CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice
Potential conflict of interest: Dr. Zhu received grants from Bayer and Onyx. Dr. Reiberger consults for Xtuit and received grants from Roche, MSD, Phenex, and Gilead. Dr. Jain consults and owns stock in Enlight, Ophthotech, and SynDevRx. He owns stock and is employed by XTuit. He received grants from Dyax, MedImmune, and PureTech. Dr. Brainard owns stock and is employed by Gilead.
Supported by National Institutes of Health grants P01-CA080124, R01-CA159258, R21-CA139168, and R01-CA126642; National Cancer Institute/Proton Beam Federal Share Program awards (to D.G.D. and R.K.J.), American Cancer Society grant 120733-RSG-11-073-01-TBG (to D.G.D.), a Max Kade Fellowship (to T.R.), and a Postdoctoral Fellowship from Astellas Foundation for Research on Metabolic Disorders, Japan (to T.H.).
Abstract
Sorafenib, a broad tyrosine kinase inhibitor, is the only approved systemic therapy for advanced hepatocellular carcinoma (HCC) but provides limited survival benefits. Recently, immunotherapy has emerged as a promising treatment strategy, but its role remains unclear in HCCs, which are associated with decreased cytotoxic CD8+ T-lymphocyte infiltration in both murine and human tumors. Moreover, in mouse models after sorafenib treatment intratumoral hypoxia is increased and may fuel evasive resistance. Using orthotopic HCC models, we now show that increased hypoxia after sorafenib treatment promotes immunosuppression, characterized by increased intratumoral expression of the immune checkpoint inhibitor programmed death ligand-1 and accumulation of T-regulatory cells and M2-type macrophages. We also show that the recruitment of immunosuppressive cells is mediated in part by hypoxia-induced up-regulation of stromal cell–derived 1 alpha. Inhibition of the stromal cell–derived 1 alpha receptor (C-X-C receptor type 4 or CXCR4) using AMD3100 prevented the polarization toward an immunosuppressive microenvironment after sorafenib treatment, inhibited tumor growth, reduced lung metastasis, and improved survival. However, the combination of AMD3100 and sorafenib did not significantly change cytotoxic CD8+ T-lymphocyte infiltration into HCC tumors and did not modify their activation status. In separate experiments, antibody blockade of the programmed death ligand-1 receptor programmed death receptor-1 (PD-1) showed antitumor effects in treatment-naive tumors in orthotopic (grafted and genetically engineered) models of HCC. However, anti-PD-1 antibody treatment had additional antitumor activity only when combined with sorafenib and AMD3100 and not when combined with sorafenib alone. Conclusion: Anti-PD-1 treatment can boost antitumor immune responses in HCC models; when used in combination with sorafenib, anti-PD-1 immunotherapy shows efficacy only with concomitant targeting of the hypoxic and immunosuppressive microenvironment with agents such as CXCR4 inhibitors. (Hepatology 2015;61:1591–1602)
Abbreviations
-
- CXCR4
-
- C-X-C receptor type 4
-
- EMT
-
- epithelial-to-mesenchymal transition
-
- FITC
-
- fluorescein isothiocyanate
-
- Gr-1
-
- myeloid differentiation antigen
-
- HCC
-
- hepatocellular carcinoma
-
- IFN
-
- interferon
-
- IL
-
- interleukin
-
- Mst
-
- mammalian sterile 20-like 1
-
- PD-1
-
- programmed death receptor-1
-
- PD-L1
-
- programmed death ligand-1 (CD274)
-
- SDF1α
-
- stromal cell–derived factor 1 alpha
-
- TAM
-
- tumor-associated macrophage
-
- TNF
-
- tumor necrosis factor
-
- Treg
-
- T-regulatory cell
-
- VEGF
-
- vascular endothelial growth factor
