Natural history and management of esophagogastric varices in chronic noncirrhotic, nontumoral portal vein thrombosis
Potential conflict of interest: Dr. Bosch consults for Gilead, Conatus, Intercept, Actelion, and Falk. He received grants from Exalenz.
Abstract
In patients with chronic noncirrhotic, nontumoral portal vein thrombosis (PVT), the usually recommended strategy for endoscopic screening and management of varices is the same as in cirrhosis. However, the efficacy of this policy in patients with PVT is unknown. We assessed the course of gastroesophageal varices in a large cohort of patients with chronic PVT. Patients prospectively registered in two referral centers for vascular liver disorders were eligible for the study. Endpoints were development and growth of varices and the incidence and outcome of portal hypertension-related bleeding. Included were 178 patients with chronic PVT. Median follow-up was 49 (1-598) months. Variceal bleeding was the initial manifestation in 27 (15%) patients. Initial endoscopy in the remaining 151 patients showed no varices in 52 (34%), small esophageal varices in 28 (19%), large esophageal varices (LEVs) in 60 (40%), and gastric varices without LEVs in 11 (7%). Ascites and splenomegaly were independent predictors for the presence of varices. In patients without varices, the probability of developing them was 2%, 22%, and 22% at 1, 3, and 5 years, respectively. In those with small esophageal varices, growth to LEVs was observed in 13%, 40%, and 54% at 1, 3, and 5 years, respectively. In patients with LEVs on primary prophylaxis, probability of bleeding was 9%, 20%, and 32% at 1, 3, and 5 years, respectively. Nine (5%) patients died after a median 51 (8-280) months, only one due to variceal bleeding. Conclusions: The course of varices in chronic noncirrhotic, nontumoral PVT appears to be similar to that in cirrhosis; using the same therapeutic approach as for cirrhosis is associated with a low risk of bleeding and death. (Hepatology 2016;63:1640-1650)
Abbreviations
-
- EBL
-
- endoscopic band ligation
-
- EV
-
- esophageal varix
-
- GOV
-
- gastroesophageal varix
-
- GV
-
- gastric varix
-
- IGV
-
- isolated gastric varix
-
- LEV
-
- large esophageal varix
-
- NSBB
-
- nonselective beta-blocker
-
- PVT
-
- portal vein thrombosis
-
- SEV
-
- small esophageal varix
Chronic noncirrhotic, nontumoral portal vein thrombosis (PVT) is a rare vascular disorder of the liver, with variceal bleeding being its main manifestation.1, 2 Indeed, several retrospective cohort studies have shown a high prevalence of esophageal varices (EVs) at the time of chronic PVT diagnosis.3, 4 Due to the low incidence and prevalence of PVT, specific studies aimed at determining adequate strategies for endoscopic screening and management of varices are scarce and small-sized. Consequently, the 2015 Baveno VI Consensus suggested applying to patients with PVT the same recommendations validated for patients with cirrhosis and portal hypertension, i.e., to perform a baseline endoscopy at diagnosis of PVT and subsequent endoscopies at 2-year or 3-year intervals in patients with no EVs or small EVs (SEVs) at baseline, to use beta-blockers or endoscopic band ligation (EBL) as a primary prophylaxis, and to use drug plus EBL to treat variceal bleeding and prevent rebleeding.5 However, whether this is also an effective and safe strategy in patients with PVT remains to be determined.
The aim of our study was to assess the course of varices in a large cohort of patients with chronic PVT with complete obstruction of the portal vein trunk and/or both branches by determining the prevalence of EVs and gastric varices (GVs) at initial endoscopy; the incidence of varices and predictive factors for its development in those without varices at initial endoscopy; the probability of, and predictive factors for, the growth of SEVs to large EVs (LEVs); and the incidence, outcome, and predictive factors of gastroesophageal variceal (GOV) bleeding, rebleeding and death.
Patients and Methods
STUDY DESIGN
Patients with PVT prospectively registered in two referral centers for vascular disorders of the liver (from July 1984 at Hospital Clinic in Barcelona and from March 1983 at Hôpital Beaujon, Clichy, Paris) were eligible for the study. All patients had given written informed consent to use their clinical data for research purposes. The guidelines of good clinical practice enumerated in the Declaration of Helsinki of 1964 and the revision in Edinburgh in 2000 were followed, and the ethical committees of the two participating hospitals approved the study protocol.
