Cancer Stem cells and their cellular origins in primary liver and biliary tract cancers
Potential conflict of interest: Nothing to report.
Supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan (15H06633) and the Uehara Memorial Foundation
Abstract
Liver and biliary tract cancers are highly aggressive, are heterogeneous in their phenotypic traits, and result in clinical outcomes that are difficult to manage. Cancers have subpopulations of cells termed “cancer stem cells” (CSCs) that share common intrinsic signaling pathways for self-renewal and differentiation with normal stem cells. These CSCs likely have the potential to evolve over time and to give rise to new genetically and functionally diverse subclones by accumulating genetic mutations. Extrinsic signaling from the tumor microenvironment, including the CSC niche, has been implicated in tumor initiation/progression and heterogeneity through dynamic crosstalk. CSCs have become recognized as pivotal sources of tumor initiation/progression, relapse/metastasis, and chemoresistance. Conclusion: The origins of CSCs are hypothesized to derive from the transformation of normal stem/progenitors and/or from the reprogramming of adult cells that converts them to stem/progenitor traits; however, the precise mechanisms have not yet been fully elucidated. (Hepatology 2016;64:645-651)
Abbreviations
-
- BTSC
-
- biliary tree stem cell
-
- CCA
-
- cholangiocarcinoma
-
- CSC
-
- cancer stem cell
-
- FL-HCC
-
- fibrolamellar HCC
-
- HCC
-
- hepatocellular carcinoma
-
- IH-CCA
-
- intrahepatic CCA
Liver cancers are among the most common cancers and the second leading cause of cancer mortality worldwide.1 Primary liver cancers include hepatocellular carcinomas (HCCs), cholangiocarcinomas (CCAs), combined HCC-CCAs, hepatoblastomas, and fibrolamellar hepatocellular carcinomas (FL-HCCs). Surgical resection and liver transplantation are the only curative treatments for early-stage patients with these cancers. However, the majority of patients are diagnosed in advanced stages of the disease during which the extant therapies are ineffective. There is accumulating evidence that cancer stem cells (CSCs) are involved in the initiation/progression of primary liver/biliary tract cancers. It is well known that cancers with stem cell-like traits exhibit poor prognoses.2, 3 Therefore, a better understanding of CSCs could lead to novel therapies.
The CSC Concept
The hierarchical CSC concept4, 5 is that CSCs have the ability for unlimited cell division comprising symmetric (self-renewal) and asymmetric (lineage restriction and differentiation) capabilities, which results in heterogeneous cell progenies. CSCs have properties that are shared with normal stem cells and with facets of embryogenesis and organogenesis that involve maturational lineages with gradients in phenotypic properties, such as cellular morphology, gene expression profiles, and paracrine signaling from lineage-dependent mesenchymal partners. The maturational lineage properties also correlate with gradients in various molecules and with intrinsic signaling pathways that are involved in self-renewal and differentiation.6 Recently, Kreso and Dick proposed an “evolutionary CSCs concept” in which intratumoral heterogeneous genetic subclones might explain tumor heterogeneity; CSCs are comprised of heterogeneous populations that over time give rise to new subclones with genetic and functional diversity due to the accumulation of genetic mutations.7 Therefore, CSCs are presumed to be factors in tumor initiation, progression, metastasis, and relapse following conventional therapies. The aggressive phenotypic traits of primary liver cancers with respect to self-renewal, tumorigenicity, invasiveness, and chemoresistance/radioresistance are assumed to be dependent on the CSC components.8, 9 Indeed, liver tumors with stem cell phenotypes in humans have been identified, as summarized by Theise and colleagues.10
The Cellular Origins of Primary Liver and Biliary Tract Cancers
Considerations about the cellular origins of primary liver/biliary tract cancers have gone through multiple phases. Originally these cancers were thought to derive from malignant transformations of mature parenchymal cells with the assumptions that hepatocytes give rise to HCCs and cholangiocytes give rise to CCAs. These assumptions gave way to assumptions that focused on origins in stem cell populations. Most recently, there has been a reconsideration of cancers as deriving from adult cells that are subjected to conditions that result in the mechanisms that give rise to cells with stem/progenitor cell traits. Notably, there are no animal models that truly reflect human hepatocarcinogenesis including the sequential steps of chronic injury and subsequent cirrhosis. Therefore, the cellular origins of primary liver/biliary cancers are still debated.11, 12
