Gab1 adaptor protein acts as a gatekeeper to balance hepatocyte death and proliferation during acetaminophen-induced liver injury in mice
Potential conflict of interest: Nothing to report.
Supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science
Abstract
Acetaminophen (APAP) overdose is the leading cause of drug-induced acute liver failure. In APAP-induced acute liver failure, hepatocyte death and subsequent liver regeneration determines the prognosis of patients, making it necessary to identify suitable therapeutic targets based on detailed molecular mechanisms. Grb2-associated binder 1 (Gab1) adaptor protein plays a crucial role in transmitting signals from growth factor and cytokine receptors to downstream effectors. In this study, we hypothesized that Gab1 is involved in APAP-induced acute liver failure. Hepatocyte-specific Gab1 conditional knockout (Gab1CKO) and control mice were treated with 250 mg/kg of APAP. After APAP treatment, Gab1CKO mice had significantly higher mortality and elevated serum alanine aminotransferase levels compared to control mice. Gab1CKO mice had increased hepatocyte death and increased serum levels of high mobility group box 1, a marker of hepatocyte necrosis. In addition, Gab1CKO mice had reduced hepatocyte proliferation. The enhanced hepatotoxicity in Gab1CKO mice was associated with increased activation of stress-related c-Jun N-terminal kinase (JNK) and reduced activation of extracellular signal-regulated kinase and AKT. Furthermore, Gab1CKO mice showed enhanced mitochondrial translocation of JNK accompanied by an increase in the release of mitochondrial enzymes into the cytosol, which is indicative of increased mitochondrial dysfunction and subsequent nuclear DNA fragmentation. Finally, in vitro experiments showed that Gab1-deficient hepatocytes were more susceptible to APAP-induced mitochondrial dysfunction and cell death, suggesting that hepatocyte Gab1 is a direct target of APAP-induced hepatotoxicity. Conclusion: Our current data demonstrate that hepatocyte Gab1 plays a critical role in controlling the balance between hepatocyte death and compensatory hepatocyte proliferation during APAP-induced liver injury. (Hepatology 2016;63:1340–1355)
Abbreviations
-
- ALF
-
- acute liver failure
-
- APAP
-
- acetaminophen
-
- CKO
-
- conditional knockout
-
- CYP2E1
-
- cytochrome P450 2E1
-
- ERK
-
- extracellular signal-regulated kinase
-
- Gab1
-
- Grb2-associated binder 1
-
- GSH
-
- glutathione
-
- HMGB1
-
- high mobility group box 1
-
- JNK
-
- c-Jun N-terminal kinase
-
- LDH
-
- lactate dehydrogenase
-
- NAPQI
-
- N-acetyl-p-benzoquinone imine
-
- ROS
-
- reactive oxygen species
-
- siRNA
-
- small interfering RNA
Acetaminophen (APAP) overdose is currently the most frequent cause of acute liver failure (ALF) in the United States and many Western countries.1 The hepatotoxicity of APAP is attributed to its conversion to a reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI), by cytochrome P450 enzymes.2, 3 Excessive NAPQI depletes liver glutathione (GSH) and subsequently forms adducts on intracellular proteins, which induces mitochondrial oxidative stress, mitochondrial dysfunction, and nuclear DNA fragmentation, leading to necrotic cell death of hepatocytes.4-6 Hepatocyte necrosis eventually induces liver regeneration.7 Thus, the balance between hepatocyte death and subsequent liver regeneration determines the prognosis of patients with APAP-induced ALF.
Recently, c-Jun N-terminal kinase (JNK) has been implicated in APAP-induced hepatotoxicity. JNK is a stress-related mitogen-activated protein kinase that is activated by reactive oxygen species (ROS), translocates to mitochondria to amplify the initial oxidative stress signal, and is required for hepatocyte death.8-10 Therefore, pharmacological inhibition of JNK is expected to be an effective therapy for APAP-induced ALF.8, 10 However, JNK inhibition may also suppress liver regeneration because JNK-mediated signals are required for hepatocyte proliferation.11 Therefore, new therapeutic targets for APAP-induced ALF need to be identified based on a detailed knowledge of both liver injury and subsequent liver regeneration.