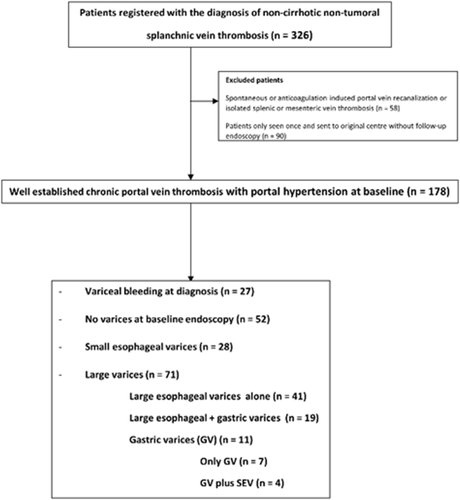
Clinical records of these patients, with special attention to reports of upper endoscopies, were reviewed and data retrospectively collected in a predesigned case report form. Patients with liver disease, spontaneous or anticoagulation-induced recanalization, partial thrombosis of the portal or splenic veins, or isolated complete thrombosis of the splenic or superior mesenteric vein with patent portal vein were excluded from the analysis (Fig. 1). Liver disease was reasonably discarded by means of (1) clinical history, (2) ruling out etiological factors for liver disease, (3) imaging studies, and (4) liver biopsy in doubtful cases. Time of inclusion into the study was considered the date of the first upper gastrointestinal endoscopy in patients with an imaging study showing PVT causing portal hypertension due to complete obstruction of the portal trunk and/or both branches of the portal vein.
Dates of first screening endoscopy and of subsequent endoscopies were recorded. Follow-up data were collected up to February 2014 or death. Endpoints evaluated during follow-up included (1) in patients without varices, the development of EVs and/or GVs during follow-up; (2) in those with SEVs, growth to LEVs; (3) and in patients with LEVs, the incidence of portal hypertension-related bleeding. Additionally, the outcome of portal hypertension-related bleeding and death were recorded.
Due to the retrospective nature of the study, time between follow-up endoscopies was not homogeneous. The median interval between follow-up endoscopies was calculated by dividing the time period between initial screening endoscopy and the latest surveillance endoscopy in relation to the studied outcome (appearance, growth of varices, or bleeding from varices) by the total number of endoscopies performed during this period of time. This interval was defined as the time/endoscopy index.
DEFINITIONS
PVT was considered acute when patients presented with abdominal pain or intestinal ischemia in the absence of clinical, endoscopic, or imaging evidence of portal hypertension. Such patients were considered to have developed stable chronic PVT and therefore, being eligible for the study, if there was no recanalization of the portal vein but development of a portal cavernoma, were confirmed at imaging investigations performed at least 6 months after the acute PVT episode. Patients with chronic PVT at diagnosis were those presenting with portal cavernoma with clinical, ultrasonographic, or endoscopic signs of portal hypertension.
EVs were defined as large or small if ≥5 mm or <5 mm, respectively.6 GVs were defined according to the classification by Sarin et al.7 Bleeding related to portal hypertension (variceal bleeding and portal hypertensive gastropathy) was defined according to Baveno VI criteria.8
In most patients an exhaustive etiological study of an underlying prothrombotic disorder was performed as described.3, 9, 10
STATISTICAL ANALYSIS
Quantitative data were expressed as means ± standard deviation or median and range, with statistical analysis performed using the Student t test or Mann-Whitney test when appropriate. Qualitative data were expressed as frequencies and percentages and analyzed using Pearson's chi-squared test or Fischer's exact test when appropriate. Backward logistic regression was used to determine independent predictors (odds ratio with 95% confidence interval) of the presence of varices at baseline. Independent predictors for variceal appearance, growth, and bleeding events were estimated as hazard ratios with 95% confidence intervals, using the Cox regression analysis and extended Cox regression analysis for time varying covariates. Age was adjusted to the outcome analyzed. The risk of variceal appearance, growth of varices, and occurrence of variceal bleeding during follow-up were described with the cumulative incidence function, taking into account death as a competing risk. This provides more accurate estimations of variceal appearance, growth of varices, and occurrence of variceal bleeding rates than censoring patients at the time of death in a Kaplan-Meier analysis.11 Competing risk analysis was performed with the R package cmprsk, with the aid of the CumIncidence function developed by Scrucca et al.12 Variables with P < 0.1 in the univariate analysis were considered for the multivariate analyses. The maximum number of variables included in the multivariate analysis was one per five to 10 outcomes. P < 0.05 was considered to be statistically significant. Data analysis was performed with SPSS version 20 (Chicago, IL) and R (www.r-project.org).