Sorafenib is a multitargeted tyrosine kinase inhibitor and a worldwide standard of care for advanced hepatocellular carcinoma (HCC) patients based on increased survival data from two phase III trials.1, 2 However, these studies also showed that HCCs rapidly become sorafenib-resistant, with a short time to progression. Due to promiscuous target inhibition by sorafenib, the mechanisms of treatment evasion are likely multifactorial. One mechanism may be the increase in tissue hypoxia after prolonged antiangiogenic therapy, which likely promotes tumor recurrence locally and at distant sites.3-5 Hypoxia can fuel resistance to treatment not only by promoting genomic instability, angiogenesis, and invasion but also by creating an immunosuppressive microenvironment.4, 6-10 Increased hypoxia results in recruitment and activation of multiple myeloid and lymphoid immune suppressor cells such as M2-type tumor-associated macrophages (TAMs), myeloid-derived suppressor cells, and T-regulatory cells (Tregs).10, 11 Indeed, we have previously found that alleviating hypoxia in breast cancers enhanced the efficacy of a vaccine therapy.12 In HCC, we demonstrated that increased hypoxia after sorafenib treatment induced stromal cell–derived 1 alpha (SDF1α) and C-X-C receptor type 4 (CXCR4) expression and myeloid differentiation antigen Gr-1+ myeloid-derived suppressor cell recruitment.13 Blockade of the SDF1α/CXCR4 axis prevented the increase in tumor desmoplasia and inhibited tumor growth despite persistent hypoxia.13
Discovery of the mechanistic link between hypoxia, inflammation, fibrosis, and HCC progression involving the SDF1α/CXCR4 pathway prompted us to test whether sorafenib also modulates the immune microenvironment in HCC through this pathway. Clinical data regarding the presence, infiltration, and function of T-infiltrating lymphocytes in HCC are limited. Case reports and a cohort study report the rare presence of T-infiltrating lymphocytes in human HCCs.14-16 Moreover, the presence and function of T-infiltrating lymphocytes may be a prognostic marker in HCC patients.17 Therefore, a combination of depletion of Tregs and concomitant stimulation of effector T cells may represent an effective strategy to reduce HCC metastasis and recurrence.18, 19 T-cell activation using antibody blockade of the immune checkpoint programmed death receptor-1 (PD-1) has been successfully used for the treatment of late-stage melanoma, and an anti-PD-1 antibody (pembrolizumab) was recently granted accelerated approval by the US Food and Drug Administration. Anti-PD-1 antibodies have also shown efficacy in other solid tumors.20 But achieving similar efficacy with immune checkpoint inhibitors in HCC will largely depend on how they are integrated with sorafenib. For example, it has also been recently shown that cancer cells, cancer-associated stromal cells, and a hypoxic tumor microenvironment can up-regulate immune regulatory proteins—immune checkpoint inhibitors such as programmed death ligand-1 (PD-L1) or its receptor PD-1—that facilitate tumor escape from immune surveillance.21, 22 Up-regulation of PD-L1/PD-1 inhibits cytotoxic CD8+ T-lymphocyte activation and proliferation and further contributes to resistance and progression of solid tumors. However, until now experimental data on the direct effects of sorafenib on cancer cells and the immune microenvironment in HCCs are lacking. Here, we used orthotopic (grafted and genetically engineered) murine models of HCC to examine the role of targeting the CXCR4 pathway in the absence or presence of PD-1 blockade on primary tumor growth, lung metastasis, and the immune microenvironment after sorafenib treatment.
Materials and Methods
Cells
We used the murine HCC cell line HCA-113 and human HCC cell lines JHH-7 and Hep3B (ATCC) (Supporting Information).
HCC Models
Orthotopic implantation was performed as described (Supporting Information).13 We also induced hepatocarcinogenesis and liver fibrosis in mammalian sterile 20-like 1 (Mst1–/–Mst2F/–) mice, as described (Supporting Information).13, 23
Imaging
The growth of intrahepatic HCCs was evaluated by high-frequency ultrasound imaging (VisualSonics, Toronto, Canada) by operators blinded to the treatment (Supporting Fig. S1). The presence of abdominal metastasis was defined as extrahepatic tumor mass infiltrating neighboring organs, significant lymph node enlargement, or peritoneal carcinomatosis. Presence and number of lung metastases were recorded for the different treatment groups as assessed macro- and microscopically in both lungs after killing the animals.