Cancer Origins from the Transformation of Stem/Progenitors
The debates regarding the cellular origin of primary liver/biliary cancers are further fueled by the findings of multiple categories of liver cancer cells with phenotypic traits that are distinct from those of classic HCCs or CCAs. For example, Allen and Lisa reported that combined HCC-CCAs have features of both HCCs and CCAs.13 Similarly, cholangiolocellular carcinomas are another subtype of CCAs that often possess both hepatocytic and cholangiocytic histological phenotypic traits.14-16 Chiba et al. demonstrated that transduction of B cell-specific Moloney murine leukemia virus integration site 1 (Bmi1) or mutant β-catenin into murine fetal hepatic stem/progenitor cells results in the enhancement of self-renewal and tumorigenicity. The histologies of tumors exhibit features with similarities to those of combined HCC-CCAs.17 Moreover, the initiation of HCCs and hepatoblastomas occurs in transgenic mice with hepatic stem/progenitor cells that overexpress β-catenin.18 Therefore, combined HCC-CCAs, cholangiolocellular carcinomas, and hepatoblastomas are thought to originate from normal hepatic stem/progenitors that are located in intrahepatic sites within the canals of Hering by the portal triads and are able to differentiate into both hepatocytes and cholangiocytes.19-22 The development of HCCs or intrahepatic CCAs (IH-CCAs) occurs in tumors from p53-deficient or liver-specific p53-mutant murine hepatic progenitors transduced with c-Myc23 or Kras,24 respectively. Recently, mutant isocitrate dehydrogenase (IDH) was demonstrated to impair hepatocyte differentiation with aberrant expansion of hepatic stem/progenitor cells through hepatocyte nuclear factor 4α (HNF4α) inhibition, and it has also been revealed that the coexistence of IDH and Kras mutations in genetically engineered mouse models results in the generation of IH-CCAs.25
Recently, collaborative efforts between Alvaro, Gaudio, Reid, and their associates resulted in the recognition of multiple subpopulations of multipotent, endodermal stem/progenitor cells throughout the biliary tree. Large numbers of these cells occur in the large intrahepatic bile ducts and the hepatopancreatic common duct.26-28 Biliary tree stem cells (BTSCs) have proven to be precursors of both the liver and pancreas and are found in peribiliary glands, which are stem cell niches that are located throughout the biliary tree (Fig. 1).26-28 Interestingly, the typical onset sites of CCAs are at the locations of peribiliary glands in the biliary tree, which include the hilum, the branching points of the biliary tree, and the periampullary region.29 Cardinale et al. described mucin-CCAs, which are subtypes of IH-CCAs, and proposed that they likely originate from BTSCs because peribiliary glands, the niches for BTSCs, are mucin-producing glandular elements in the biliary tree.29
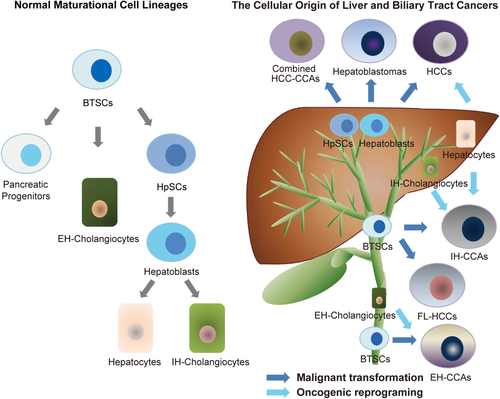
The BTSC populations have proven to be relevant to FL-HCCs, a category of primary liver cancer that is of considerable concern due to its occurrence in young people without evidence of chronic liver disease and unknown epidemiological factors.30 Genetic analyses have revealed that FL-HCCs are quite distinct from other liver cancers with respect to genetic and transcriptomic traits.30-32 A striking example is the finding of a fusion gene, i.e., the DNAJB1-PRKACA chimera, which is a unique feature of FL-HCCs that is likely contributory to their pathogenesis.31, 32 FL-HCCs express hepatic, biliary, and endocrine marker traits.32 A human patient-derived xenograft tumor model of FL-HCC that is maintained in immunocompromised hosts has been demonstrated to be highly enriched in CSCs, and its global transcriptomic expression profile most closely resembles that of BTSCs, which are newly identified subpopulations that are precursors of both the liver and pancreas.33 This finding clarifies the findings of others that some FL-HCCs exhibit hepatic traits, some exhibit pancreatic traits, and, as most recently revealed, some exhibit intestinal traits33; the traits exhibited by FL-HCCs are dictated by which of the BTSC subpopulations are most closely related to the origin for the tumor.