Results
A total of 326 patients with PVT were prospectively registered in the two referral centers with the diagnosis of splanchnic vein thrombosis, and 148 patients were excluded: 90 had no follow-up endoscopy data after the first visit as they were referred back to the primary center, 42 had spontaneous or anticoagulation-induced total recanalization of the PVT, 11 had partial thrombosis of the splanchnic vessels not causing portal hypertension, and five had isolated complete thrombosis of the splenic or superior mesenteric vein. Finally, 178 patients were included in the study (Fig. 1). Baseline characteristics and etiological factors are shown in Table 1. An inherited prothrombotic factor and at least one acquired systemic prothrombotic factor were detected in 29% and 48% of evaluated patients, respectively (Table 1). Median (range) follow-up was 49 (1-598) months.
| n, Mean | %, ±SD | |
|---|---|---|
| Male gender | 96 | 54% |
| Age (years) | 41 | ±16 |
| Hematocrit (%) | 38 | ±7 |
| Platelet count | 283 | ±269 |
| <100 × 109/L | 21 | 14% |
| <150 × 109/L | 45 | 29% |
| Prothrombin time (%) | 76 | ±22 |
| ALT (U/L) | 43 | ±38 |
| AST (U/L) | 34 | ±23 |
| Alkaline phosphatase (U/L) | 194 | ±204 |
| GGT (U/L) | 95 | ±128 |
| Albumin (g/L) | 40 | ±6 |
| Known acute PVT at diagnosis | 37 | 21% |
| Inheriteda prothrombotic factor (evaluated in 158 patients) | 42 | 27% |
| Acquiredb prothrombotic factor (evaluated in 168 patients) | 81 | 50% |
|
Myeloproliferative disease (evaluated in 165 patients) |
64 | 39% |
|
Any inherited or acquired prothrombotic factor (evaluated in 158 patients) |
94 | 59% |
| Two or more prothrombotic factors (evaluated in 158 patients) | 30 | 19% |
| PVT without either SV or SMV thrombosis | 63 | 35% |
| PVT plus SV thrombosis without SMV thrombosis | 23 | 13% |
| PVT plus SMV thrombosis without SV thrombosis | 25 | 14% |
| PVT plus SV and SMV thrombosis | 67 | 38% |
| Ascites at baseline (evaluated 174 patients) | 37 | 21% |
| Splenomegaly (evaluated in 156 patients) | 111 | 71% |
| Splenectomy | 15 | 9% |
| Anticoagulation | 84 | 51% |
- a Prothrombin gene mutation, Factor V Leiden mutation, protein C and S deficiency, antithrombin III deficiency.
- b Myeloproliferative disorders, antiphospholipid syndrome, paroxysmal nocturnal hemoglobinuria and excluding local factors, oral contraceptives, and pregnancy.
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase; SD, standard deviation; SMV, superior mesenteric vein; SV, splenic vein.
Variceal bleeding was the first manifestation of PVT in 27 patients (15%) (EVs in 26 and GVs in one). In the remaining 151 patients, no varices were present in 52 (34%), SEVs (all without red signs) in 28 (19%), and LEVs in 60 patients (40%, in 19 of them with associated GVs). In 11 (7%) additional patients, there were large GVs (in four associated with SEVs) (Supporting Fig. S1). In summary, 71 patients had LEVs and/or GVs susceptible to primary prophylaxis (47% of patients not presenting with variceal hemorrhage). Among them, red signs were not mentioned in 12 patients and were present in 20 of the remaining 60 patients (33%). Ascites was present in 37/174 (21%) patients but was usually mild, only detectable at imaging studies in 25. During the follow-up 17 additional patients developed ascites.
At the time of inclusion in the study 84 patients were receiving anticoagulation, and 43 additional patients received anticoagulation subsequently during follow-up. The reasons for anticoagulation were prothrombotic conditions in 83 patients (myeloproliferative disorders in 57, genetic or other acquired thrombophilic factors in 26) and rethrombotic events without an identified thrombophilic factor in 11 and 33 patients presenting with a severe acute PVT episode. During follow-up anticoagulation was stopped in 22 patients, in six of them due to complications attributed to it. Anticoagulation was restarted later due to new thrombotic events in six patients.