Grb2-associated binder 1 (Gab1) was originally identified as a scaffolding adaptor protein downstream of growth factor and cytokine receptors, such as insulin receptor, epidermal growth factor receptor, c-Met, and gp130.12-16 In response to stimuli, Gab1 is tyrosine-phosphorylated and transmits downstream to mitogenic signaling pathways, including the extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase or phosphoinositide 3-kinase/AKT (also known as protein kinase B) pathways.14-17 We previously showed that Gab1 regulates mouse embryo development through these pathways in vivo.18 More recently, we demonstrated that in hepatocytes Gab1 is essential for protection against liver fibrogenesis in mice.19 Furthermore, others have shown that Gab1 promotes hepatocyte proliferation after partial hepatectomy in mice,20 indicating that Gab1 is crucial for the repair process following liver injury. Gab1 has also been implicated in the response to several stressors, including oxidative stress,21 ultraviolet irradiation,22 and cardiac ischemia/reperfusion injury.23 These findings suggest that hepatocyte Gab1 might be involved in the pathogenesis of APAP-induced liver injury, which is associated with both stress and mitogenic signaling. However, to date, the role of Gab1 in this process remains unclear.
Based on these backgrounds, we hypothesized that Gab1 would be involved in the pathophysiology of APAP-induced ALF. To test this hypothesis, we used a loss-of-function mouse model and investigated the molecular mechanisms responsible for the functional role of Gab1 in APAP-induced hepatotoxicity.
Materials and Methods
Mice
The generation of C57BL/6 mice carrying a Gab1 gene with two loxP sequences flanking exon 2 (Gab1flox/flox) has been described.19 Hepatocyte-specific Gab1 conditional knockout (Gab1CKO) mice were generated by crossing Gab1flox/flox C57BL/6 mice with albumin promoter-driven Cre recombinase (Alb-Cre) transgenic C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME). All mice used for the experiments were 8-10 weeks old and male. All animal experiments were approval by the Animal Care and Use Committee of Osaka University Medical School and performed according to institutional guidelines.
Induction of Liver Injury
APAP (Sigma-Aldrich, St. Louis, MO) was dissolved in phosphate-buffered saline at 55°C and cooled to 37°C before use. All experiments were performed by fasting mice at 7:00 pm and administering APAP intraperitoneally at a dose of 250 mg/kg at 5:00 pm on the following day, except in the experiments shown in Supporting Fig. 1A-C. Some mice were evaluated for mortality, while others were sacrificed between 1.5 hours and 24 hours after APAP treatment for biochemical, histological, and immunological analyses. For the assessment of liver regeneration, control and Gab1CKO mice were also treated with a sublethal dose (150 mg/kg) of APAP and sacrificed at 24 hours and 48 hours after APAP treatment for further analysis. Hepatocytes per view field were counted at ×100 magnification.
Small Interfering RNA-Mediated Depletion of Gab1 in Mouse Hepatocyte Cell Lines and Human Liver-Derived Cell Lines
AML12 cells were obtained from ATCC (Manassas, VA) and maintained as described.24 HepaRG cells were obtained from Biopredic International (Rennes, France), seeded at 1 × 105 cells/cm2 on type I collagen-coated dishes, and cultured in William's E medium containing 1% glutamine (Invitrogen Life Technologies, Carlsbad, CA) and the appropriate medium supplement described in the manufacturer's instructions. To deplete Gab1, AML12 cells were transfected with either 20 nM of murine Gab1 small interfering RNA (siRNA) or control siRNA using Lipofectamine RNAiMAX (Invitrogen Life Technologies). HepaRG cells were also transfected with 20 nM of human Gab1 siRNA or control siRNA after 5 days of culture. At 48 hours after transfection, AML12 cells and HepaRG cells were treated with H2O2 (Nakalai Tesque) or APAP, respectively, and analyzed.
Isolation and Culture of Primary Mouse Hepatocytes
Hepatocytes were isolated from control and Gab1CKO mice using the two-step pronase-collagenase perfusion method and cultured as described.19 Hepatocyte preparations with viability of 90% or higher, as determined by trypan blue exclusion, were used for experiments. Hepatocytes were seeded at 4 × 104 cells/cm2 on type I collagen-coated dish in William's E medium containing 10% fetal bovine serum, 2 mM l-glutamine, 10-7 M insulin, 10-7 M dexamethasone, 100 U/mL penicillin, and 100 U/mL streptomycin.
