Association between nucleos(t)ide analog and tumor recurrence in hepatitis B virus–related hepatocellular carcinoma after radiofrequency ablation
Potential conflict of interest: Nothing to report.
This work was supported, in part, by the National Health Research Institutes (PH-104-PP-23) and Taichung Veterans General Hospital (104DHA0500198), Taiwan.
Abstract
Radiofrequency ablation (RFA) is the best choice for curative treatment of hepatocellular carcinoma (HCC) cases not suitable for surgical intervention, but efforts should be made to reduce the risk of tumor recurrence. We aimed to investigate the association between nucleos(t)ide analog (NA) therapy for hepatitis B virus (HBV) and the risk of HCC recurrence post-RFA. Using the Taiwan National Health Insurance Research Database between July 1, 2004 and December 31, 2012, we screened 48,807 patients with newly diagnosed HBV-related HCC. We identified 850 patients (200 patients who used NAs for more than 90 days and 650 who never used NA post-RFA) who received RFA as a potentially curative treatment for HCC. Patients in the NA-treated cohort were randomly matched 1:2 with patients in the untreated cohort by age, sex, cirrhosis, and the time period between RFA and initiation of NA therapy. Finally, 133 patients were recruited in the NA-treated group and 266 in the untreated group for analysis. Cumulative incidences of and hazard ratios (HRs) for HCC recurrence were analyzed after adjusting for competing mortality. The HCC recurrence rate of the NA-treated group was significantly lower than that of the untreated group (2-year recurrence rate: 41.8%; 95% confidence interval [CI]: 32.9-50.6 vs. 54.3%; 95% CI: 48.0-60.6; modified log-rank test: P < 0.05). In modified Cox's regression analysis, NA therapy was independently associated with a decreased risk of HCC recurrence (HR, 0.69; 95% CI: 0.50-0.95; P < 0.05). Multivariate stratified analyses verified the association of NA therapy and decreased HCC recurrence in almost all patient subgroups. Conclusion: NA therapy was associated with a decreased risk of HCC recurrence among patients with HBV-related HCC post-RFA. (Hepatology 2016;63:1517-1527)
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- CI
-
- confidence interval
-
- COPD
-
- chronic obstructive pulmonary disease
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HIV
-
- human immunodeficiency virus
-
- HR
-
- hazard ratio
-
- ICD-9
-
- International Classification of Diseases, Ninth Revision
-
- NA
-
- nucleos(t)ide analog
-
- NHI
-
- Taiwan National Health Insurance program
-
- NHIRD
-
- the National Health Insurance Research Database in Taiwan
-
- NSAIDs
-
- nonsteroidal anti-inflammatory drugs
-
- RCIPD
-
- the Registry for the Catastrophic Illness Patient Database
-
- RFA
-
- radiofrequency ablation
-
- TAE
-
- transarterial embolization
Hepatocellular carcinoma (HCC) remains the third-most common cause of cancer death worldwide, and HCC-related deaths have been estimated to be approximately 700,000 per year.1 With a low survival rate of less than 12% in 5 years, HCC is the fastest rising cause of cancer death in the United States.2 Although HCC surveillance for high-risk populations may contribute to early tumor treatment and reduced mortality rates,3 the clinical outcomes of patients receiving curative treatments remain unsatisfactory.4 Among HCC patients receiving potentially curative surgical resection, tumor recurrence rates can be as high as 50% in 3 years,5 and most tumor recurrences are clustered within 2 years of surgery.6 Further improvement in the prevention of tumor recurrence after curative treatment is imperative.
Radiofrequency ablation (RFA) is a highly effective curative treatment for HCC, especially for small tumors.7 However, RFA is an alternative choice to surgical resection because of a relatively higher local recurrence rate compared to that of surgery.8, 9 In current clinical practice guidelines, RFA is the standard of care for early-stage HCC cases not suitable for surgical intervention.10, 11 However, even with a higher tumor recurrence rate, RFA has become more popular in recent years because of the advantages of an excellent safety rate, rapid wound recovery, and acceptable efficacy.7 Consequently, HCC patients who may benefit most from RFA are severely with cirrhosis and/or elderly.12 RFA now plays a central role in the curative treatment of HCC, and efforts should be made to reduce the risk of tumor recurrence post-RFA.
