The profile of platelet α-granule released molecules affects postoperative liver regeneration
Potential conflict of interest: Nothing to report.
This work was supported by the Austrian Society of Surgical Oncology (ACO-ASSO) with the “Georg-Stumpf Grant 2012” and the Association of Research on the Biology of Liver Tumors (to P.S.) and Austrian Science Fund FWF-P24978 (to A.A.).
Abstract
Platelets promote liver regeneration through site-specific serotonin release from dense granules, triggering proliferative signaling in hepatocytes. However, the effects of factors derived from platelet α-granules on liver regeneration are unclear, because α-granules contain bioactive molecules with opposing functions. Because α-granule molecules are stored in separate compartments, it has been suggested that platelets selectively release their α-granule content dependent on the environmental stimulus. Therefore, we investigated the pattern of circulating α-granule molecules during liver regeneration in 157 patients undergoing partial hepatectomy. We measured plasma levels of α-granule-derived factors in the liver vein at the end of liver resection, as well as on the first postoperative day. We observed a rapid accumulation of platelets within the liver after induction of liver regeneration. Platelet count and P-selectin (a ubiquitous cargo of α-granules) were not associated with postoperative liver dysfunction. However, low plasma levels of vascular endothelial growth factor (VEGF), but high levels of thrombospondin 1 (TSP-1), predicted liver dysfunction after resection. Patients with an unfavorable postoperative α-granule release profile (high TSP-1/low VEGF) showed substantially worse postoperative clinical outcomes. The unfavorable postoperative α-granule release profile was associated with increased postoperative portal venous pressure and von Willebrand factor antigen levels as a marker for intrahepatic endothelial dysfunction. Conclusion: The postoperative profile of circulating platelet-derived factors correlates with the ability of the remnant liver to regenerate. Portal venous pressure and intrahepatic endothelial dysfunction might account for the selective granule release profile. Selective modulation of platelet α-granule release in patients may represent an attractive target for therapeutic interventions to improve liver regeneration and clinical outcomes after partial hepatectomy. (Hepatology 2016;63:1675-1688)
Abbreviations
-
- ADP
-
- adenosine diphosphate
-
- AUC
-
- area under the curve
-
- CCC
-
- cholangiocarcinoma
-
- CD62P
-
- P-selectin
-
- HCC
-
- hepatocellular carcinoma
-
- HPF
-
- high power field
-
- HVPG
-
- hepatic venous pressure gradient
-
- ISGLS
-
- International Study Group of Liver Surgery
-
- ICG
-
- indocyanine green
-
- LD
-
- liver dysfunction
-
- LR
-
- liver regeneration
-
- LSECs
-
- liver sinusoidal endothelial cells
-
- PDR
-
- plasma disappearance rate
-
- PF-4
-
- platelet factor 4
-
- POD
-
- postoperative day
-
- PT
-
- prothrombin time
-
- PVE
-
- portal venous embolization
-
- PVP
-
- portal venous pressure
-
- ROC
-
- receiver operating characteristic
-
- R15
-
- retention rate at 15 minutes
-
- SB
-
- serum bilirubin
-
- TSP-1
-
- thrombospondin 1
-
- TGF-β
-
- transforming growth factor beta
-
- VEGF
-
- vascular endothelial growth factor
-
- vWF-Ag
-
- von Willebrand factor antigen
Platelets are critically involved not only in hemostasis, but also in inflammation, wound healing and angiogenesis. Through release of their granule content, they orchestrate signal responses in a plethora of target cells, including hepatocytes. Platelets are potent inducers of liver regeneration (LR) after partial hepatectomy and platelet activation as well as granule release increase after liver resection.1, 2 Platelet activation results in the release of dense granules, followed by secretion of α-granule content.3 Exocytosis of α-granules represents a highly regulated process, involving distinct packaging and agonist-dependent α-granule subset release, which allows platelets to exert specific functions (e.g., pro- and antiangiogenic effects) in response to different stimuli.4
Thrombocytopenia negatively correlates with LR, and accumulating evidence suggests that dense granule-derived serotonin represents a major inducer of LR.2, 5-7 Platelet α-granules also contain several proteins involved in LR, but in contrast to dense granules, these bioactive molecules have multiple and opposing functions. Whereas vascular endothelial growth factor (VEGF) promotes LR,7-9 thrombospondin 1 (TSP-1) suppresses hepatocyte growth through a transforming growth factor beta (TGF-β)-dependent pathway.10 Accordingly, a reduction of platelet count would affect both pro- and antiregenerative bioactive molecules.
In this study we assessed released components of platelet α-granules in patients undergoing liver resection. We were able to show that the profile of released α-granule content affects postoperative LR. Furthermore, we provide evidence that increased postoperative portal venous pressure (PVP) is associated with an unfavorable α-granule release profile.
Materials and Methods
Study Population
In this prospective study 157 patients undergoing hepatic resection were analyzed (see Table 1). Blood was collected preoperatively, and on the first and fifth postoperative day (POD).