Assessment of Released Lactate Dehydrogenase Activity
The amount of cell death was assessed by measuring lactate dehydrogenase (LDH) in cell culture medium, using the LDH-Cytotoxic Test (Wako, Kyoto, Japan). Triton X-100, 1% (w/v), was used as a positive control. LDH (percentage released) was determined as a ratio of LDH released by the material to LDH released by the Triton X-100 solution. Primary hepatocytes were treated with 0.2-1.6 mM of H2O2 for 6 hours after 16 hours of culture. Primary hepatocytes were also treated with 0.8 mM of APAP for 12 hours to 36 hours after 8 hours of culture.
Assessment of Mitochondrial Function
To assess the extent of decrease in mitochondrial membrane potential in Gab1 siRNA-transfected HepaRG cells treated with 2.5-5 mM of APAP for 16 hours, the JC-10 Mitochondrial Membrane Potential Assay Kit (Abcam, Cambridge, MA) was used according to the manufacturer's instructions. For the measurement of mitochondrial ROS generation, Gab1 siRNA-transfected HepaRG cells treated with 5 mM of APAP for 8 hours were loaded with MitoSOX Red mitochondrial superoxide indicator (Molecular Probes, Eugene, OR), and live cell imaging was performed using a fluorescence microscope (Keyence, Osaka, Japan).
Statistical Analysis
Statistical analysis was performed using JMP Pro 11.0 software (SAS Institute Inc., Cary, NC). Values for all measurements are presented as mean ± standard error of the mean. Statistical analyses were performed using the Student t test. The log-rank test was also used for the statistical analysis of the survival rate. P values <0.05 were considered statistically significant. See Supporting Information for a more detailed description of materials and methods.
Results
Hepatocyte-Specific Gab1CKO Mice Show Enhanced Liver Injury and Higher Mortality After APAP Treatment
To determine the functional role of Gab1 in APAP-induced hepatotoxicity, we used Gab1flox/flox;Alb-Cre+/- hepatocyte-specific conditional knockout (Gab1CKO) and Gab1flox/flox;Alb-Cre-/- (control) mice, both of which were intraperitoneally injected with 250 mg/kg of APAP. Gab1 was tyrosine-phosphorylated in the livers of control mice but not of Gab1CKO mice (Fig. 1A). We next examined the survival rate and the extent of liver injury in Gab1CKO and control mice after APAP treatment. Gab1CKO mice had a significantly lower survival rate than control mice after APAP treatment (25% in Gab1CKO versus 75% in control; Fig. 1B). Gab1CKO mice also had significantly increased alanine aminotransferase levels compared to control mice (Fig. 1C).
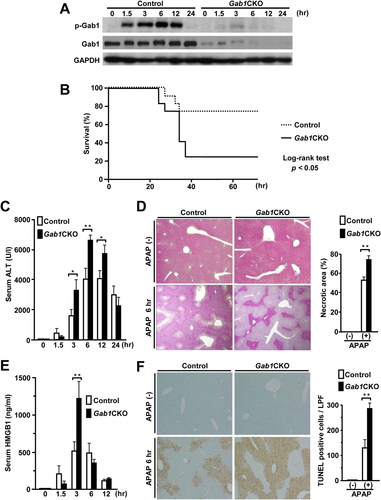
Histological examination of livers revealed that Gab1CKO mice had a larger area of centrilobular necrosis compared to control mice after APAP treatment (Fig. 1D). Gab1CKO mice exhibited a more than two-fold increase in the level of serum high mobility group box 1 (HMGB1), a well-characterized marker of necrotic cell death,25 at 3 hours after APAP treatment (Fig. 1E). In addition, the extent of hepatocyte DNA fragmentation, as assessed by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling staining, was significantly increased in liver sections from Gab1CKO mice at 6 hours after APAP treatment (Fig. 1F). Higher susceptibility to APAP of Gab1CKO mice was confirmed by another experimental protocol (Supporting Fig. S1A-C). Furthermore, the enhanced liver injury in Gab1CKO mice was also accompanied by an exacerbation of inflammation, as evidenced by increased hepatic expression and serum levels of interleukin-6 or interleukin-1β (Supporting Fig. S2A,B). Collectively, these findings indicate that the loss of hepatocyte Gab1 results in enhanced liver injury with widespread hepatocyte necrosis and higher mortality after APAP treatment.