Hepatitis B virus (HBV) and hepatitis C virus (HCV) have been identified as the most important risk factors in the development of HCC,13, 14 and antiviral therapies can prevent HCC development.15-18 Moreover, HBV and HCV have been related to HCC recurrence after curative therapies,19-22 and antiviral therapies may prevent HCC recurrence after liver resection.23-25 In our previous studies of HCC after liver resection, antiviral therapies for chronic hepatitis B or C were associated with a lower risk of HCC recurrence.23, 26 However, in HBV-related HCC post-RFA, the association between nucleos(t)ide analog (NA) therapy and HCC recurrence has not been investigated. We hypothesized that NA therapy could effectively reduce HCC recurrence after curative RFA, so we conducted a cohort study to evaluate the association between NA therapy and the risk of HCC recurrence.
Patients and Methods
Study Design
In this cohort study, the medical records of patients who received RFA for HCC were retrieved from the National Health Insurance Research Database (NHIRD) in Taiwan. The NHIRD contains health care data from more than 99% of the entire population of 23.38 million in Taiwan. The NHIRD comprises comprehensive information, including demographic data, dates of clinical visits, diagnostic codes, operation codes, and details of prescriptions, among others, as described in our previous studies.18, 23, 26-30 This study has been approved by the research ethics committee of the National Health Research Institutes in Taiwan.
Study Population
The patient selection process is shown in Fig. 1. We included all hospitalized patients who had received RFA as a potentially curative treatment for their newly diagnosed HCCs between July 1, 2004 and December 31, 2012. All patients who were admitted with a primary diagnosis of HCC were identified by the International Classification of Diseases, Ninth Revision (ICD-9), diagnosis code 155.0. The accuracy of HCC diagnosis was confirmed by the inclusion of patients in the Registry for the Catastrophic Illness Patient Database (RCIPD). The RCIPD is an official subsystem of the NHIRD in which histopathological confirmation or typical HCC image characteristics are required for the diagnosis of HCC.18, 23, 26, 29
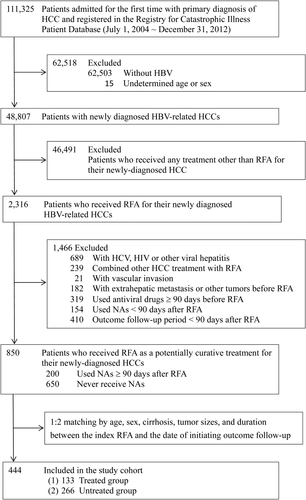
Only patients with HBV infection (ICD-9 codes 070.2, 070.3, and V02.61) were included; patients with HCV infection, human immunodeficiency virus (HIV) infection, or other viral hepatitis were excluded. We also excluded patients who received combination therapies (liver resection, transarterial embolization [TAE], or intra-arterial chemotherapy) with RFA, patients with any vascular invasion (ICD-9 codes 452, 453.0, and 453.2), extrahepatic metastasis, or other malignant tumor (ICD-9 codes 140-208) before and at the first diagnosis of HCC, and patients who received antiviral therapy (lamivudine, entecavir, adefovir, telbivudine, tenofovir, or interferon) for 90 days or more before the index admission. Antiviral therapy for chronic hepatitis B has been covered by the Taiwan National Health Insurance (NHI) program since October 1, 2003, but reimbursement for antiviral therapy requires patients to fulfill certain criteria, such as elevated serum alanine aminotransferase (ALT; ≥2× upper limit of normal) and elevated HBV-DNA titers (>2,000 IU/mL).18 In the clinical practice of RFA treatment for HCC in Taiwan, patients would receive the secondary HCC treatment within 3 months after the potentially curative RFA if any viable tumor was found.31, 32 Using the definition of potentially curative RFA in the present study, that is, no tumor recurrence within 3 months post-RFA, patients with an outcome follow-up period less than 90 days were excluded.