| Variables | Total (N = 157) | LD (N = 18) | No LD (N = 135) | P Value |
|---|---|---|---|---|
| N (%), Median (Range) | N (%), Median (Range) | N (%), Median (Range) | ||
| Sex | 0.879 | |||
| male | 98 (62.4) | 11 (61.1) | 85 (63.0) | |
| female | 59 (37.6) | 7 (38.9) | 50 (37.0) | |
| Age, years | 64 (22-86) | 64 (37-81) | 64 (22-86) | 0.522 |
| Neoplastic entity | ||||
| mCRC | 113 (72.0) | 9 (50) | 102 (75.6) | 0.022a |
| HCC | 21 (13.4) | 6 (33.3) | 15 (11.1) | 0.010a |
| CCC | 14 (8.9) | 2 (11.1) | 10 (7.4) | 0.583 |
| Non-neoplastic | 7 (4.5) | 0 (0) | 7 (5.2) | 0.323 |
| Others | 2 (1.3) | 1 (5.6) | 1 (0.7) | 0.091 |
| Preoperative CTx | ||||
| Yes | 71 (45.2) | 6 (33.3) | 63 (46.7) | 0.286 |
| Cirrhosis | ||||
| Yes | 21 (14.6) | 6 (33.3) | 15 (12.1) | 0.022a |
| Resection | 0.043a | |||
| Major | 72 (45.9) | 12 (66.7) | 56 (41.5) | |
| Minor | 85 (54.1) | 6 (33.3) | 79 (58.5) | |
| PVE | 0.669 | |||
| Yes | 6 (3.9) | 1 (5.9) | 5 (3.7) | |
| Preoperative paramters | ||||
| SB mg/dL | 0.62 (0.19-6.64) | 0.57 (0.27-6.64) | 0.62 (0.19-3.17) | 0.462 |
| PT % | 105 (40-150) | 105 (62-150) | 105 (40-150) | 0.586 |
| ALP U/L | 91 (38-396) | 100 (51-396) | 91 (38-375) | 0.372 |
| GGT U/L | 49 (11-1,041) | 53 (21-492) | 46 (11-1,041) | 0.278 |
| AST U/L | 29.50 (8-404) | 30 (14-208) | 30 (8-404) | 0.692 |
| ALT U/L | 27 (7-346) | 31 (13-120) | 26 (7-346) | 0.300 |
| Albumin g/L | 42.05 (30.8-49.6) | 39.10 (31.5-47.3) | 42.40 (30.8-49.6) | 0.033a |
| HVPG mm Hga | 4 (2-9) | 5 (4-8) | 3.5 (2-9) | 0.328 |
| Platelets (×10^3/µL) | 217 (62-503) | 249 (119-337) | 210 (62-503) | 0.169 |
- a N = 17; if metric parameters are indicated, median with (range) is illustrated. A total of 153 patients were assessable for postoperative LD.
- Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTx, chemotherapy; GGT, gamma-glutamyltransferase; mCRC, metastatic colorectal cancer; n.a., not assessable.
We further assessed platelet activation and degranulation during “early LR” in a subset of 26 patients undergoing hemihepatectomy. Because this procedure triggers the nonligated lobe of the remaining liver to regenerate already during surgery, we collected blood from the portal and liver vein after parenchymal dissection (n = 26) to determine changes of platelet activation and platelet-stored granule proteins. Furthermore, liver tissue samples were retrieved at the beginning of surgery and after parenchymal transection (n = 10) to evaluate platelet accumulation within the liver. Specific characteristics of patients were prospectively recorded (Table 1). Extent of resection was classified according to the International Hepato-Pancreato-Biliary Association Brisbane 2000 nomenclature (<3 segments = minor; ≥3 segments = major). The study was approved by the Institutional ethics committee (#424/2010;#2032/2013) and registered at the clinical trials registry (ClinicalTrials.gov Identifier: NCT01700231; NCT02113059). All patients gave written informed consent.
Definition and Classification of Postoperative Liver Dysfunction and Morbidity
We used the criteria published by the International Study Group of Liver Surgery (ISGLS) to evaluate liver dysfunction (LD; i.e., posthepatectomy liver failure).11 Of note, patients who reached normal serum bilirubin (SB) or prothrombin time (PT) values before POD5 (and hence no further blood collection was performed on or after POD5) were considered as “no LD.”
To evaluate postoperative morbidity, the classification described by Dindo et al.12 was applied and the severity of postoperative complications recorded in grade I to V. In case of multiple complications per patient, the most serious one was considered for statistical analysis.
Preparation of Platelet-Free and -Rich Plasma and Serum
Because the investigated growth factors are stored in platelet α-granules, an optimized plasma preparation technique was applied as previously described.14 Briefly, blood was drawn into prechilled tubes containing citrate, theophylline, adenosine, and dipyridamole and immediately placed on ice and further processed within 30 minutes. After an initial centrifugation step at 1,000g and 4°C for 10 minutes, the plasma supernatant was subjected to further centrifugation at 10,000g and 4°C for 10 minutes (to remove remaining platelets).
To obtain platelet-rich plasma, citrated blood (1:10 volume of 3.2% [w/v] trisodium citrate) was centrifuged at room temperature for 20 minutes at 125g without brakes.
Serum samples were retrieved by blood collection without the addition of anticoagulants and by centrifugation 30 minutes after collection (at 1,000g and room temperature for 10 minutes). All supernatants were stored at −80°C.
Flow Cytometric Analysis of Platelets
In a subset of 26 patients, basal and agonist (adenosine diphosphate [ADP] 50 µM)-induced platelet α-granule release before and on the day after liver resection were determined by quantification of surface P-selectin (CD62P) in platelet-rich plasma using a directly conjugated anti-CD62P-PE antibody (BioLegend, San Diego, CA), as previously described.16 and analyzed by flow cytometry using a BD Accuri flow cytometer with BD Accuri C6 analysis software (Becton Dickinson, Franklin Lakes, NJ).
Quantification of Soluble VEGF, TSP-1, Platelet Factor 4, and Soluble CD62P
Plasma and serum samples were analyzed for human VEGF, TSP-1, platelet factor 4 (PF-4), and CD62P by enzyme-linked immunosorbent assay (all Quantikine; R&D Systems, Minneapolis, MN), according to manufacturer's instructions.