Treatment
The maximal tumor diameters were measured in two dimensions to calculate tumor volume by the formula (a/2 × b/2 × b/2) × 4/3 × Pi. Tumor dimensions were either measured by calipers at the experimental end point (after 2 weeks of treatment) or by ultrasound starting 1 week after implantation, where tumor size was typically 3 × 3 mm (corresponding to a tumor volume of ∼14 mm3). At that time, mice were randomized according to tumor size to the treatment groups to ensure similar tumor sizes at baseline (pretreatment) when treatment was started. Treatment included daily gavage with phosphate-buffered saline/vehicle (control group) or sorafenib at a dose of 50 mg/kg (in phosphate-buffered saline/1% Tween80; MGH Pharmacy) and with the CXCR4 inhibitor AMD3100 (10 mg/kg body weight, subcutaneously by osmotic minipumps; Sigma). In separate experiments, these treatments were combined with antibody against murine PD-1 (100 µg intraperitoneally every 3 days, for a total of five times).
Cell Viability Assays
Cell viability was assessed using the nucleic acid stain SYTO-60 (Invitrogen). Cells (3000 per well) were seeded into 96-well plates, allowed to adhere overnight, and exposed to a range of drug concentrations. After 72 hours, cells were fixed in 4% formaldehyde and stained with SYTO-60 and cell viability was assessed by fluorescence measurement using a SpectraMax M5 microplate reader (excitation 630 nm, emission 695 nm; Molecular Devices). The fraction of control was calculated by dividing the fluorescence obtained from the drug-treated cells by the fluorescence obtained from the control (no drug)–treated cells.
Assessment of Apoptosis by Terminal Deoxynucleotidyl Transferase–Mediated Deoxyuridine Triphosphate Nick-End Labeling Staining and Cleaved Caspase-3 Staining
Frozen sections of HCC tissues were stained using the TACS TdT Kit (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol. Apoptotic cells were counted in four randomly selected visual fields for each sample. The apoptotic index was calculated as the fraction of apoptotic nuclei.
Immunohistochemistry and Immunofluorescence
For evaluation of vascular density, frozen tumor sections (7-8 µm thick) were immunostained with primary antibodies against CD31 (for endothelial cells) and counterstained with fluorescent secondary antibodies. Samples were imaged by using an Olympus confocal microscope and quantified using four or five random fields per sample. Pimonidazole (Hypoxyprobe; Hypoxyprobe, Inc.) was used as a marker of hypoxia. Hypoxyprobe (60 mg/kg body weight) was injected intraperitoneally 30 minutes before the animals were killed. Tumors were harvested and processed immediately. Hypoxia was assessed in frozen tissue sections by immunofluorescence staining of pimonidazole by anti-Hypoxyprobe-fluorescein isothiocyanate (FITC)–labeled antibody.
To evaluate the number and distribution of CD8+ T lymphocytes, frozen sections of HCC samples were stained for CD8+ cells and nuclear staining with 4′,6-diamidino-2-phenylindole. Whole tumor sections were scanned using mosaic confocal microscopy. The number of CD8+ T lymphocytes was recorded both in the tumor margin (200 µm) and in the tumor center (three tumor samples per group were assessed, six random regions were selected both for tumor margin and for tumor center: four groups × three mice × six peripheral × six central tumor regions). In addition, we performed immunostaining for CD8 and PD-L1/CD274 in human HCC samples (n = 16), obtained under an approved institutional review board protocol. We evaluated the number and distribution of intratumoral CD8+ T lymphocytes (margin versus center) in a similar manner.
Flow Cytometry
Tumor tissues were harvested and prepared for flow cytometry as previously described (Supporting Information).12 Flow-cytometric data were obtained using an LSR-II flow cytometer (Becton Dickinson) and analyzed with FACSDiva software. We used the following monoclonal anti-mouse antibodies: CD4-FITC, CD4-PE-Cy7, CD8a-FITC, CD8a-PE, CD45-PE, CD45-PE-Cy7, CD25-APC-Cy7, FoxP3-APC, Gr-1-APC, and CD11b-APC-Cy7 (BD Biosciences), as well as F4/80-FITC and F4/80-PE (eBioscience) (see Supporting Fig. S2).