Study Cohorts
Patients who received RFA as a potentially curative treatment for their newly diagnosed HCCs were divided into two cohorts according to their use of NAs. The treated cohort comprised patients who were treated with NAs for at least 90 days, and the untreated cohort consisted of patients who were never treated with NA therapy. Patients who received NA therapy for less than 90 days were excluded. Each patient in the treated cohort was randomly matched with 2 patients in the untreated cohort by age, sex, cirrhosis, and tumor size. To eliminate the immortal time bias, the untreated controls were also matched for the time period between the index RFA and the initiation of NA therapy for the treated patients.26, 33 Untreated controls were selected by matching the dates of index RFA with those of the treated patients (±180 days), and they were followed up from the same dates of NA therapy initiation for the treated patients.
Main Outcome Measurements
Treated and untreated patients were followed up for HCC recurrence after initiation of NA therapy and the date of matched post-RFA duration, respectively. HCC recurrence was defined as application of any treatment modality for HCC recurrence (liver resection, percutaneous ethanol injection, RFA, liver transplantation, TAE, or chemotherapy) after the date of initiation of outcome follow-up during the study period. Patients were followed up until the date of HCC recurrence, death, or December 31, 2012. Death was defined as withdrawal of the patient from coverage in the Taiwan NHI program.
Prognostic Factor Assessment
In addition to age and sex, several other important prognostic factors were evaluated. Information regarding the size of ablated HCC tumors was retrieved from the treatment database, and tumor sizes were divided into three categories, as follows: <3, 3-5, and >5 cm. Major coexisting diseases that might confound the outcome of interest were evaluated by ICD-9 codes at or before the index RFA, including liver cirrhosis (571.5), liver decompensation (789.5, 572.2, and 572.4), hypertension (401-405), diabetes mellitus (250), peptic ulcer disease (531-534), chronic obstructive pulmonary disease (COPD; 490-505, 506.4), acute coronary syndrome (410-414), cerebrovascular accident (430-436), chronic renal failure (585), and hyperlipidemia (272.0-272.2). All diseases listed in the Charlson comorbidity index were analyzed except for HIV infection, metastatic tumor, and concurrent other malignancies owing to the exclusion criteria of the present study.34
Medication information was retrieved from the pharmacy prescription database. Any antiviral therapy for chronic hepatitis B that was started during the study period was analyzed according to the design of this study. In addition, certain potentially chemopreventive medications, including nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, statins, and metformin, were also analyzed. Drug users were defined as those who used more than 1 tablet per month during the study period. Propensity scores were measured using a logistic model with age, sex, tumor size, cirrhosis, underlying comorbidities, and use of potentially chemoprotective drugs.
Statistical Analysis
Descriptive data were presented as median values (25%-75% interquartile ranges), and case numbers and percentages were used for study subjects in the treated and untreated groups. Cumulative incidences of HCC recurrence post-RFA were calculated, and calculated results were expressed as the estimated number along with the 95% confidence interval (CI). Because death from underlying comorbidities led us to apply informative censoring in calculating HCC incidence, patient mortality preceding HCC recurrence was considered to be a competing risk event. Cumulative incidences in competing risk data ratios were calculated and compared by using a modified Kaplan-Meier's method and Gray's method.35 After adjusting competing morality, differences in the full time-to-event distributions between the treated and untreated groups were compared by a modified log-rank test.23
After adjusting for age, sex, tumor size, cirrhosis, diabetes mellitus, and use of NSAIDs or aspirin, statins, and metformin, multivariate regression analyses were conducted to determine the independent prognostic factors for HCC recurrence. Hazard ratios (HRs) were determined by modified Cox's proportional hazard models for analysis of competing risk events. For the estimations of cumulative incidences under competing risk events, Cox's models were constructed by using the “cmprsk” package for R (http://cran.r-project.org/web/packages/cmprsk/index.html). All data were analyzed by SAS software (version 9.3; SAS Institute, Inc., Cary, NC).