Hepatocyte Gab1 Is Dispensable for the Metabolism of APAP
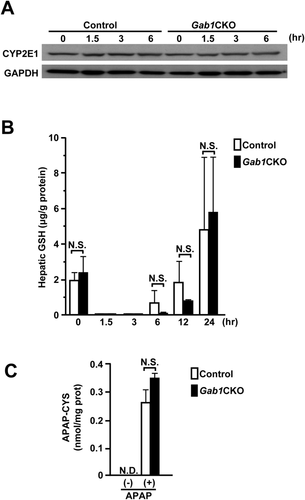
To exclude the possibility that the enhanced liver injury in Gab1CKO mice was due to altered APAP metabolism, we first examined the expression of cytochrome P450 2E1 (CYP2E1), the principal enzyme responsible for the conversion of APAP to its toxic metabolite NAPQI.2, 3, 26 The expression of CYP2E1 was nearly equivalent between the two groups (Fig. 2A). Hepatic GSH depletion is a hallmark of excessive NAPQI generation during APAP-induced liver injury.26, 27 We found that there was no significant difference in the extent of hepatic GSH depletion at early time points (1.5 hours and 3 hours) as well as at baseline between the two groups (Fig. 2B). At later time points (6 hours and 12 hours), Gab1CKO mice tended to show slower GSH recovery compared with control mice (Fig. 2B). The reactive metabolite NAPQI is known to bind to cysteine groups of proteins, resulting in the formation of APAP protein adducts.28, 29 Consistently, we showed that there was no significant difference in the content of intrahepatic APAP-protein adducts (APAP-cysteine adducts) between the two groups (Fig. 2C). Together, these results indicate that hepatocyte Gab1 is not associated with drug metabolism in APAP-induced hepatotoxicity.
Hepatocyte Proliferation Is Suppressed in Hepatocyte-Specific Gab1CKO Mice After APAP Treatment
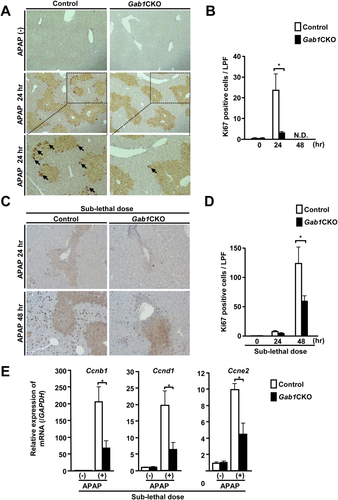
Liver regeneration is also known to be important for survival after APAP overdose.7, 30 In mice, the peak of liver regeneration occurs later than 48 hours after APAP treatment.7, 31, 32 In this study, approximately 80% of Gab1CKO mice died between 24 hours and 36 hours after APAP treatment; therefore, we evaluated the proliferation of hepatocytes in Gab1CKO mice at 24 hours after APAP treatment. Ki67 staining showed that hepatocyte proliferation was significantly reduced in the livers of Gab1CKO mice compared to control mice (Fig. 3A,B). Histological examination at 12 hours and 24 hours after APAP treatment revealed a larger area of centrilobular necrosis compared to control mice (Supporting Fig. S3). To confirm the mitogenic function of Gab1, we also used a sublethal dose of APAP (150 mg/kg) to evaluate its effect in live mice at a later time point. At 48 hours after treatment with a sublethal dose of APAP, Gab1CKO mice showed significantly reduced hepatocyte proliferation (Fig. 3C,D) and hepatic expression of cell cycle-related genes, such as Ccnb1, Ccnd1, and Ccne2 (Fig. 3E). Consistently, Gab1CKO mice showed a larger area of centrilobular necrosis compared with control mice at 12 hours, 24 hours, 48 hours, and 72 hours after treatment with a sublethal dose of APAP, whereas control mice showed complete resolution of necrosis at 72 hours after APAP treatment (Supporting Fig. S4). Together, these data support the mitogenic function of Gab1 in liver regeneration and wound-healing processes after APAP treatment.