Histopathological Investigation
Liver tissue samples were retrieved at the beginning of surgery and after parenchymal transection (n = 10) to evaluate platelet accumulation within the liver during the initial period of LR. Characteristics of these patients are listed in Supporting Table 2. Liver tissue samples were stained for CD61 to evaluate platelet accumulation within the liver after induction of LR using automated staining protocols. In brief, before antibody exposure, tissue slides were pretreated using heat-induced epitope retrieval for 20 minutes with BOND Epitope Retrieval Solution 2. Subsequently, tissue sections were stained automatically by LEICA BOND III Immunhistostainer using a monoclonal mouse anti-human CD61 antibody (Novocastra, NCL-CD61-308; Leica Biosystems, Buffalo Grove, IL) at a 1:1,600 dilution in BOND Primary Antibody Diluent. For antibody detection, a BOND Polymer Refine Red Detection Kit (DS9390) was used. CD61-positive cells were manually counted per high power field (HPF) in the region of most intense platelet accumulation by three independent and blinded investigators. Additionally, CD61-positive cells were manually counted in 10 randomly chosen HPFs within the liver tissue specimen.
Indocyanine Green: Clearance Test
Perioperative liver function was evaluated by ICG clearance testing. Briefly, pulse spectrometry was used to quantify patients’ blood ICG concentration (0.25 mg/kg body weight). Plasma disappearance rate (PDR) and retention at 15 minutes (R15) were recorded with a Limon Technology device (PULSION Medical Systems AG, Feldkirchen, Germany) and automatically calculated in accord with the time course of the blood ICG concentration.
Statistical Analysis
Statistical analyses were carried out with SPSS software (version 20; SPSS, Inc., Chicago, IL) and were based on nonparametric tests (Mann-Whitney's U test and Wilcoxon's test). Logistic regression analysis was used to evaluate the predictive potential of basic patient characteristics and growth factors and our subsequently defined high-risk group. A receiver operating characteristic (ROC) analysis was performed to assess the potential of circulating VEGF and TSP-1 plasma levels to define suitable cut-off values in order to identify patients with postoperative LD with a specificity of >80%. Box plot illustrations are given without outliers and extreme values to improve the resolution of interquartile ranges. P values <0.05 were considered statistically significant.
Results
Patients’ Demographics
In this study, 157 patients scheduled for major (n = 72) and minor (n = 85) liver resection were prospectively included. Basic characteristics, including sex, tumor type, preoperative chemotherapy, extent of resection, portal venous embolization (PVE), age, and preoperative blood as well as liver function parameters, are illustrated for the entire cohort, as well as for patients with and without postoperative LD, in Table 1. When we compared patients suffering from postoperative LD with the subset without complications (153 patients were assessable for this analysis), we observed several differences that were consistent with previously published observations (Table 1). LD occurred significantly more frequently in patients with hepatocellular carcinoma (HCC) and less frequently in patients with colorectal cancer liver metastases (presumably a result of less frequent underlying liver disease compared to HCC patients). Moreover, patients with LD suffered from cirrhosis more frequently and had slightly decreased preoperative albumin levels. Whereas LD was more frequent in patients undergoing major resection, chemotherapy or radiation did not affect postoperative LD (Table 1; for details on chemotherapy regimens, see Supporting Table 1). No patients had clinically significant portal hypertension (hepatic venous pressure gradient [HVPG] >10 mm Hg). For more detailed analyses, 26 patients undergoing hemihepatectomy consented to additional blood sampling from the portal and hepatic vein, and among those, samples from 10 patients were used to assess intrahepatic platelets in liver resection samples. None of the patients received platelet transfusions, and no intraoperative cell save device was used.
Circulating α-Granule Contents Increase After Liver Resection
Initially, we aimed to evaluate whether circulating α-granule proteins increase after liver resection. Accordingly, we examined circulating VEGF and TSP-1 levels, which are both platelet α-granule derived,14, 17, 18 before and after surgery. Platelet count decreased after partial hepatectomy and tended to recover until POD5 (platelets median: pre-OP = 217 g/L, POD1 = 165 g/L [P < 0.001 vs. pre], POD5 = 182 g/L [P <0.001 vs. pre, P < 0.001 vs. POD1]; Fig. 1A). Plasma levels of TSP-1 and VEGF were found to be significantly increased after liver resection. VEGF and TSP-1 showed different kinetics: The postsurgically elevated VEGF levels significantly decreased until POD5, whereas TSP-1 remained elevated until POD5 (TSP-1 median: pre-OP = 32.8 ng/mL, POD1 = 50.23 ng/mL, POD5 = 49.0 ng/mL; pre-OP vs. POD1: P < 0.001; POD1 vs. POD5: P = 0.174; pre-OP vs. POD5: P = 0.016; VEGF median: pre-OP = 22.3 pg/mL, POD1 = 28.4 pg/mL, POD5 = 16.4 pg/mL; pre-OP vs. POD1: P = 0.038; POD1 vs. POD5: P < 0.001; pre-OP vs. POD5: P = 0.409; Fig. 1B, C).
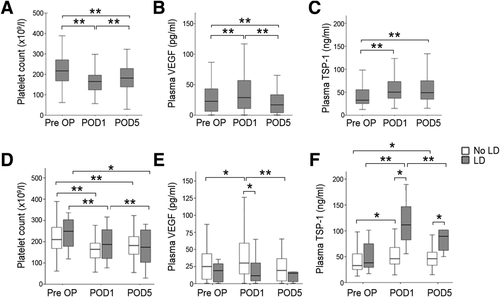
Perioperative time course of platelets and platelet-stored growth factors and their relation to postoperative LD. Platelet counts (A), circulating plasma TSP-1 (B), and VEGF (C) before (Pre OP), 1 day (POD1), as well as 5 days (POD5) after liver resection are shown and are further stratified in patients with and without postoperative LD (D-F). *P < 0.05; **P < 0.005.