Results
Baseline Demographic Characteristics of the Study Subjects
We initially identified 111,325 potentially eligible patients admitted for the first time with primary diagnosis of HCC and registered in the RCIPD between July 1, 2004 and December 31, 2012 (Fig. 1). After excluding 62,503 patients who did not have HBV infection, 15 patients whose age or sex was undetermined, and 46,491 patients who received any HCC treatment other than RFA, 2,316 patients who received RFA for their newly diagnosed HBV-related HCCs were identified. We further excluded patients who had HCV, HIV, or other viral hepatitis, who received combination therapy with RFA, who were found to have vascular invasion, extrahepatic metastasis, or concurrent malignancies before or at the diagnosis of HCC, who used NA or interferon ≥90 days before RFA, who used NAs <90 days after RFA, and who had an outcome follow-up period <90 days after RFA. We identified a total of 850 patients (200 patients who used NAs for more than 90 days and 650 patients who never used NA after RFA) who received RFA as a potentially curative treatment for their newly diagnosed HCCs. Furthermore, patients in the NA-treated cohort were randomly matched 1:2 with patients in the untreated cohort by age, sex, cirrhosis, and the time period between RFA and initiation of NA therapy. Finally, a total of 133 patients were recruited in the NA-treated group and 266 in the untreated group for analysis.
Baseline demographic characteristics of study subjects in the NA-treated and untreated groups are presented in Table 1. There were no significant differences in the prognostic factors between the two groups, including age, sex, tumor size, liver cirrhosis, liver decompensation, major coexisting diseases such as diabetes mellitus, modified Charlson score, use of NSAIDs or aspirin, metformin, statins, and propensity score. The median age was around 60 years in both groups, and around 80% of patients were male. Tumor size in patients receiving RFA was generally small, and more than 70% of patients had tumors <3 cm. The median interval to start antiviral therapy after the index RFA was 0.42 (interquartile range: 0.04-1.21) years among the NA-treated patients. The median duration of NA exposure was 1.48 (interquartile range: 0.62-2.39) years during the study period, and the median duration of NA therapy was 1.24 (interquartile range: 0.38-2.15) years during the outcome follow-up period (3 months after NA initiation). However, more than 70% of patients had underlying liver cirrhosis before RFA, and around 10% of patients had liver decompensation. A proportion of patients (21.8% in the NA-treated group and 24.1% in the untreated group) had underlying diabetes mellitus, and more than half of diabetic patients (13.5% in the NA-treated group and 18.1% in the untreated group) were metformin users.
| Characteristics | Treated n = 133 | Untreated n = 266 | P Value |
|---|---|---|---|
| Age, years | 60.0 (52.0-67.8) | 59.9 (53.0-68.3) | 0.32 |
| Sex, n (%) | 1.00 | ||
| Male | 109 (81.9) | 218 (81.9) | |