Western Blotting
We used antibodies for PD-L1 (Abcam, Cambridge, MA) to measure the levels of this immune checkpoint in HCC tissues and HCA-1 cells (Supporting Information).
Quantitative Reverse-Transcriptase Polymerase Chain Reaction
Total RNA was extracted with the RNeasy Mini-Kit (Qiagen) from flow cytometry–sorted viable TAMs (7AAD–CD45+F4/80+Gr-1– cells). Complementary DNAs were synthesized using the TaqMan RT Kit (Applied Biosystems, Branchburg, NJ). We used specific primers for β-actin, arginase-1, interleukin-1β (IL-1β), IL-10, IL-12, tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase, chemokine C-C motif ligands 17 and 22 (CCL17, CCL22), CXCR4, chemokine C-X-C motif ligand 10 (CXCL10), matrix metalloproteinase-9, and transforming growth factor-β and determined the relative level of gene expression using the Real-Time SBYR Green PCR master mix (Applied Biosystems) on a Stratagene Mx3000P qPCR System, as described.12
Protein Measurements Using a Multiplexed Enzyme-Linked Immunosorbent Assay
Frozen tumors of each group were prepared using a lysis cocktail buffer (radioimmunoprecipitation assay buffer; Boston Bioproducts, Inc., Boston, MA) including phosSTOP, cOmplete (Roche, Indianapolis, IN). Tumors were homogenized with a tissue homogenizer (Qiagen, Valencia, CA), and the suspensions were sonicated using a sonicator (Branson, Notre Dame, IN). All samples were prepared at a protein concentration of 2 µg/mL. The proinflammatory Panel 1 Mouse V-PLEX kit was used to measure the concentration of several cytokines: IL-1β, IL-2, IL-6, IL-8, IL-12, interferon-γ (IFN-γ), TNF-α, and KC-Gro (Meso Scale Discovery, Rockville, MD).
Statistical Analysis
All statistical analyses were performed by Graph Pad Prism software. The Student t test or the Mann-Whitney U test was used according to data distribution. Numbers of animals (proportions) with metastases were compared by chi-squared test. P < 0.05 was considered statistically significant.
Results
CXCR4 Inhibition During Sorafenib Treatment Has Antivascular and Antimetastatic Effects and Delays HCC Progression
Blockade of the SDF1α/CXCR4 axis by continuous infusion of AMD3100 using osmotic pumps significantly delayed the primary growth of orthotopic HCA-1 tumors in immunocompetent C3H mice when combined with daily sorafenib treatment compared to either treatment alone (Fig. 1A). In the HCA-1 HCC model, mice develop spontaneous lung metastases that are detectable 28 days after intrahepatic tumor implantation. Despite delaying the primary tumor growth, sorafenib did not affect metastasis formation or overall survival compared to control-treated mice (Fig. 1B,C). In contrast, the addition of AMD3100 treatment significantly inhibited lung metastasis and led to an increase in overall survival (Fig. 1B,C). The tumor growth delay was seen after combination therapy was reproduced in orthotopic human HCC xenograft models using Hep3B and JHH-7 cells (Supporting Fig. S3). Of interest, tumor growth inhibition occurred in the face of minor direct effects of recombinant SDF1α or AMD3100 on HCC or endothelial cell proliferation after sorafenib treatment in vitro (Supporting Fig. S4). However, sorafenib treatment significantly reduced intratumoral microvascular density and increased hypoxia and SDF1α expression, and addition of AMD3100 to sorafenib enhanced the antivascular effects of treatment (Supporting Fig. S5). Moreover, we found that CXCR4 inhibition prevented the increase in epithelial-to-mesenchymal transition (EMT) markers in HCC cells cultured in hypoxic conditions, in a dose-dependent manner (Supporting Fig. S6). Thus, the inhibition of HCC progression induced by sorafenib and AMD3100 in these HCC models is at least in part due to tumor microenvironment–mediated effects.