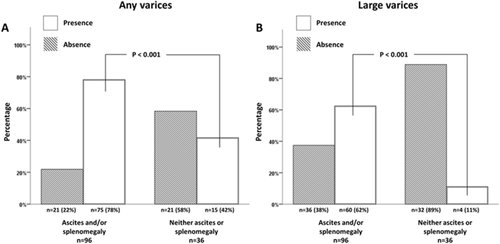
In patients without variceal bleeding at diagnosis, the presence of ascites and of splenomegaly was the only independent predictor for the presence of varices of any size at baseline endoscopy (Table 2 and Fig. 2). They were also independent predictors of the presence of large GOVs requiring primary prophylaxis. To exclude the potential confounding factor of the presence of splenomegaly in patients with a myeloproliferative neoplasm, the analysis was repeated excluding these patients. Ascites and splenomegaly still were the only independent predictors of the presence of varices at baseline endoscopy. However, EVs were present in 11% of patients without either ascites or splenomegaly.
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | |
| Hematocrit (%) | 0.94 (0.89-0.99) | 0.027 | ||
| Platelet count (×109/L) | 1 (1-1) | 0.108 | ||
| Platelet count <150 × 109/L | 1.87 (0.84-4.19) | 0.127 | ||
| Prothrombin time (%) | 0.98 (0.97-1) | 0.078 | ||
| ALT (U/L) | 1.01 (1-1.02) | 0.204 | ||
| Albumin (g/L) | 0.93 (0.87-0.99) | 0.021 | ||
| Known acute PVT at diagnosis | 2.98 (1.41-6.32) | 0.004 | ||
|
Inheriteda prothrombotic factor (evaluated in 104 patients) |
1.08 (0.5-2.35) | 0.846 | ||
|
Acquiredb prothrombotic factor (evaluated in 138 patients) |
1.18 (0.59-2.35) | 0.633 | ||
| PVT without either SV or SMV thrombosis | 0.93 (0.48-1.83) | 0.837 | ||
| PVT plus SV and SMV thrombosis | 0.76 (0.39-1.47) | 0.410 | ||
| Ascites at baseline | 3.34 (1.22-9.14) | 0.019 | 4.05 (1.26-13.03) | 0.019 |
| Splenomegaly (evaluated in 132 patients) | 4.35 (2.04-9.3) | <0.001 | 3.91 (1.77-8.66) | 0.001 |
- a Prothrombin gene mutation, Factor V Leiden mutation, protein C and S deficiency, antithrombin III deficiency.
- b Myeloproliferative disorders, antiphospholipid syndrome, paroxysmal nocturnal hemoglobinuria and excluding local factors, oral contraceptives, and pregnancy.
- Abbreviations: ALT, alanine aminotransferase; CI, confidence interval; SMV, superior mesenteric vein; SV, splenic vein.
DEVELOPMENT OF VARICES IN PATIENTS WITHOUT VARICES AT INITIAL ENDOSCOPY
Forty out of the 52 (77%) patients without EVs or GVs at initial endoscopy had at least one surveillance endoscopy. Surveillance endoscopy was not performed in the remaining 14 patients due to a follow-up of <2 years in nine patients and for unknown reason in five. The median elapsed time for development of varices was 37.5 (range 7-166) months, the median time/endoscopy index was 17.6 (range 4-94) months, and the median number of follow-up endoscopies performed was two (range one to nine).
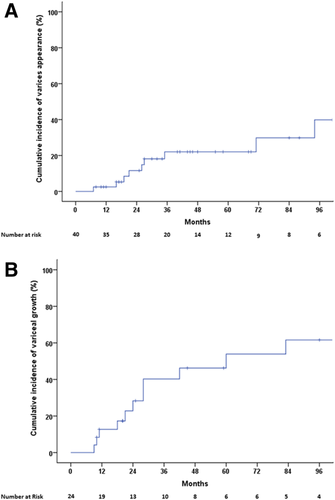
Varices developed in 10 (25%) patients (SEV in five, LEV in four, and isolated gastric varix 1 [IGV1] alone in one), with an actuarial probability of 2%, 22%, and 22% at 1, 3, and 5 years, respectively (Fig. 3A). At univariate Cox regression analysis, including the use of anticoagulation, only splenomegaly had a trend toward an association with a higher risk of variceal formation (Supporting Table S1). Due to the retrospective nature of the study, the potential impact of rethrombosis on the splanchnic area could not be evaluated.
EV GROWTH
Thirty-three patients had SEVs (in 28 at initial endoscopy and in five additional patients during follow-up endoscopies). In 24 of them (73%), at least one follow-up endoscopy was performed. Follow-up endoscopy was not performed due to follow-up time <2 years in five patients and for unknown reasons in four. Median elapsed time was 27 months (range 9-218) months, the time/endoscopy index was 11 (range 5-83) months, and the median number of endoscopies performed was two (range 1-19).