Hepatocyte-Specific Gab1CKO Mice Show Altered Mitogenic or Stress Signaling Pathways in the Liver After APAP Treatment
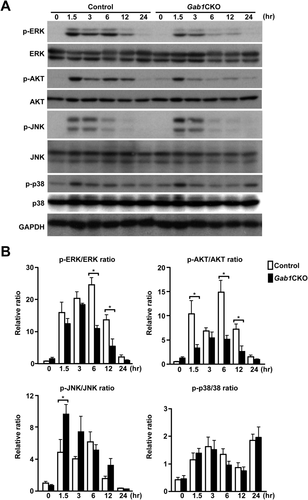
To investigate the molecular mechanism underlying the enhanced hepatotoxicity in Gab1CKO mice, we examined mitogenic and stress signaling pathways in the liver after APAP treatment. Gab1CKO livers exhibited a reduced activation of mitogenic signals, including ERK and AKT, known targets of Gab1,14, 15, 33 throughout the observation period (Fig. 4A,B). Regarding the stress signals evaluated, the phosphorylation of p38 was almost equivalent between the two groups. In contrast, Gab1CKO mice demonstrated an increase in the activation of JNK, a critical regulator of APAP-induced liver injury. These data implicate stress-related JNK and mitogenic ERK and AKT signaling as potential mechanisms underlying the enhanced liver injury observed in Gab1CKO mice.
Hepatocyte-Specific Gab1CKO Mice Show Enhanced Mitochondrial Dysfunction During APAP-Induced Hepatotoxicity
It is well established that in the process of APAP-induced liver injury, oxidative stress induces JNK activation and mitochondrial translocation, leading to mitochondrial dysfunction, nuclear DNA fragmentation, and subsequent hepatocyte necrosis.9, 10 Therefore, we investigated whether mitochondrial translocation of JNK was altered in the livers of Gab1CKO mice. Gab1CKO mice had significantly more mitochondria-localized, phosphorylated JNK compared to control mice (Fig. 5A,B). In contrast, mitochondrial translocation of Bax, another regulator of APAP-induced liver injury,34 was almost equivalent between the two groups. Gab1CKO mice also released significantly more mitochondrion-specific enzymes, such as apoptosis-inducing factor and endonuclease G, into the cytosol, which could be a trigger of nuclear DNA fragmentation (Fig. 5C,D).35, 36 Furthermore, Gab1CKO mice displayed a statistically significant increase in biomarkers of mitochondrial toxicity, such as glutamate dehydrogenase and mitochondrial DNA (Fig. 5E,F).6 Together, these data suggest that enhanced hepatocyte necrosis in Gab1CKO mice is accompanied by JNK-mediated mitochondrial dysfunction.
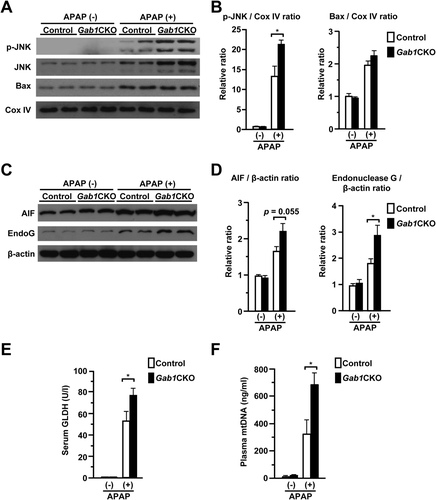
Loss of Hepatocyte Gab1 Exacerbates APAP-Induced Mitochondrial Damage and Promotes Cell Death In Vitro
To confirm the protective role of Gab1 in APAP-induced hepatotoxicity, primary hepatocytes from Gab1CKO and control mice were isolated and treated with APAP in vitro. Gab1-deficient hepatocytes exhibited increased cytotoxicity following APAP treatment (Fig. 6A,B). Gab1-deficient hepatocytes also showed an increase in the amount of LDH released into the cell culture medium compared to control hepatocytes (Fig. 6C). The protective role of Gab1 in APAP-induced cytotoxicity was further confirmed using human liver-derived cells, HepaRG cells, which retain adequate drug-metabolizing capacity (Supporting Fig. S5A,B).37, 38 These data indicate that Gab1 is crucial for APAP-induced cytotoxicity in both human and mouse hepatocytes.