| Female | 24 (18.1) | 48 (18.1) | |
| Tumor size, cm, n (%) | 0.88 | ||
| <3 | 101 (75.9) | 196 (73.7) | |
| 3-5 | 28 (21.1) | 62 (23.3) | |
| >5 | 4 (3.0) | 8 (3.0) | |
| Interval to start NA therapy, years | 0.42 (0.04-1.21) | — | |
| NA exposure during study period, years | 1.48 (0.62-2.39) | — | |
| NA therapy during outcome follow-up, years | 1.24 (0.38-2.15) | — | |
| Major coexisting diseases, n (%) | |||
| Liver cirrhosis | 97 (72.9) | 194 (72.9) | 1.00 |
| Liver decompensation | 18 (13.5) | 25 (9.4) | 0.21 |
| Hypertension | 41 (30.8) | 93 (35.0) | 0.41 |
| Diabetes mellitus | 29 (21.8) | 64 (24.1) | 0.62 |
| Peptic ulcer disease | 36 (27.1) | 71 (26.7) | 0.94 |
| COPD | 7 (5.3) | 22 (8.3) | 0.28 |
| Acute coronary syndrome | 0 (0.0) | 6 (2.3) | 0.18 |
| Cerebral vascular disease | 11 (8.3) | 20 (7.5) | 0.79 |
| Renal failure | 1 (0.8) | 6 (2.3) | 0.43 |
| Hypercholesterolemia | 0 (0.0) | 1 (0.4) | 1.00 |
| Modified Charlson score | 1 (1-2) | 1 (1-2) | 0.79 |
| Drug users, n (%) | |||
| NSAIDs or aspirin | 49 (36.8) | 88 (33.1) | 0.46 |
| Statins | 5 (3.8) | 18 (6.8) | 0.22 |
| Metformin | 18 (13.5) | 48 (18.1) | 0.25 |
| Propensity score | 0.34 (0.31-0.36) | 0.33 (0.31-0.35) | 0.24 |
- Data are expressed as median (interquartile range) or number (percentage). NA therapy during outcome follow-up: NA use was calculated from 3 months post-NA initiation.
Cumulative Incidences of HCC Recurrence
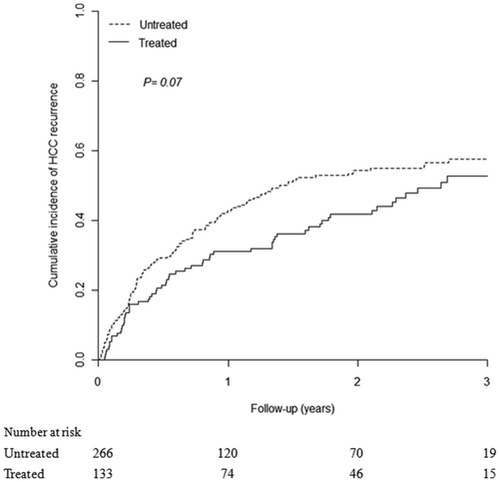
Cumulative incidences of HCC recurrence in the NA-treated and untreated groups were calculated, and a total of 188 patients (47.1%) were found to have developed HCC recurrence in 2 years: 52 (39.1%) in the NA-treated group and 136 (51.1%) in the untreated group (P < 0.05). However, a high percentage of patients had underlying cirrhosis in this study; 12 (9.0%) in the NA-treated group and 25 (9.4%) in the untreated group died before HCC recurrence. After adjusting for competing mortality, the HCC recurrence rate of the NA-treated group was significantly lower than that of the untreated group (2-year recurrence rate: 41.8%; 95% CI: 32.9-50.6 vs. 54.3%; 95% CI: 48.0-60.6; modified log-rank test: P < 0.05; Supporting Fig. 1). Although the 3-year HCC recurrence rate of the NA-treated group was lower than that of the untreated group (52.7%; 95% CI: 42.7-62.8 vs. 57.6%; 95% CI: 50.9-64.2), statistical significance was not reached (P = 0.07; Fig. 2).
Cumulative Incidences of Overall Mortality
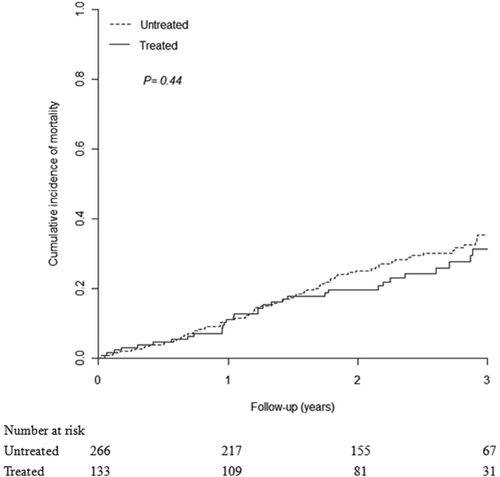
Cumulative incidences of overall mortality in the NA-treated and untreated groups are shown in Fig. 3. Although the overall mortality rate of the NA-treated group was lower than that of the untreated group (2-year mortality rate: 19.6%; 95% CI: 12.5-26.7 vs. 25.0%; 95% CI: 19.5-30.5), the difference was not statistically significant. In addition, the 3-year overall mortality rate of the NA-treated group was consistently lower than that of the untreated group (3-year mortality rate: 31.4%; 95% CI: 21.4-41.3 vs. 35.3%; 95% CI: 28.4-42.3), but the difference was still not statistically significant.