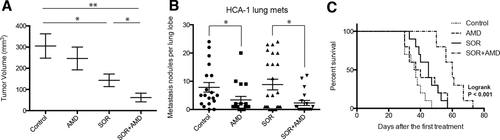
Treatment with the SDF1α/CXCR4 inhibitor AMD3100 plus sorafenib inhibits primary tumor growth and incidence of lung metastasis formation and improves overall survival in orthotopic HCC models. (A) While sorafenib (SOR) treatment alone marginally delays HCC growth, the addition of AMD3100 (AMD) to SOR—but not AMD alone—induces an additional significant delay in tumor growth (n = 8). (B) The number of lung metastatic nodules is significantly reduced in AMD-treated mice. (C) Overall survival is significantly prolonged only in orthotopic HCC-bearing mice treated with SOR and AMD. Data are representative of at least two independent experiments and are presented as mean ± SEM (n = 10), *P < 0.05, **P < 0.01.
CXCR4 Inhibition Prevents the Polarization Toward an Immunosuppressive HCC Microenvironment During Sorafenib Treatment
We next examined the effects of sorafenib treatment on tumor inflammatory cell infiltration by flow-cytometric analyses of enzymatically digested HCC tissue. While the fraction of CD45+ immune cells of the total number of viable cells did not change significantly, we found that sorafenib increased the numbers of F4/80+ TAMs and CD11b+Gr-1+ and CD45+CXCR4+ myeloid cells in both HCA-1 and JHH-7 HCC models (Fig. 2A-C, Supporting Fig. S7). Moreover, sorafenib treatment resulted in an increase in the fraction of tumor-infiltrating CD4+CD25+FoxP3+ Tregs in HCA-1 tumors (P < 0.05) (Fig. 2D). Addition of AMD3100 to sorafenib significantly decreased the fraction of F4/80+ TAMs, CD11b+Gr-1+ myeloid cells, and CD4+CD25+FoxP3+ Tregs in the orthotopic HCA-1 model to levels comparable to those of treatment-naive (control) HCCs (Fig. 2).
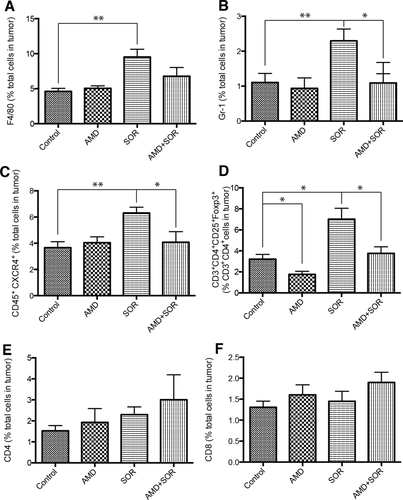
Sorafenib treatment induces a polarization toward a proimmunosuppressive environment in orthotopic HCA-1 tumors, which is prevented by CXCR4 inhibition in the face of persistent hypoxia. (A-D) Changes in viable tumor-infiltrating immune cells in HCA-1 tumors from mice treated with sorafenib with or without AMD3100 versus control, analyzed by flow cytometry. The number of 7AAD–CD45+F4/80+ tumor-associated macrophages (A), 7AAD–CD11b+Gr-1+ monocytes (B), 7AAD–CD45+CXCR4+ cells (C), and 7AAD–CD4+CD25+FoxP3+ Treg cells (D) significantly increased in sorafenib-treated HCCs. Combining AMD3100 treatment with sorafenib prevents these effects. (E-F) The number of 7AAD–CD4+CD3+ (E) and 7AAD–CD8+CD3+ (F) T lymphocytes was not significantly different between the four treatment groups in HCA-1 HCCs. Data are shown as mean ± SEM, *P < 0.05.