TSP-1 and VEGF exhibit opposing functions in LR. Therefore, we addressed the perioperative time course of platelets, TSP-1, and VEGF in patients developing LD after partial hepatectomy. Whereas we found no significant difference in the perioperative platelet counts between patients with and without postoperative LD (platelet median: pre-OP: LD = 249 g/L, no LD = 210 g/L, P = 0.169; POD1: LD = 187 g/L, no LD = 164 g/L, P = 0.195; POD5: LD = 174 g/L, no LD = 182 g/L, P = 0.805; Fig. 1D), VEGF and TSP-1 showed an opposing time course in patients suffering from impaired postoperative liver function recovery: Circulating VEGF only increased in patients without postoperative LD (TSP-1 median: pre-OP: LD = 38.1 ng/mL, no LD = 32.8 ng/mL, P = 0.222; POD1: LD = 111.5 ng/mL, no LD = 46.0 ng/mL, P < 0.001; POD5: LD = 89.4 ng/mL, no LD = 46.0 ng/mL, P = 0.001; Fig. 1E). In contrast, patients with postoperative LD had higher postsurgical levels of TSP-1 than patients without postoperative LD (VEGF median: pre-OP: LD = 18.5 pg/mL, no LD = 24.5 pg/mL, P = 0.294; POD1: LD = 11.1 pg/mL, no LD = 29.6 pg/mL, P = 0.010; POD5: LD = 14.7 pg/mL, no LD = 18.8 pg/mL, P = 0.403; Fig. 1F).
The Profile of Circulating α-Granule Released Molecules Is Associated With Postoperative LD
We compared circulating α-granule proteins at the onset of LR (POD1) in patients who later suffered from postoperative LD and in patients with uncomplicated postoperative liver function recovery. Patients suffering from postoperative LD showed increased plasma levels of antiproliferative TSP-1, PF-4, and TGF-β, whereas proteins derived from proproliferative granules, such as VEGF and fibrinogen, were reduced in the plasma of these patients (plasma TSP-1 median: LD = 111.5 ng/mL, no LD = 46.0 ng/mL, P < 0.001; plasma PF-4 median: LD = 18.0 ng/mL, no LD = 8.81 ng/mL, P = 0.047; plasma TGF-β median: LD = 3,228 pg/mL, no LD = 1,636 pg/mL, P = 0.021; plasma VEGF median: LD = 11.1 pg/mL, no LD = 29.6 pg/mL, P = 0.010; plasma fibrinogen median: LD = 286 mg/dL, no LD = 339 mg/dL, P = 0.040; Fig. 2A-E).
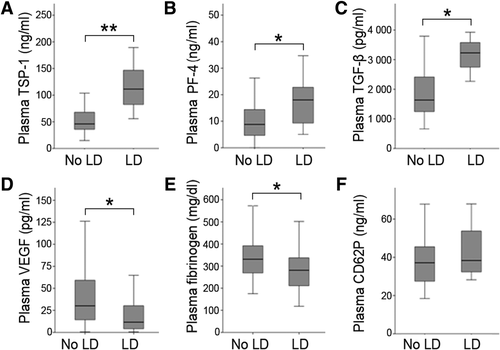
Circulating platelet-derived α-granule proteins 1 day after liver resection in patients who developed LD and patients without LD. Plasma TSP-1 (A), plasma PF-4 (B), total plasma TGF-β (C), plasma VEGF (D), plasma fibrinogen (E), and plasma CD62P (F) are illustrated according to postoperative LD. *P < 0.05; **P < 0.005.
Of note, postoperative plasma CD62P (which is stored in all α-granule subtypes) and serum growth factor levels, reflecting all potentially released platelet stored factors, did not differ in patients with and without postoperative LD (plasma CD62P median: LD = 37.2 ng/mL, no LD = 38.4 ng/mL, P = 0.344, Fig. 2F; serum VEGF median: LD = 511.7 pg/mL, no LD = 360.6 pg/mL, P = 0.128; serum CD62P median: LD = 56.5 ng/mL, no LD = 57.5 ng/mL, P = 0.658; serum PF-4 median: LD = 146.6 ng/mL, no LD = 194.5 ng/mL, P = 0.302; serum TSP-1 median: LD = 10,955.8 ng/mL, no LD = 7,665 ng/mL, P = 0.268; Supporting Fig. 1A-D).
We further assessed the potential of preoperative α-granule contents to predict postoperative LD. We were unable to detect any significant differences in preoperative α-granule proteins in patients with and without postoperative LD (plasma VEGF median: LD = 18.5 pg/mL, no LD = 24.5 pg/mL, P = 0.294, plasma TSP-1 median: LD = 38.1 ng/mL, no LD = 32.8 ng/mL, P = 0.222; plasma PF-4 median: LD = 15.6 ng/mL, no LD = 11.6 ng/mL, P = 0.502; plasma CD62P median: LD = 32.06 ng/mL, no LD = 32.08 ng/mL, P = 0.679).
Platelets Selectively Accumulate at the Site of LR Early After Partial Hepatectomy
We investigated whether platelets accumulate within the liver upon induction of LR during hemihepatectomy. Already 1-2 hours after intraoperative portal vein ligation, we found an increase in intrahepatic platelets, determined by CD61 positivity, in liver tissue (Fig. 3A). Intrahepatic platelets significantly increased (median, 57-73.5 CD61+cells/HPF) when we evaluated the area of most intense platelet accumulation (P = 0.037; Fig. 3B), as well as when 10 randomly selected HPFs were assessed (median, 46.6-69.3 CD61+cells/HPF; P = 0.037). We further determined the difference in platelet count between the portal vein and the liver vein. In the majority of patients, we indeed observed a decrease in platelet count in the liver vein compared to the portal vein, indicating that platelets do adhere in the hepatic vasculature and remain in the liver (Fig. 3C). We then evaluated whether platelet activation was altered in platelets derived from the liver vein compared to the portal vein. Basal platelet activation (as determined by surface expression of CD62P) in a subset of 26 patients was not significantly increased after platelets passed the hepatic microcirculation (Fig. 3D). We observed no significant difference in platelet responsiveness in liver vein platelets compared to platelets derived from the portal vein, indicating that at early time points after liver resection, activated platelets accumulated in the liver and could therefore not be detected in the circulation. In contrast, circulating platelet-derived growth factors increased significantly in the liver vein (TSP-1: median peripheral blood = 39.8 ng/mL, median liver vein = 77.1 ng/mL, P = 0.002; Fig. 3E; VEGF: median peripheral blood = 30.5 pg/mL,median liver vein = 42.2 pg/mL, P = 0.001; Fig. 3F).