Multivariate Analysis of Prognostic Factors
The multivariate regression analysis for determining independent prognostic factors for HCC recurrence is shown in Table 2. Antiviral therapy with NAs was an independent prognostic factor that was associated with a decreased risk of HCC recurrence (HR, 0.69; 95% CI: 0.50-0.95; P = 0.02). In contrast, liver cirrhosis was an independent prognostic factor that was associated with an increased risk of HCC recurrence (HR, 1.64; 95% CI: 1.13-2.38; P < 0.01). Other potential prognostic factors, such as age, sex, tumor size ≥3 cm, diabetes mellitus, use of NSAIDs or aspirin, use of statins, or use of metformin, were not significantly associated with HCC recurrence in this study.
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Antiviral therapy | 0.68 (0.50-0.94) | 0.02 | 0.69 (0.50-0.95) | 0.02 |
| Age per year | 1.00 (0.99-1.02) | 0.65 | 1.00 (0.98-1.01) | 0.68 |
| Male sex | 0.85 (0.60-1.21) | 0.37 | 0.93 (0.64-1.36) | 0.71 |
| Tumor size ≥3 cm | 1.17 (0.84-1.63) | 0.35 | 1.25 (0.89-1.75) | 0.20 |
| Liver cirrhosis | 1.62 (1.13-2.32) | <0.01 | 1.64 (1.13-2.38) | <0.01 |
| Diabetes mellitus | 1.15 (0.82-1.60) | 0.42 | 1.04 (0.68-1.59) | 0.87 |
| NSAIDs or aspirin use | 1.04 (0.78-1.40) | 0.79 | 1.03 (0.75-1.41) | 0.87 |
| Statin use | 0.90 (0.47-1.72) | 0.74 | 0.82 (0.43-1.58) | 0.56 |
| Metformin use | 1.29 (0.90-1.86) | 0.17 | 1.26 (0.77-2.04) | 0.35 |
Multivariate Stratified Analyses in All Subgroups of Patients
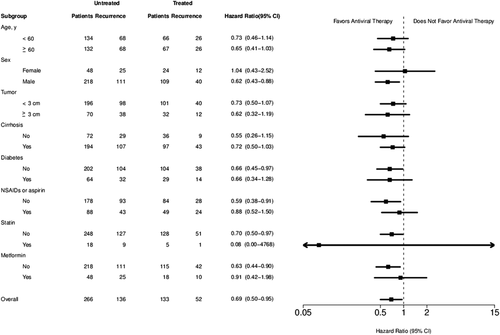
Multivariate stratified analysis was performed in all subgroups of patients, including those ages < or ≥60 years, both sexes, those with tumor size < or ≥3 cm, underlying liver diabetes mellitus, and those who used NSAIDs or aspirin, statins, or metformin. Multivariate stratified analyses verified the association of NA therapy and decreased HCC recurrence in almost all patient subgroups (HR < 1.0; Fig. 4). These observations further confirmed the association between NA therapy and decreased risk of HCC recurrence in HCC patients after curative RFA. Furthermore, regarding the association between NA therapy and reduced HCC recurrence, statistical significance was reached in several subgroups, including male patients (HR, 0.62; 95% CI: 0.43-0.88), those without diabetes mellitus (HR, 0.65; 95% CI: 0.45-0.97), those who did not use NSAIDs or aspirin (HR, 0.59; 95% CI: 0.38-0.91), statins (HR, 0.70; 95% CI: 0.50-0.97), and metformin (HR, 0.63; 95% CI: 0.44-0.90).