Growth from SEV to LEV occurred in 10/24 (42%) patients, four of whom also developed GVs (GOV2 in two, GOV1 in one, IGV1 in one). Additionally, two patients maintained SEVs but developed large GVs (Supporting Fig. S1). Actuarial probability of variceal growth (EV or GV) was 13%, 40%, and 54% at 1, 3, and 5 years, respectively (Fig. 3B). At univariate Cox regression analysis there were no significant factors associated with variceal growth, including anticoagulant therapy and coexisting myeloproliferative disorders. Interestingly, in three patients (12.5%) SEVs disappeared during follow-up. Only one of them was on anticoagulation. Variceal disappearance was confirmed during follow-up at 25, 114, and 125 months during which three, five, and three follow-up endoscopies were performed, respectively.
Preventing First Variceal Bleeding
PATIENTS WITH LEVs
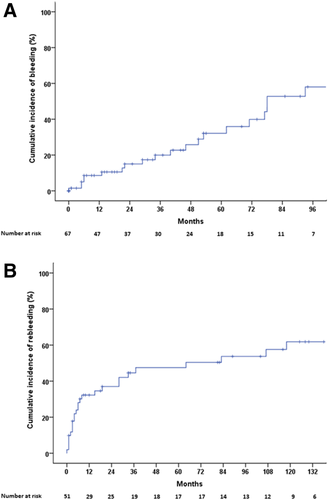
LEVs were identified in 74 patients. Twenty-three patients also have concomitant GVs (GOV2 n = 13, GOV1 n = 8, and IGV1 n = 2). In 60/74 patients LEVs were found at the baseline endoscopy, and in the remaining 14 patients they appeared during follow-up after a median of 27.5 (range 9-94) months. In three of them LEVs were identified during a variceal bleeding episode at 28, 60, and 94 months after the previous endoscopy which showed SEVs in two and no varices in one patient, respectively. Therefore, 71 patients were candidates for primary prophylaxis. Four patients were not treated, for unknown reasons (three of them [75%] developed portal hypertensive bleeding episodes). Sixty-seven patients received primary prophylaxis: 59 only with nonselective beta-blockers (NSBBs), five with NSBBs + EBL, and three only with EBL because of contraindications/side effects of NSBBs. Median dose of NSBB was 100 mg (range 10-240). Twenty-one of the 67 patients (31%) had a portal hypertensive hemorrhage. The actuarial probability of bleeding was 9%, 20%, and 32% at 1, 3, and 5 years, respectively (Fig. 4A). Bleeding rates were similar among patients receiving only NSBBs (19/59, 32%) or EBL (alone or with NSBB; 2/8, 25%). One patient bled during the first prophylactic EBL session. Sources of bleeding were EV in 16, GV in two, portal hypertensive gastropathy in two, and ectopic varices in one. Univariate Cox regression analysis, including treatment with anticoagulants and coexisting myeloproliferative disorders, did not identify any predictive factor for first bleeding in this population (Supporting Table S2)
GVs
Fourteen patients had GV: IGV1 in six patients, IGV2 (gastric corpus) in four, and GOV2 in four. Six patients had concomitant SEVs, and eight did not. In one patient GOV2 was diagnosed at variceal bleeding. In four patients GVs were small and primary prophylaxis was not initiated. Thus, only nine of these patients received primary prophylaxis with NSBBs. None of these patients bled during a median follow-up of 46 (6-248) months. As mentioned, 23 additional patients had GVs in association with LEVs and were submitted to primary prophylaxis; two bled from GVs.
Acute Bleeding Episodes
FIRST BLEEDING EPISODE
At least one portal hypertensive bleeding episode occurred in 57 patients (at diagnosis in 28 patients and in 29 additional patients during follow-up). Source of bleeding was EVs in 48 patients, GVs in four, ectopic varices in one, and portal hypertensive gastropathy or enteropathy/colopathy in four. Clinical data of the gastrointestinal bleeding episode are summarized in Table 3. Twenty-one (37%) patients were on primary prophylaxis, and 15 (26%) were receiving anticoagulants (Coumadin in 11, low molecular weight heparin in four) at the time of upper gastrointestinal bleeding; 75% of patients received blood with a median of four packed blood cell units (range 1-40). Hemostatic endoscopic therapy was the mainstay of treatment (Table 3). Failure to control bleeding or early rebleeding occurred in nine (17%) patients. Six of them underwent emergency surgery as rescue therapy (derivative in five and nonderivative in one). Despite surgery, three patients had further rebleeding finally controlled by adding NSBBs. There was no mortality related to this first gastrointestinal bleeding episode despite 15 patients receiving anticoagulation when they bled.