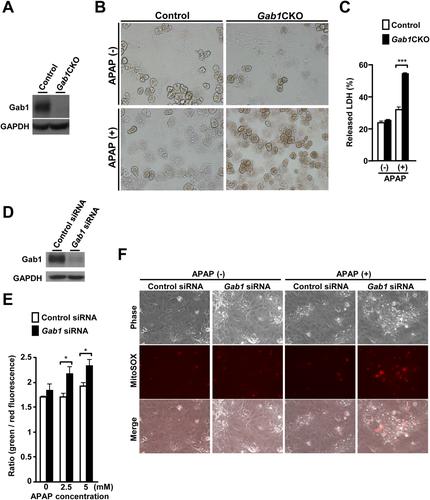
To examine the effect of hepatocyte Gab1 on mitochondrial function in vitro, we first evaluated loss of mitochondrial membrane potential in HepaRG cells transfected with Gab1-specific siRNA or control siRNA, using the fluorescent probe JC-10. Following APAP treatment, Gab1-depleted HepaRG cells exhibited a significant increase in the ratio of green to red fluorescence intensity compared to control cells (Fig. 6D,E), which is indicative of a greater extent of mitochondrial membrane depolarization. Furthermore, Gab1-depleted HepaRG cells also showed a marked increase in mitochondrial ROS accumulation compared to control cells as assessed by MitoSOX Red fluorescent probe following APAP treatment (Fig. 6F). These results indicate that the enhanced cytotoxicity in Gab1-depleted liver-derived cells was associated with exacerbated mitochondrial oxidative stress and damage.
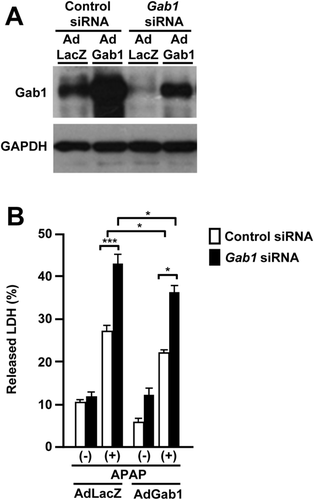
Overexpression of Gab1 Restores APAP-Induced Cytotoxicity in Gab1-Depleted Liver-Derived Cells
To confirm the contribution of Gab1 in APAP-induced liver injury, we also examined the effect of Gab1 protein overexpression on APAP-induced liver injury using an adenovirus-mediated overexpression system. The adenovirus expressing Gab1 (AdGab1) effectively rescued its protein expression in Gab1 siRNA-transfected HepaRG cells, whereas the adenovirus expressing β-galactosidase (AdLacZ) did not (Fig. 7A). The administration of AdGab1 resulted in a statistically significant reduction of the LDH released into the supernatant of Gab1 siRNA-transfected HepaRG cells treated with APAP compared with that of AdLacZ (Fig. 7B), supporting the protective role of Gab1 in APAP-induced hepatotoxicity.
Loss of Hepatocyte Gab1 Enhances ROS-Induced JNK Activation During Cell Death
Because the generation of ROS and the resulting oxidative stress has been suggested to be the primary cause of APAP-induced liver damage,5 we tested the involvement of Gab1 in ROS-induced cytotoxicity using Gab1CKO and control hepatocytes. Gab1CKO hepatocytes showed increased cell death induced by hydrogen peroxide (H2O2), which is a ROS (Fig. 8A). Gab1CKO hepatocytes also had an increase in LDH released into the culture medium compared to control hepatocytes (Fig. 8B). This increase in ROS-mediated cytotoxicity was confirmed using the murine hepatocyte AML12 cell line (Supporting Fig. S5C,D), further supporting the protective role of Gab1 in ROS-induced as well as APAP-induced hepatotoxicity.