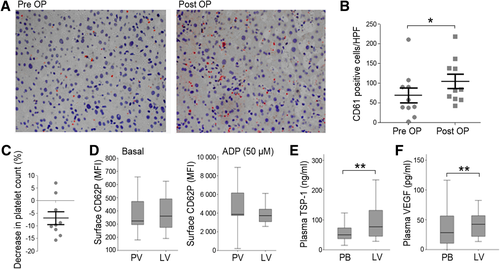
Platelet accumulation at the site of liver regeneration and the timely release of intraplatelet growth factors. A representative sample of immunohistochemistry slides of the liver before liver resection as well as 1.5 hours after the induction of LR is given in (A). (B) illustrates CD61-positive cells per HPF of all evaluated patients (n = 10). Differences in platelet count in blood samples derived from liver vein relative to portal vein (n = 10) (C). Basal platelet activation as well as reactivity in response to 50 μM of ADP, determined by surface expression of CD62P, in portal vein (PV) and liver vein (LV) blood are shown in (D). Plasma levels of preoperative TSP-1 (E) and VEGF (F) in peripheral blood (PB) and liver vein 1.5 hours after induction of LR. *P < 0.05; **P < 0.005.
Postoperative Circulating Platelet α-Granule Contents Show a Predictive Potential for Postoperative LD
To characterize the association of platelet α-granule-derived factors with LD and to compare the predictive potential of α-granule proteins, we performed a logistic regression analysis also including basic characteristics of our cohort (Table 2). Besides several well-established predictors of postoperative LD (see Table 2), we found a significant predictive value for lower VEGF and higher TSP-1 values at POD1. To further define a high-risk group (low VEGF and high TSP-1) within our patient cohort, we aimed at defining a cut-off level for TSP-1 and VEGF using ROC analyses. We found a significant association of proproliferative VEGF (VEGF: area under the curve [AUC] = 0.707, P = 0.010; Fig. 4A) and antiproliferative TSP-1 on POD1 (TSP-1: AUC = 0.894, P < 0.001; Fig. 4B) with postoperative LD. Using ROC curve analysis, we defined our cutoffs to detect postoperative LD with a specificity of >80% (TSP-1 = 80 ng/mL; 87.3% specificity; VEGF = 8 pg/mL; 82.2% specificity). We then tested the predictive potential of our high-risk group using logistic regression including all significant variables or variables of interest close to significance and were able to demonstrate that patients within our high-risk group had a substantially elevated risk to suffer from postoperative LD (Table 2). To further document that our high-risk group was independently associated with postoperative LD, we performed a multivariate logistic regression analysis for all variables that reached significance upon univariate regression. Importantly, we found our high-risk group to be independently associated with postoperative LD (Table 2).
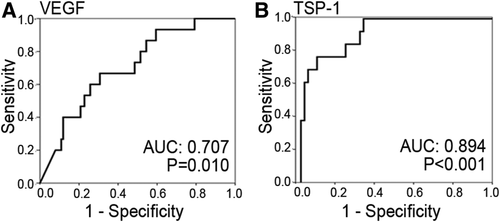
Analysis of the predictive value of circulating platelet-stored α-granule proteins one day after liver resection for postoperative LD. ROC curves of circulating growth factors are illustrated for VEGF (A) and TSP-1 (B).
| Univariate Logistic Regression | Multivariate Logistic Regression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Total N (%), Median (Range) | No LD N (%), Median (Range) | LD N (%), Median (Range) | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value |
| VEGF pg/mL (POD1) | 28.4 (0.0-163.1) | 29.6 (0.0-163.1) | 11.1 (0.0-64.32) | 0.010** | 0.967 | 0.939-0.996 | 0.024a | |||
| TSP-1 ng/mL (POD1) | 50.2 (14.8-390.9) | 46.03 (14.8-390.9) | 111.5 (55.7-189.5) | <0.001*** | 1.017 | 1.005-1.028 | 0.004*a | |||
| VEGFlow + TSP-1high (POD1) | 8 (7.4) | 3 (37.5) | 5 (62.5) | <0.001*** | 19.167 | 3.857-95.251 | <0.001*** | 14.156 | 2.156-92.96 | 0.006** |
| Sex, male | 98 (62.4) | 85 (63.0) | 11 (61.1) | 0.879 | ||||||
| Age, years | 64 (22-86) | 64 (22-86) | 64 (37-81) | 0.522 | ||||||
| Neoplastic entity | ||||||||||
| mCRC | 113 (72.0) | 102 (75.6) | 9 (50) | 0.022a | 0.324 | 0.119-0.883 | 0.028a | 0.510 | 0.039-6.641 | 0.607 |
| HCC | 21 (13.4) | 15 (11.1) | 6 (33.3) | 0.010** | 4.000 | 1.309-12.227 | 0.015a | 0.972 | 0.068-13.932 | 0.983 |
| CCC | 14 (8.9) | 10 (7.4) | 2 (11.1) | 0.583 | ||||||
| Non- | 7 (4.5) | 7 (5.2) | 0 (0) | 0.323 | ||||||
| Neoplastic | ||||||||||