| n, Mean | %, SD | |
|---|---|---|
| Male gender | 34 | 60% |
| Age (years) | 42 | 19 |
| Bleeding under primary prophylaxis | 21 | 37% |
| Bleeding under anticoagulation | 15 | 26% |
| Percentage of patients requiring blood transfusion | 75% | |
| Number of packed RBC units | 4 | 7 |
| Treatment used (% of patients) | ||
| Only vasoactive drugs | 30% | |
| EBL and vasoactive drugs | 23% | |
| EIS and vasoactive drugs | 26% | |
| Cyanoacrylate injection and vasoactive drugs | 2% | |
| Not mentioned | 19% | |
| Failure to control bleeding episode | 16% | |
| Rescue surgical shunt | 11% | |
| Mortality | 0 |
- Abbreviations: EIS, endoscopic injection sclerotherapy; SD, standard deviation.
SECONDARY PROPHYLAXIS AND REBLEEDING
Of the 51 patients surviving the first bleeding episode without need of rescue surgery, 30 began endoscopic therapy (EBL or endoscopic injection sclerotherapy) alone or with concomitant NSBBs, 16 were treated only with NSBBs, four did not receive secondary prophylaxis for unknown reasons, and one had elective shunt surgery. The median dose of propranolol was 80 mg (range 10-360). Twenty-four (47%) patients rebled on secondary prophylaxis. Actuarial probabilities of rebleeding on secondary prophylaxis at 1, 3, and 5 years were 32%, 45%, and 47%, respectively (Fig. 4B), without significant differences between patients in whom bleeding was the first manifestation of PVT (n = 24) and those in whom the first bleeding developed during follow-up (n = 27). Sources of rebleeding were EVs in 17, GVs in two, post-EBL ulcers in three, ectopic varices in one, portal hypertensive colopathy and enteropathy in one patient each, and unknown origin in one patient. Blood transfusions were given in 73% of patients with a median of four packed blood cell units (range 1-21). Failure to control rebleeding occurred in four patients, with rescue surgery required to control the bleeding episode in three patients. Ten additional patients were submitted to elective surgery (six nonderivative and four derivative) to prevent further rebleeding. Only one patient, refusing rescue surgery, died as a consequence of variceal rebleeding. Univariate Cox regression analysis did not identify any clinical or imaging factor predicting rebleeding in this population. Again, neither anticoagulant therapy nor myeloproliferative disorders were associated with higher rebleeding rate (Supporting Table S3).
SURVIVAL
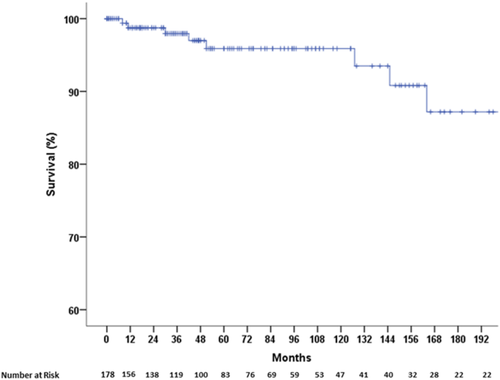
During follow-up, nine (5%) patients died. The 1-year, 3-year, and 5-year actuarial probabilities of survival were 99%, 98%, and 96%, respectively (Fig. 5). Four patients died due to multiorgan failure, two due to multiple extrasplanchnic thrombotic events, one to extrahepatic malignancy, one from a malabsorptive syndrome of unknown origin, and one because of variceal rebleeding. Age, altered liver enzymes, and presence of ascites at baseline were significantly associated with mortality at univariate Cox regression analysis. Due to the low number of events, no multivariate analysis was performed.