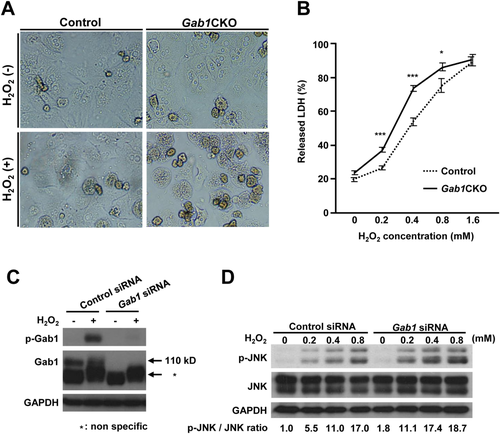
Finally, we examined the role of Gab1 in ROS-induced JNK activation in vitro, using AML12 cells transfected with Gab1-specific siRNA or control siRNA. Following stimulation with H2O2, Gab1 was tyrosine-phosphorylated (Fig. 8C). Furthermore, Gab1-depleted AML12 cells exhibited enhanced phosphorylation of JNK after H2O2 treatment (Fig. 8D). These results indicate that the increased cytotoxicity in Gab1-deficient hepatocytes was accompanied by the enhancement of ROS-induced JNK activation.
Discussion
Identification of the molecular mechanisms orchestrating hepatocyte death and proliferation will provide promising therapeutic avenues for the treatment of APAP overdose.7, 26 In this study, we demonstrated, for the first time, that hepatocyte Gab1 is crucial for protection against APAP-induced hepatotoxicity. After APAP treatment, Gab1CKO mice showed enhanced hepatocyte death and higher mortality. Furthermore, Gab1CKO mice displayed reduced hepatocyte proliferation. During these processes, Gab1CKO mice had increased activation of stress-related JNK and reduced activation of mitogenic ERK and AKT signaling in their livers, suggesting that Gab1 is a dual regulator of both mitogenic and stress signaling in APAP-induced hepatotoxicity. Our current data clearly show that Gab1 is crucial for stress-induced hepatocyte death and subsequent liver regeneration during APAP-induced liver injury.
Gab1 serves as a scaffolding adaptor protein that transmits receptor-mediated intracellular signaling cascades downstream of growth factors or cytokines.14-16 Previous studies have demonstrated an essential role for Gab1 in transmitting hepatocyte growth factor/c-Met39 and epidermal growth factor family/epidermal growth factor receptor signals33 in vivo. In fact, we showed that complete knockout of murine Gab1 results in embryonic lethality with developmental abnormalities resulting from global defects in growth factor and cytokine signaling.18 Gab1 is also tyrosine-phosphorylated in response to H2O221 or ultraviolet irradiation22 in mouse embryonic fibroblasts in vitro and has been implicated in cardiac ischemia/reperfusion injury.23 In this study, we showed that Gab1 is tyrosine-phosphorylated after APAP treatment, in which oxidative stress is involved at the initial injury step.26 These data indicate that Gab1 is involved in stress signaling during liver injury, particularly in APAP-induced hepatotoxicity.
In APAP hepatotoxicity, it is widely accepted that the metabolism of APAP to the reactive metabolite NAPQI by CYP2E1 represents the initial step of liver injury and that the initial GSH depletion reflects the amount of excessive NAPQI generation in the liver.27 In this study, loss of hepatocyte Gab1 did not affect the initial depletion of GSH levels, nor did it affect hepatic expression of CYP2E1 protein during APAP-induced liver injury, suggesting that hepatocyte Gab1 would be dispensable for APAP metabolism. In contrast, Gab1CKO mice also showed slower recovery of hepatic GSH levels at later time points during APAP-induced liver injury. One possible explanation for this divergence is that slower recovery of hepatic GSH levels might be the result of enhanced liver injury in Gab1CKO mice. Further study will be needed to elucidate this issue.
Previous reports have shown that Gab1 is involved in caspase-dependent apoptosis in several cell types.21, 23, 40 However, the predominant mode of cell death in APAP-induced hepatotoxicity is believed to be oncotic necrosis rather than apoptosis.6, 41 Consistent with this notion, we found that Gab1CKO mice had enhanced hepatocyte death with a significant increase in serum HMGB1, a well-documented biomarker of APAP-induced hepatocyte necrosis in both mice and humans.25, 42 In addition, the livers of Gab1CKO mice showed no activation of apoptotic caspase 3 or 7 at any time after APAP treatment (Supporting Fig. S6). Thus, Gab1 regulates caspase-independent hepatocyte necrosis during APAP-induced liver injury.