| Others | 2 (1.3) | 1 (0.7) | 1 (5.6) | 0.091 | 7.882 | 0.471-131.89 | 0.151 | |||
| Preoperative CTx | 71 (45.2) | 63 (46.7) | 6 (33.3) | 0.286 | ||||||
| Cirrhosis | 21 (14.6) | 15 (12.1) | 6 (33.3) | 0.022a | 3.964 | 1.278-12.291 | 0.017a | 0.972 | 0.068-13.932 | 0.983 |
| Resection (major) | 72 (45.9) | 56 (41.5) | 12 (66.7) | 0.043a | 2.821 | 0.999-7.967 | 0.050a | 2.336 | 0.505-10.813 | 0.278 |
| PVE | 6 (3.9) | 5 (3.7) | 1 (5.9) | 0.669 | ||||||
| Preoperative paramters | ||||||||||
| SB mg/dL | 0.62 (0.19-6.64) | 0.62 (0.19-3.17) | 0.57 (0.27-6.64) | 0.462 | ||||||
| PT % | 105 (40-150) | 105 (40-150) | 105 (62-150) | 0.586 | ||||||
| ALP U/L | 91 (38-396) | 91 (38-375) | 100 (51-396) | 0.372 | ||||||
| GGT U/L | 49 (11-1,041) | 46 (11-1,041) | 53 (21-492) | 0.278 | ||||||
| AST U/L | 29.50 (8-404) | 30 (8-404) | 30 (14-208) | 0.692 | ||||||
| ALT U/L | 27 (7-346) | 26 (7-346) | 31 (13-120) | 0.300 | ||||||
| Albumin g/L | 42.05 (30.8-49.6) | 42.40 (30.8-49.6) | 39.10 (31.5-47.3) | 0.033a | 0.858 | 0.754-0.976 | 0.020a | 0.820 | 0.690-0.973 | 0.023a |
| HVPG mm Hga | 4 (2-9) | 3.5 (2-9) | 5 (4-8) | 0.328 | ||||||
| Platelets (×10^3/µL) | 217 (62-503) | 210 (62-503) | 249 (119-337) | 0.169 | ||||||
- VEGF and TSP-1 were not carried on to MVA because our high-risk group (VEGFlow + TSP-1high) was defined by these variables and accordingly depend on each other.
- a N = 17; if metric parameters are indicated, median with (range) is illustrated. A total of 153 patients were assessable for postoperative LD.
- Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTx, chemotherapy; GGT, gamma-glutamyltransferase; mCRC, metastatic colorectal cancer; n.a., not assessable.
To further confirm the relevance of our findings, we additionally performed a subgroup analysis singularly including colorectal cancer liver metastases patients, to achieve a more homogenous patient cohort. Accordingly, despite the reduction of statistical power, we were able to document that the postoperative α-granule release profile was the only parameter being significantly associated with postoperative LD (Supporting Table 3).
Patients With an Antiproliferative α-Granule Profile Suffer From an Increased Incidence of Postoperative Morbidity and Prolonged Hospitalization
To determine the clinical relevance of our observations, we further defined three subgroups reflecting the postoperative (POD1) circulating α-granule protein pattern: a low-risk group (VEGFhigh+TSP-1low [66.7%]); an intermediate-risk group (either VEGFhigh+ TSP-1high or VEGFlow+ TSP-1low [25.9%]); and a high-risk group (VEGFlow + TSP-1high [7.4%]). Strikingly, high-risk patients had an increased incidence of postoperative LD (62.5%) as compared to the intermediate-risk (21.4%; P = 0.026) and to the low-risk (2.8%; P < 0.001) groups (Fig. 5A).
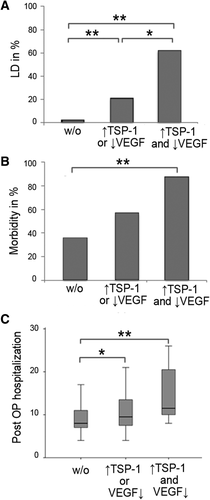
Patients exhibiting an unfavorable profile of postoperative α-granule protein secretion and postoperative clinical outcome. Patients were classified in high-, intermediate-, and low-risk groups according to their postoperative profile of α-granule protein secretion. Accordingly, patients are divided in groups with low TSP-1 and high VEGF (low-risk – w/o), either high TSP-1 or low VEGF (intermediate-risk), and high TSP-1 and low VEGF (high-risk). Incidence of postoperative LD (A), morbidity (B), and postoperative hospitalization (C) are illustrated according to groups.*P < 0.05; **P <0.005.
In line with this observation, postoperative morbidity was also highest in the high-risk group (87.5%), followed by the intermediate-risk group (57.1%; P = 0.115) and the low-risk group (36.1%; P = 0.005; Fig. 5B).
Furthermore, the high-risk profile resulted in a significantly prolonged postoperative hospital stay (median high-risk: 12 days; median intermediate-risk: 10 days; median low-risk: 8 days; low-risk vs. high-risk group: P = 0.002; low-risk vs. intermediate-risk group: P = 0.043; intermediate-risk vs. high-risk group: P = 0.099; Fig. 5C).