Cancer Origins from the Oncogenic Reprogramming of Differentiated Cells to Generate Stem/Progenitors
Stem/progenitor cell functions during liver development have been well characterized and have long been thought to be related to the origins of liver cancers.22, 28, 34 The ductular reactions that occur in chronic liver disease are hypothesized to involve a pool of stem/progenitors that replenish the tissue during liver regeneration or to give rise to tumors especially following adult chronic liver injury.35-38
In recent years, alternative interpretations that suggest that liver regeneration derives entirely from mature hepatocytes through mechanisms of cell plasticity or reprogramming events have emerged.39, 40 However, these proposals of adult cells as the primary or even sole source of postnatal regeneration and as the sources of tumors are being questioned due to new realizations about stem/progenitor cell sources. The demands of the quiescent liver and mild regenerative states appear to be met by periportal, unipotent hepatocytic progenitors in combination with Axin2+ diploid hepatocytic progenitors that are located pericentrally around the central vein and triggered by Wnt signaling.41 The periportally committed progenitors (so-called hybrid hepatocytes) are assumed to be intermediates that are derived from hepatic or biliary tree stem/progenitors and are a source for liver replenishment but not for the initiation of HCCs.42 In contrast, severe liver injuries result in responses from periportal, multipotent hepatic/biliary tree stem/progenitors and are correlated with dramatic architectural changes in the biliary tree.43 These reports from lineage tracing studies in mice suggest that periportal multipotent stem/progenitors are not relevant to liver regeneration or to HCCs.44, 45 With respect to tumor formation, two groups using cell fate lineage tracing in mice have claimed that the simultaneous activation of Notch and Akt signaling induces the malignant cholangiocellular differentiation/transformation of hepatocytes and that these hepatocytes give rise to IH-CCAs. Thus, the authors of these studies have concluded that IH-CCAs originate from hepatocytes rather than cholangiocytes or hepatic stem/progenitors.46, 47 An alternative conclusion was reached by Forbes and associates, who demonstrated that IH-CCAs can arise from biliary epithelia with chronic inflammation and the loss of p53 using cholangiocyte lineage tracing in mice.48 It is possible that HCCs and IH-CCAs can arise from any type of liver cell due to the cellular plasticity that might be caused by reprogramming mechanisms. However, these conclusions are dependent on an excessive reliance on data from murine models of Cre-mediated-lineage tracing systems and the known technical limitations of these models.38, 49 Moreover, there are no murine models that mimic human cancers in terms of the major pathways of hepatocarcinogenesis and arise from chronic liver disease with cirrhosis. Therefore, the establishment of such animal models is needed to accurately represent bona fide human cancers.
Evidence for Reprogramming Phenomena
Several studies have reported the induction/generation of CSCs from both transformed cells50 and normal cells, including murine induced pluripotent stem cells51 and human fibroblasts,52 through oncogenic reprogramming. Recent work has demonstrated spontaneous dedifferentiation from basal cell-like human mammary epithelial cells into stem-like cells. This dedifferentiation was enhanced by oncogenic transformation, which indicated that the transformed cells might partially reflect the differentiation capacity of the original cells.53 Gastric intestinal metaplasia caused by Helicobacter pylori infection is known to be a risk factor for gastric adenocarcinoma, and the demonstration of the mechanism of the conversion of the gastric epithelium to an intestinal phenotype was the first evidence of aberrant reprogramming during the disease progression process.54 Thus, it has become evident that oncogenic reprogramming can be induced in differentiated cells by intrinsic and extrinsic factors.