Discussion
This study clearly demonstrated that HCC recurrence rates were significantly lower in the NA-treated group compared with rates in the untreated group after RFA, and NA therapy was an independent prognostic factor associated with a reduced risk of HCC recurrence. To the best of our knowledge, this is the first study to report on the positive effect of NA therapy on the prevention of HCC recurrence after RFA treatment. The findings of the present study support the speculation that NA therapy could improve clinical outcomes after curative RFA, and further prospective studies are mandatory for confirmation of our findings.
HBV is one of the most important risk factors for HCC development, and a high viral load has been associated with increased risk of HCC development and HCC recurrence after curative treatment.36-38 Several studies have reported that NA therapy can reduce the risk of HCC development.18, 39 In the control of chronic HBV infection for HCC patients receiving liver resection, NA therapy is more reliable than interferon therapy owing to its potent antiviral effect and minimal side effects.40 In our previous study, we found that NA therapy for patients with HBV-related HCC after liver resection was associated with a lower risk of HCC recurrence, and NA therapy was an independent prognostic factor (HR, 0.67; 95% CI: 0.55-0.81).23 In the present study, NA therapy was also associated with a lower risk of HCC recurrence post-RFA among patients with HBV-related HCC, and the protective effect of NA therapy (HR, 0.69; 95% CI: 0.50-0.95) was similar to that of our previous study.23 These consistent results suggest NA therapy could effectively improve outcomes of HCC patients after curative surgery or tumor ablation.
According to current clinical practice guidelines, RFA is recommended for early HCC cases not suitable for surgical intervention, and the characteristics of HCC patients receiving RFA could be very different from those receiving liver resection.10, 11 HCC patients who receive RFA may be severely cirrhotic and/or elderly.12 Compared to the HCC patients who received liver resection in our previous study,23 the mean age of HCC patients who received RFA in this study was significantly higher (59.9 vs. 54.6 years), and the proportion of patients with clinical cirrhosis was much higher (72.9 vs. 39.8%). Moreover, around 10% of patients in this study suffered from decompensated liver cirrhosis before the index RFA. Our data support that elderly and/or HCC patients with cirrhosis could gain from receiving NA therapy after curative treatments.
HCC patients with small tumors who were resection candidates might be treated with RFA instead of liver resection in real-world practice, but they could not be separated out for analysis owing to limitations of the Taiwan NHIRD. However, underlying cirrhosis is the most important concern when considering surgical eligibility of HCC patients in current HCC practice guidelines,10, 11 and patients with or without cirrhosis were separately evaluated in this study. However, the sample size of cohort without cirrhosis was small, so the protective effect of NA therapy was not statistically significant. Further large-scale studies are needed for confirmation of the benefits of NA therapy.
In this study, we decided to focus on the 2-year follow-up data owing to several limitations. First, RFA became a popular HCC treatment in Taiwan in the latter part of the study period, and more than 70% of study patients were followed up for HCC recurrence for only 2 years or less (Fig. 2). The case numbers became sparse after follow-up for more than 2 years. Second, the median duration of NA therapy was only 1.48 (interquartile range: 0.62-2.39) years in the treated group, and the effect of NA therapy could be precisely evaluated in the first 2 years of patient follow-up. Third, owing to the limitations of Taiwan NHI reimbursement for NA therapy during the study period, NA therapy might have been discontinued in some patients of the treated group 1-3 years after initiation of NA therapy. The protective effects of NA therapy might thus be weakened in the subsequent years, especially among patients with cirrhosis who were at high risk of HCC recurrence.6
Mortality is another important outcome in the treatment of HCC. Although the overall mortality rates of the NA-treated group were lower than those of the untreated group, the differences were not statistically significant. However, compared to the HCC patients in our previous liver resection study,23 patients in this RFA study cohort were older and more with cirrhosis, and they also had more coexisting diseases, such as diabetes mellitus (23.3 vs. 16.1%), hypertension (33.6 vs. 23.8%), and cerebrovascular accident (7.8% vs. 3.4%). The higher mortality rates among the older patients in this study cohort might reduce the statistical differences of overall mortality between the treated and untreated groups. In addition, a relatively small sample size of this RFA study cohort might also account for the lack of statistical significance. RFA has become a popular treatment for HCC in recent years, and a study with a larger cohort may confirm the survival benefit of NA therapy in the future.