Markers of Portal Hypertension After Liver Resection Are Increased in Patients With an Unfavorable α-Granule Protein Profile
To elucidate potential causes for this selective α-granule release, we evaluated markers of PVP after liver resection by determining HVPGs in a subset of 17 patients undergoing liver resection (colorectal cancer liver metastases in 4 patients [23.5%], HCC in 12 patients [70.6%], and cholangiocarcinoma [CCC] in 1 patient [5.9%]). Patients within our high-risk group (VEGFlow + TSP-1high) had elevated HVPG values before liver resection (median HVPG before LR: low-risk group = 2.5 mm Hg; intermediate-risk group = 4 mm Hg; high-risk group = 7 mm Hg; low-risk vs. high-risk group: P = 0.048; low-risk vs. intermediate-risk group: P = 0.234; intermediate-risk vs. high-risk group: P = 0.667; Fig. 6A), with a consistent pattern being observed after surgery (median HVPG after LR: low-risk group: 3 mm Hg; intermediate-risk group: 6 mm Hg; high-risk group: 12.5 mm Hg; low-risk vs. high-risk group: P = 0.095; low-risk vs. intermediate- risk group: P = 0.177; intermediate-risk vs. high-risk group: P = 0.286; Fig. 6B). Moreover, the increase of HVPG after liver resection was most pronounced in patients with an unfavorable α-granule secretion profile (median HVPG increase after LR: low-risk group: 0 mm Hg; intermediate-risk group: 1.5 mm Hg; high-risk group: 5 mm Hg; low-risk vs. high-risk group: P = 0.190; low-risk vs. intermediate-risk group: P = 0.537; intermediate-risk vs. high-risk group: P = 0.429; Fig. 6C).
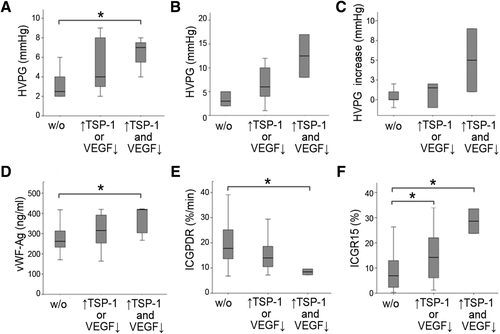
Patients exhibiting an unfavorable profile of postoperative α-granule protein secretion and postoperative PVP. Patients were divided in low- (low TSP-1 and high VEGF – w/o), intermediate- (either high TSP-1 or low VEGF), and high-risk (high TSP-1 and low VEGF) groups. HVPG before (A) and after liver resection (B) as well as postresectional increase of HVPG (C) are illustrated for all risk groups. Furthermore, postoperative values of noninvasive markers, known to reflect PVP, are illustrated in D (vWF-Ag), E (ICG-PDR), and F (ICGR15). *P < 0.05; **P < 0.005.
Because von Willebrand factor antigen (vWF-Ag) and PDR/R15 are clinically relevant markers of portal hypertension and more easily and widely available than HVPG measurements, we used these noninvasive parameters to assess postoperative PVP in our patients according to their postoperative α-granule secretion profile. In line with our HVPG results, we found that, in our overall cohort, the high-risk group significantly differed in postoperative vWF-Ag (low-risk group = 262 ng/mL; intermediate-risk group = 316 ng/mL; high-risk group =419 ng/mL; low-risk vs. high-risk group: P = 0.012; low-risk vs. intermediate-risk group: P = 0.123; intermediate-risk vs. high-risk group: P = 0.179; Fig. 6D), ICG-PDR (low-risk group: 17.8%/min; intermediate-risk group: 14.0%/min; high-risk group: 8.5%/min; low-risk vs. high-risk group: P = 0.030; low-risk vs. intermediate-risk group: P = 0.063; intermediate-risk vs. high-risk group: P = 0.105; Fig. 6E), and ICG-R15 (low-risk group: 6.9%; intermediate-risk group: 14.2%; high-risk group: 28.6%; low-risk vs. high-risk group: P = 0.030; low-risk vs. intermediate-risk group: P = 0.044; intermediate-risk vs. high-risk group: P = 0.105; Fig. 6F). Furthermore, levels of vWF-Ag significantly correlated with the ratio of α-granule stored growth factors (TSP-1/VEGF) on POD1 (see Supporting Fig. 2).
Discussion
Here, we present the first in human data showing that platelet α-granule contents might modify postoperative LR. In particular, the profile of released pro- and antiproliferative granule components seemed to determine the ability of the remnant liver to regenerate, reflected by the incidence of postoperative LD and clinical outcomes (morbidity and hospitalization). These in vivo data provide first evidence that a differential α-granule release by platelets may be a relevant mechanism influencing LR after liver resection. Moreover, we demonstrate that disturbances of hepatic microcirculation, as reflected by preexisting portal hypertension and liver disease, as well as more pronounced increases in HVPG after resection, might trigger selective α-granule release.
Platelets have been shown to accumulate in the liver immediately after hepatectomy, where large numbers translocate into the space of Disse in the liver sinusoids.22 Transmission electron microscopy in mice has been used to document direct contact between platelets and hepatocytes as early as 5 minutes after resection.22 We were able to show similar results in human patients after liver resection, where intrahepatic platelets accumulated within the first 2 hours of LR. Intriguingly, no increase in platelet activation after passing the hepatic microcirculation was observed. However, platelet count decreased (delta portal vein to hepatic vein), suggesting that platelets adhere in the hepatic sinusoids and permanently remain within the liver as previously suggested.23, 24 Excessive intraoperative blood loss, as reflected by the use of intraoperative red blood cell transfusion, was not associated with platelet counts or dynamics (data not shown), excluding a possible bias by blood dilution or bleeding.
Hepatic accumulation of platelets was accompanied by increased plasma VEGF and TSP-1 levels in the liver vein, as compared to systemic levels already 1-2 hours after LR induction (hemihepatectomy). This rapid increase strongly favors a specific release of these growth factors rather than an induction of protein synthesis. Because platelets are known to be a major source of circulating VEGF17 and a main storage pool for TSP-1,15, 25 platelets are most likely the major contributor for the increase in circulating VEGF and TSP-1.
