in the tumor) and uridine diphosphate glucuronosyl transferases and cytochrome P4503As polymorphisms (conversion in the liver), are very heterogeneous in the patient population,45 a personalized approach taking these parameters into account should be envisaged.
PDXs are robust and reliable surrogates of human tumors that are being adopted as preclinical reference worldwide. The continuously growing availability of molecular data on cancer clearly indicates tumor heterogeneity as the major challenge for the identification of common treatment protocols. To cope with this observation, different strategic approaches can be envisaged, such as the generation of large PDX panels for preclinical mouse trials aimed at identifying treatments with wide-range efficacy28 or use PDXs as avatars to tailor personalized treatments.46 Our approach tries to conciliate these two lines. The long-term aim of this study is to establish PDXs covering progressively tumor heterogeneity and analyze these models at molecular and pharmacological levels to identify novel therapeutic options and associate molecular biomarkers predictive of tumor response. Besides the need to identify common molecular characteristics in tumors that show similar response to anticancer therapies, preclinical panels for rare tumor types could be very useful to guide the repositioning of treatments already available in the clinic. This is particularly true for pediatric tumors. This panel of PLC-PDXs reflecting the patient population diversity will provide clinicians and pharmaceutical companies with a powerful preclinical tool that will allow robust information to (1) understand the molecular basis of the disease, (2) identify predictive markers for better patient stratification, and (3) allow “preclinical-like” evaluation of drugs not primarily developed in this specific indication.
Acknowledgments
The authors are grateful to all the technicians from XenTech for their assistance in the study, all the families that decided to sign the informed consent thus rendering this study possible, and the Macy Easom Cancer Research Foundation for their fundamental involvement the PLC-PDX program.
REFERENCES
Author names in bold designate shared co-first authorship.