Forbes and associates observed that opposing mechanisms between Notch and macrophage-derived Wnt signaling orchestrate cell fate decisions in hepatic progenitors in chronic liver disease.55 This phenomenon was recently confirmed in liver cancers.56 These authors demonstrated that the loss of p53 contributes to the dedifferentiation of mature hepatocytes into Nestin+ progenitor-like cells and results in the transformation into HCCs or CCAs with lineage-specific mutations that target Wnt and Notch signaling, respectively.56 This study also expanded previous findings that p53 loss contributes to the enhancement of reprogramming57 and the acquisition/maintenance of CSC properties.58 Notably, Thorgeirsson and associates elegantly described confirmed tumor initiation from all murine hepatic lineage stage cells (i.e., hepatic stem/progenitor cells, lineage-committed hepatoblasts, and differentiated adult hepatocytes) transduced with oncogenic H-Ras and SV-40LT along with acquisition of stem cell-like properties due to reprogramming events.59 These authors demonstrated that all lineage stages of hepatic cells have the potential for oncogenic reprogramming into CSCs with the activation of lineage-specific pathways, especially adult hepatocytes in which c-Myc acts as the driver of this reprogramming. Interestingly, tumors can give rise to other tumors from these three independent lineage stages of cell origins to yield the phenotypic diversity of HCCs and CCAs. Lineage-committed hepatoblasts are predominantly reprogrammed into CCA-like tumors, hepatic stem/progenitor-derived tumors exhibit epithelial-mesenchymal transition-like phenotypes, and those from differentiated adult hepatocytes preferentially exhibit HCC-like phenotypes.59 This study also indicated that tumors derived from hepatic stem/progenitor cells exhibit striking enrichment in CSCs compared with those from differentiated cells.
Based on the above evidence, the phenotypic features of tumors may have roots in the origins of the cells that were malignantly transformed. In human primary liver/biliary cancers, it is plausible that the cellular origin of most HCCs derives from dysplastic nodules in liver cirrhosis that are subjected to reprogramming events, whereas stem/progenitor cells that are malignantly mutated might yield tumors with stem cell-like traits and poor prognoses, such as subtypes of HCCs, CCAs,60 combined HCC-CCAs, hepatoblastomas, and FL-HCCs (Fig. 1).33
Tumor Microenvironment
The microenvironments of stem cell niches are strictly controlled by intrinsic and extrinsic programs of soluble signals that work synergistically with extracellular matrix components to regulate the homing/proliferation/differentiation of stem cells.61 It is possible that stem cells might be susceptible to malignant transformation with genetic or epigenetic modifications when the mechanisms underlying the maintenance of normal stem cell homeostasis are dysregulated.
The niche for liver CSCs has not yet been elucidated, but it is assumed that mechanisms similar to those acting in the niches of normal stem cells exist to control cell proliferation, migration, invasion, apoptosis resistance, and/or the epithelial-mesenchymal transition of CSCs by direct or indirect factors.62 Interestingly, He et al. demonstrated the existence of HCC progenitors with a gene expression profile that is similar to that of normal hepatobiliary progenitors within the foci of altered hepatocytes, which are premalignant, dysplastic regions in cirrhotic livers. The tumorigenicity of these cells has been demonstrated to be driven by autocrine interleukin-6 signaling through LIN28 up-regulation,63 which indicates that foci of altered hepatocytes act as the niche for liver CSCs and interleukin-6 signaling might be needed for malignant progression with the acquisition of stemness. The extracellular matrix is a major component of the niche and has been described to play a pivotal role in normal stem cells or CSCs.61 Dysfunctions of the extracellular matrix occur during the initiation and development processes of cancer and modulate not only the transformation of cells but also the dysregulation of stromal cells. These modulations result in cancer development and contribute to angiogenesis, vasculogenesis, and hypoxia during the generation of the tumor microenvironment.64 Similar results have also been demonstrated for HCCs.65
Concluding Remarks
- What oncogenic events and/or signaling pathway(s) are involved in the pathogenic process?
- In what type(s) of cells, i.e., stem/progenitors or mature cells, do these events occur?
- What is the location of the niche/microenvironment that is associated with the pathogenic process?
- Is there robust evidence for reprogramming, and how common is this process in pathogenesis?
- If one considers evolving CSCs to be relevant, when does malignant transformation occur during tumorigenesis?
The elucidation of cellular origin and molecular mechanisms underlying the orchestration of CSCs with robust complexity and diversity could shed light on the development of future therapeutic modalities against these aggressive cancers.
Acknowledgment
I thank Dr. Lola M. Reid (University of North Carolina School of Medicine, Chapel Hill, NC) for useful discussions and for editing the manuscript in English.