Discussion
The current study includes a large cohort of consecutive patients with chronic PVT followed in two referral centers for patients with portal hypertension and vascular liver disorders. The strengths of the study derive from (1) endoscopic follow-up performed according to a relatively standardized schedule in patients without varices or with SEVs, (2) primary and secondary prophylaxis of variceal bleeding applied in a relatively uniform manner in almost all patients with adequate indication, and (3) an etiologic workup of prothrombotic factors in most patients and anticoagulant therapy given according to a uniform management protocol.10
Variceal bleeding was the first manifestation of PVT in 27 patients (15%), similar to what was reported in other previous studies in patients without cirrhosis or malignancy.3, 13
It is important to note that the number of patients in whom variceal bleeding was the first manifestation of PVT markedly decreased with time. Indeed, only seven of the 120 (6%) patients diagnosed with PVT after 2001 had variceal bleeding as the presenting symptom of chronic PVT. This likely reflects the fact that currently PVT is diagnosed earlier in its course by highly sensitive and now widely available imaging studies in patients with no or few clinical manifestations.
At the first upper endoscopy 71% of our patients had EVs. This percentage was 55% (98/178) if bleeding varices or those at high risk of bleeding only were considered. Considering only the 151 patients without variceal bleeding at diagnosis of PVT, 34% of them did not have varices, 19% had SEVs, and 47% had LEVs requiring primary prophylaxis (40% EVs and 7% GVs). The percentage of varices found in our cohort of patients at baseline endoscopy is similar to that found at screening endoscopy in previous studies in patients with cirrhosis.14-16 However, in these studies the proportions of small and large varices differed14, 16 or were not specified.15 Therefore, a comparison with the prevalence of small and large varices detected in our study cannot be performed.
Ascites and splenomegaly were found to be independent predictors of the presence of GOVs at baseline endoscopy. GOVs were present in >85% of patients with ascites and in 74% of those with splenomegaly. However, among patients without ascites or splenomegaly still 42% had GOVs, although only 11% had large GOVs requiring primary prophylaxis. Thus, although the risk of having large varices is low in patients without ascites or splenomegaly, the absence of splenomegaly and/or ascites cannot be safely used as a criterion to rule out the presence of high-risk varices. This finding supports the recent Baveno VI meeting recommendations that it is mandatory to perform screening endoscopy in all patients at diagnosis of chronic PVT.5
Actuarial probability of development of varices in patients without varices at initial endoscopy was 2% at 1 year and 22% at 3 and 5 years, respectively. Our data differ from the study by Amitrano et al.,13 where no development of varices was observed during the follow-up in 20 patients with portal and/or splenic vein thrombosis. We have no clear explanation for this discrepancy; however, the larger number of patients included in our study and the longer follow-up of our patients may explain the difference. Our findings support the current empirical recommendation that patients with PVT without varices need to be submitted to follow-up screening endoscopies. Indeed, the observed incidence of EVs in our study is almost identical to that found in the prospective timolol study17 and only slightly lower than the 5%, 17%, and 28% at 1, 2, and 3 years, respectively, reported in patients with cirrhosis by Merli et al.18 Although ours was not a prospective study, in our cohort of patients the median interval time between baseline and follow-up endoscopies was 17.6 months, lower than the 2-year to 3-year interval recommended to assess variceal development in patients with compensated cirrhosis.5
There are scarce data on the probability of variceal growth in patients with PVT. Amitrano et al. reported variceal growth of SEVs during follow-up in three out of eight patients with PVT.13 In the current study, the actuarial probability of growth of SEVs requiring primary prophylaxis was 13%, 40%, and 54% at 1, 3, and 5 years, respectively. This rate was similar to that reported by Merli et al.18 in which the majority of patients with cirrhosis belonged to Child-Pugh classes A and B (41% at 3 years and 51% at 5 years). The median interval time between baseline endoscopy showing SEVs and the one detecting variceal growth divided by the total number of endoscopies performed during this period of time was 11 months, which is close to the 12-18 months recommended for evaluating the potential for growth of small varices in patients with cirrhosis.5 Interestingly, in three (11%) patients, SEVs disappeared during follow-up. Thus, our results strongly suggest that patients with PVT and SEVs should undergo follow-up endoscopies to identify variceal growth/regression.
In our study, three patients bled during follow-up before identifying LEVs and, therefore, without receiving primary prophylaxis. All of them had an initial endoscopy that showed no or small varices over 2 years before. This finding further supports the need to perform endoscopies at the 1-year to 2-year interval recommended for patients with cirrhosis.6
Once varices become large, the actuarial probability of bleeding despite primary prophylaxis was 9%, 20%, and 32% at 1, 3, and 5 years, respectively, a figure that is comparable to bleeding rates in patients with cirrhosis and large varices on primary prophylaxis19-23 and clearly lower than the observed incidence of bleeding in the four patients of our cohort who did not receive prophylaxis (three of four). Thus, although the number of patients without prophylaxis was very low, our data suggest that the recommendation for primary prophylaxis of variceal bleeding in cirrhosis is valid for patients with chronic PVT. It is important to remark that no robust data on the spontaneous bleeding risk in patients with chronic PVT and large varices exist. Rate of first bleeding was similar in patients receiving only NSBBs or EBL (with or without NSBBs). However, because most patients received NSBBs, our study cannot provide strong information regarding comparison of efficacy between the two methods in primary prophylaxis.