It is important to note that antiviral therapy is not the only factor affecting outcomes, and HCC can develop even when HBV has been controlled.39 Comparable to the findings of previous studies,6 the results of the present study showed that cirrhosis was an independent risk factor for HCC recurrence. The rates of tumor recurrence are very high among patients with cirrhosis with HBV-related HCC after curative liver resection, and the cumulative incidence could be more than 40% in 2 years.41 Regression of liver fibrosis has been observed after long-term NA therapy for HBV, but a certain percentage of cirrhosis cases cannot be reversed.42 Investigations that focus on improving the reversibility rate of cirrhosis will be crucial in the future. In addition, tumor size could be another important prognostic factor, but it did not reach statistical significance in this study. This result might be attributed to a relatively small sample size of patients with large tumors. Moreover, some potentially chemopreventive drugs, such as NSAIDs or aspirin, statins, and metformin, have been interested in lowering the risk of HCC recurrence,23, 26, 43 but their effects were not significant in the present study. Further well-controlled researches should be carefully designed for investigation of the chemopreventive effects of these drugs.
There were several limitations in this study. First, although we followed some of the steps used in randomized, controlled trials and used many methods to match potential confounders, a causal relationship between NA therapy and risk of HCC recurrence cannot be directly inferred based on the results of this observational study. A well-designed prospective study would be helpful for confirmation of our findings. Second, even though this study enrolled what is, to date, the largest cohort for analysis of NA therapy in prevention of HCC recurrence post-RFA, the sample size is not large enough for a long-term follow-up. RFA has become a popular HCC treatment in Taiwan in recent years, so a significant proportion of data were treated as censored in this study. However, our results clearly demonstrate the effect of 2 years of NA therapy. Third, our database does not provide detailed laboratory data, such as ALT level and HBV viral load. However, patients in the NA-treated group had more severe hepatitis than those in the untreated group according to the health insurance reimbursement criteria; for example, patients with cirrhosis who are eligible for NA therapy must have blood HBV-DNA titers >2,000 IU/mL. The protective effect of NA therapy might be underestimated, but the conclusion of this study remained unchanged. Fourth, by the definition of HCC recurrence in this study, it is possible that HCC recurrence was not detected if patients did not receive any treatment for the recurrent tumors. However, most patients who underwent RFA as a curative treatment were in early stage, and rapid progression to untreatable disease was probably very unlikely. In addition, patients in the treated group received regular follow-up for NA refills, but some patients in the untreated group might be followed up irregularly. The diagnosis or treatment for HCC recurrence was more likely to be missed in the untreated group. The differences of tumor recurrence between both study groups might have been underestimated, but the major finding of the present study was not affected. Last, we could not rule out the possibility that some patients paid out of pocket for NA therapy and were classified into the untreated cohort. However, even if misclassification did result in underestimation of the protective effect of NA therapy, the conclusion of this study was not changed.
In summary, NA therapy was associated with a decreased risk of HCC recurrence among patients with HBV-related HCC post-RFA, and the protective effect could be observed even in elderly and patients with cirrhosis.
Acknowledgment
This work was conducted using data retrieved from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, the Department of Health, and managed by the National Health Research Institutes, Taiwan. The interpretation and conclusions presented herein do not represent those of the Bureau of National Health Insurance, the Department of Health, or the National Health Research Institutes, Taiwan. There are no conflicts of interest to declare.