Mitochondrial dysfunction is a critical event in the pathophysiology of APAP-induced hepatotoxicity in both mice and humans.6, 43 The activation and mitochondrial translocation of JNK is a key mediator of this process and causes impaired mitochondrial respiration and the generation of ROS.9, 10, 44 Mitochondrial generation of ROS in turn induces activation and translocation of JNK to mitochondria, forming a vicious ROS-JNK feed-forward loop.44 This loop eventually results in the amplification of oxidative stress, perpetuating further mitochondrial dysfunction and necrotic cell death.9, 10, 44 In agreement with these findings, Gab1CKO mice had an enhanced activation and increased mitochondrial translocation of JNK in their livers after APAP treatment, indicating that the deletion of Gab1 causes enhanced mitochondrial dysfunction, leading to hepatocyte necrosis. In vitro experiments using Gab1-deficient or Gab1-depleted hepatocytes confirmed that Gab1 protects from APAP-induced mitochondrial dysfunction and hepatocyte death. At this stage, the detailed mechanism by which Gab1 enhances mitochondrial translocation of JNK in APAP-induced hepatotoxicity remains unclear. The mitochondrial fraction did not express Gab1 protein, whereas the cytosolic fraction expressed Gab1 protein regardless of APAP treatment, suggesting that Gab1 might regulate JNK activation and its mitochondrial translocation indirectly during APAP-induced liver injury (Supporting Fig. S7). Glycogen synthase kinase 3β promotes the activation of JNK and its mitochondrial translocation at early time points (1 hour and 2 hours) after APAP treatment in mice,45 whereas apoptosis signal-regulating kinase 1 is required for the prolonged activation of JNK at later time points (3 hours and 6 hours) after APAP treatment in mice.46 One possible mechanism by which hepatocyte Gab1 could affect JNK activation and its mitochondrial translocation is through regulating upstream modulators of JNK. Further studies will be needed to elucidate the precise mechanisms in this process. Taken together, these data indicate that hepatocyte Gab1 is a critical regulator of the initial liver injury phase of APAP-induced ALF.
It is believed that cell death can stimulate the compensatory proliferation of surrounding normal cells to promote the wound-healing response in mammals and other species.32, 47, 48 During liver injury, as the extent of hepatocyte death increases, compensatory liver regeneration is more likely to be promoted.32 Interestingly, in spite of enhanced hepatocyte death, compensatory hepatocyte proliferation was significantly reduced in Gab1CKO mice during the regeneration phase. Because most Gab1CKO mice died before the peak of liver regeneration, the impaired hepatocyte proliferation might have a minor effect on their higher mortality. Nevertheless, our data are consistent with previous reports showing that hepatocyte Gab1 stimulates hepatocyte proliferation after bile duct ligation19 or partial hepatectomy.20 Reduced ERK and AKT activation in the livers of Gab1CKO mice might contribute to the impaired hepatocyte proliferation after APAP treatment. In addition, Gab1CKO mice showed increased HMGB1 levels compared with control mice after APAP treatment. Previous publications have shown that administration of anti-HMGB1 antibody49 or genetic ablation of hepatic HMGB150 promotes liver regeneration after APAP treatment in mice, suggesting that HMGB1 itself impairs liver regeneration and worsens liver injury induced by APAP. Therefore, the increased HMGB1 levels in Gab1CKO mice after APAP treatment might contribute to the impaired liver regeneration in these mice. Others have shown that pharmacological inhibition of JNK protects against APAP-induced hepatotoxicity in mice.8 However, JNK inhibitors might impair hepatocyte proliferation in the regenerating liver.11 Our current data indicate that Gab1 can regulate both hepatocyte death and liver regeneration during APAP-induced liver injury, suggesting that strategies targeting hepatocyte Gab1 could overcome the shortcomings associated with JNK inhibitors.
In conclusion, we have demonstrated that hepatocyte Gab1 plays a pivotal role in the protection against APAP-induced hepatotoxicity by inhibiting hepatocyte necrosis in the initial liver injury phase. Because Gab1 also transmits mitogenic signals in the liver regeneration phase, Gab1 could serve as a gatekeeper to control the balance between hepatocyte death and subsequent liver regeneration in APAP-induced hepatotoxicity. Thus, our results suggest that hepatocyte Gab1 would be a potential therapeutic target for APAP-induced liver injury.
References
Author names in bold designate shared co-first authorship.