It is important to remark that our study was unable to identify any relationship between the use of anticoagulation and the course of esophageal varices. Thus, there were no significant differences in variceal development, growth, or bleeding in patients receiving or not anticoagulation.
Overall, at least one portal hypertensive bleeding episode occurred in 57 (32%) patients. Although transfusion of blood products was required in 75% patients and rescue shunt surgery due to failure to control bleeding in 6/57 (11%) patients, there were no deaths related to the first bleeding episode, which is in accordance with previous data3, 11, 24, 25 on the extremely low mortality of variceal bleeding in patients with PVT. This low mortality occurred despite 26% of patients bleeding while under anticoagulation treatment, confirming previous observations in an independent population25, 26 that anticoagulation did not have a major impact on the outcome of variceal bleeding in patients with PVT. A similar observation has been reported in patients with cirrhosis.27
In our cohort of patients with previous variceal bleeding, despite the use of secondary prophylaxis, the actuarial probability of rebleeding at 1, 3, and 5 years was 32%, 45%, and 47%, respectively, a rebleeding rate similar to that reported in the literature for variceal rebleeding during secondary prophylaxis in patients with cirrhosis.28-31 Rebleeding rates in our study were lower than those reported in a large study by Spaander et al.25 but within the range reported in a smaller study by the same group where patients were submitted to secondary prophylaxis with EBL32 and in the study by Orr et al.33 By contrast, our rebleeding rates were slightly higher than those reported by Sarin et al., although they included patients with both PVT and noncirrhotic portal hypertension.24 Regardless, the observed rebleeding rate clearly shows that there is still room for improvement.
Although gastrointestinal bleed at diagnosis of PVT,13 anticoagulant therapy,25 extension of thrombosis to the splenic vein, presence of gastric fundal varices,32 and thrombosis of the superior mesenteric vein34 have all been described as predictive factors associated with rebleeding episodes during follow-up, we could not confirm these findings in our study. Finally, in our cohort of patients with PVT the mortality was very low, only occurring in nine patients (5%) with an actuarial probability of survival of 99% and 96% at 1 and 5 years, respectively, confirming the good prognosis of these patients.13, 25, 33, 35 In our study, by univariate Cox regression analysis, age, altered liver enzymes, and ascites were significantly associated with mortality, as observed by Janssen et al.25, 34 and Orr et al.33
In this study, selection bias was minimized by including all patients with noncirrhotic, nontumoral PVT registered and followed at the two centers. The long study period of over 20 years could be associated with a change in the management of patients seen early in the study compared to those diagnosed more recently, with the latter patients being more likely to receive anticoagulation and consequently a greater possibility of portal vein recanalization.36 However, this potential bias was reduced by only including patients with well-developed chronic portal vein thrombosis with portal hypertension. Finally, and despite the presence of an underlying liver disease being plausibly discarded, we cannot completely exclude that in a minority of patients an unrecognized liver disease may have been missed.
In conclusion, the study confirms that GOVs are a frequent finding in patients with chronic PVT. They are especially frequent in patients with ascites, even detected only at imaging studies or with splenomegaly. Varices at high risk of bleeding are infrequent but not rare in the absence of these two factors. Most progression, indicated by development of varices in patients without varices at diagnosis and variceal growth in patients with small varices, takes place early in the course of PVT. The risk appears to be similar to that of patients with cirrhosis and therefore calls for a similar schedule of follow-up endoscopies—in short, every 2-3 years in patients without varices and every 1-2 years in those with small varices. The risks of first variceal bleeding on primary prophylaxis and of rebleeding are also similar to those observed in cirrhosis provided a similar therapeutic approach based on NSBB and endoscopic therapy is used. Anticoagulation does not seem to be associated with higher risk of bleeding and rebleeding. Mortality of bleeding in patients with PVT is very low and mostly related to conditions not directly related to PVT.
Acknowledgment
We thank Kamal Zekrini for assistance in data collection.