Whereas VEGF is proregenerative, TSP-1 has negative effects on LR.10, 26 Of note, it has been proposed that pro- and antiangiogenic proteins are organized into separate platelet α-granules and are differentially released upon activation.4 In vitro data revealed that different α-granule subsets are selectively released by specific agonists, as suggested by agonist-specific α-granule secretion kinetics.4, 27, 28 However, to date no in vivo evidence exist that platelets are capable of selectively releasing α-granule proteins and that this has an impact on clinical outcomes. Intriguingly, we found a specific α-granule release pattern in patients with LD, which precisely followed the previously observed clustering: a “proangiogenic” α-granule subgroup, containing VEGF and fibrinogen, whereas “antiangiogenic” factors were stored in other, distinct granules. In line with these observations, we show that patients suffering from postoperative LD had lower circulating postoperative VEGF and fibrinogen levels. In contrast, anti-angiogenic TSP-1, PF-4, and total TGF-β were increased in these patients. Importantly, we found that the total amount of circulating platelets, as well as plasma levels of soluble CD62P, which is stored in all α-granule subtypes,4 were comparable in patients with and without LD.
Furthermore, we observed that patients suffering from postoperative LD failed to release VEGF, whereas antiproliferative TSP-1 markedly increased on POD1. However, patients with an increase in plasma VEGF, but no induction of TSP-1 release, on POD1 were at lower risk to develop postoperative LD. Of note, platelet packing within the bone marrow was suggested to be affected in patients with liver cirrhosis.21 Accordingly, we evaluated preoperative serum growth factor levels of VEGF and TSP-1. Neither of these growth factors was significantly different between patients with and without postoperative LD, indicating that patients with LD do store balanced amounts of VEGF and TSP-1, and that it is rather a specific postoperative platelet granule exocytosis, which seems to determine the unfavorable profile observed in these patients.
Furthermore, using the profile of circulating growth factors on POD1, we were able to document major clinical relevance of this α-granule release pattern. Patients with an unfavorable profile (high TSP-1 and low VEGF) on POD1 had an increased risk of postoperative LD (more than 60%), postoperative morbidity (more than 80%), and hospitalization compared to patients with high VEGF and low TSP-1. Furthermore, the predictive value of the α-granule release pattern was documented upon multivariate analysis as well as within a subgroup without preexisting liver disease (metastatic colorectal cancer patients).
We further aimed to determine possible mechanisms that could account for the observed specific α-granule release resulting in an unfavorable growth factor profile. A priming event in LR is the increase of PVP immediately after partial hepatectomy.29, 30 This increase of intrahepatic vascular pressure has been shown to immediately affect the lining of liver sinusoidal endothelial cells (LSECs), most likely by increased shear stress. In particular, the highly organized fenestrations of the liver sinusoids are disrupted shortly after hepatectomy, triggering an accumulation of platelets within the liver sinusoids.23, 24
Whereas this initial event is essential for initiation of LR, an excessive increase of PVP is associated with an impaired ability of the remnant liver to regenerate. Indeed, a pharmacological reduction of postresectional PVP was associated with accelerated postoperative LR.31
Accordingly, an unfavorable profile of α-granule release was associated with an increase in pre- and postoperative PVP, measured by HVPG. Importantly, the relative increase of HVPG after resection was also associated with unfavorable α-granule secretion profile, suggesting that postresectional PVP might be a relevant trigger for specific α-granule secretion. Indeed, experimental studies suggest a central relevance of portal blood flow to platelet activation. Whereas shear stress itself affects platelet activation, endothelial cell damage caused by increased intrahepatic vascular pressure can additionally modify platelet activation.32
However, preoperative HVPG was also elevated in our high-risk patients, suggesting that preexisting portal hypertension might also contribute to specific postoperative platelet activation. PVP is usually increased as a result of preexisting underlying liver disease. It is well known that platelet activation is altered in patients with liver cirrhosis33 and liver infection.34 Whereas platelet adherence is defective, platelets of patients with cirrhosis frequently suffer from a storage pool defect, including α-granule proteins such as PF-4.21 The relevance of preexisting liver disease is further supported by the fact that 50% of the patients within our high-risk group suffered from liver cirrhosis, which was significantly more often than in our low-risk group (low-risk group: 16%; P = 0.010, data not shown). However, to exclude the possibility that our results were solely a result of preexisting liver disease, a multivariate analysis was performed demonstrating that the postoperative α-granule release pattern independently predicted postoperative LD. Furthermore, when only patients without preexisting liver disease (metastatic colorectal cancer patients) were included, we observed a similar predictive value.
An unfavorable α-granule release profile correlated with circulating vWF-Ag levels, a surrogate marker for postoperative PVP, endothelial dysfunction, and portal hypertension35 on POD1. Platelet aggregation is not only affected by vWF, but also by platelet interaction with LSECs.36 Accordingly, endothelial cell dysfunction itself could contribute to specific α-granule release, which, in turn, affects LR. Of note, platelets can directly interact with LSECs and thereby modify hepatocyte proliferation, as recently reviewed by Meyer et al.37
A potential weakness of our study is that, apart from PF-4, not all platelet-stored factors measured are platelet specific, but can also be produced by other cell types. However, the rapid increase of VEGF and TSP-1 in the liver vein and the fast intrahepatic accumulation of platelets after liver resection support the hypothesis of a specific growth factor release from the largest readily available pool—platelet α-granules. Furthermore, the profile of circulating growth factors followed previously published selective release of α-granule content and clinical observations support a critical role of platelets in postoperative LR.4, 5
Taken together, we present the first clinical evidence that the profile of circulating platelet α-granule proteins seem to affect postoperative LR in patients after liver resection. Furthermore, we provide the first in vivo evidence that selective α-granule release might modify pathophysiological processes involved in LR. Further exploring the trigger of platelet activation in this clinical setting will be of utmost importance to potentially modulate platelet α-granule release in patients. Targeting specific intrahepatic platelet granule release may represent a powerful and attractive target for therapeutic interventions to improve LR after liver resection.
Acknowledgment
The authors thank Angela Knöbl and Susanne Humpeler for the technical assistance.