Both vascular endothelial growth factor (VEGF) and SDF1α have been reported to mediate the trafficking and retention of tumor-infiltrating myeloid (bone marrow–derived) cell populations (F4/80+ TAMs, Gr-1+ monocytic and granulocytic populations) in other tumor types and in the normal liver.24-28 However, it is currently unknown how inhibitors of these pathways affect bone marrow–derived cell activation and functional cytokine secretion. To examine this, we sorted F4/80+ TAMs using flow cytometry from digested HCC tissue after 14 days of treatment, extracted mRNA, and performed quantitative polymerase chain reaction analysis to measure widely accepted markers of angiogenesis and of M1 and M2 types of activation.8, 29 The TAMs from sorafenib-treated HCCs expressed more than two-fold higher levels of CXCR4 and VEGF as well as the M2-type markers CCL22 and arginase-1 compared to those from control HCCs (Supporting Table S1). On the other hand, the expression of the M1-type markers was not significantly altered in TAMs in sorafenib-treated HCCs (Supporting Table S1). AMD3100 alone or in combination with sorafenib reduced the expression of M2-type markers in TAMs without affecting that of M1-type markers (Supporting Table S1). To examine whether sorafenib and AMD3100 treatments also affect the infiltration and proliferation of T lymphocytes in HCC, we further assessed the fraction of tumor-infiltrating CD4+ and CD8+ T lymphocytes. Combination treatment with sorafenib and AMD3100 or sorafenib alone did not increase the fraction of CD4+ and CD8+ T lymphocytes (Fig. 2E,F). Thus, blockade of the SDF1α/CXCR4 axis may inhibit sorafenib-induced immunosuppression in HCC but is insufficient to enhance T-lymphocyte infiltration.
PD-L1 Is Expressed by HCC Cells and Is Increased After Sorafenib Treatment
Hypoxia has been shown to upregulate the expression of immune regulatory proteins such as PD-1/PD-L1, which inhibit cytotoxic CD8+ T-lymphocyte proliferation and activation. We next evaluated if treatment-induced hypoxia increases PD-L1 expression in HCA-1 tumors. Immunocompetent mice bearing orthotopic HCA-1 tumors were treated when the tumor reached a size of 14 mm3. Tumors were treated with sorafenib for 28 days, and then the mice were killed and the tumors collected. Western blot analysis showed that sorafenib treatment resulted in increased PD-L1 expression in HCA-1 tumors compared with control treated tumors (Fig. 3A). We next examined the cell types that express PD-L1 in HCC. We found that cultured HCA-1 cells expressed detectable levels of PD-L1 (Supporting Fig. S8A). By flow cytometry of digested tumor tissue, we detected, as expected, expression of PD-L1 on multiple hematopoietic cells, including on a fraction of Gr-1+ cells, TAMs, dendritic cells, and lymphocytes (Supporting Table S2). In addition, analysis of resected human HCC tissues showed PD-L1 expression in both cancer cells and immune cells infiltrating the tumor stroma (Supporting Fig. S8B,C). Finally, immunofluorescence analysis of HCA-1 tumors showed colocalization of PD-L1 expression with pimonidazole staining of hypoxic tissue (Supporting Fig. S8D). These data suggest that PD-L1 expression by the cancer cells and HCC stromal cells, particularly in hypoxic regions, may play a role in immunosuppression after sorafenib treatment.

In grafted and genetically engineered models PD-1 blockade is active against HCC and is facilitated by sorafenib when combined with CXCR4 inhibition. (A) Orthotopic HCA-1 tumors were treated when the tumor reached a size of 14 mm3 with sorafenib for 28 days, and then the tumors were collected. Western blot analysis shows that PD-L1 expression is increased in HCC tissue after sorafenib treatment compared to control treated tumors. (B) Either PD-1 blockade alone or the combination of sorafenib/AMD3100 significantly delayed tumor growth (both P < 0.05 versus control). The most effective tumor growth delay was achieved by the triple-combination therapy of sorafenib/AMD3100/anti-PD-1 (P = 0.013 versus control). (C) While the number of lung metastases was significantly reduced by anti-PD-1 treatment or the combination of sorafenib/AMD3100, the triple therapy of sorafenib/AMD3100/anti-PD-1 resulted in the most pronounced decrease in lung metastases incidence. (D) In the Mst-mutant mouse model PD-1 blockade alone (P = 0.04 versus control), but not in combination with SOR, significantly delayed HCC growth (not significant). The most effective tumor growth delay and regression were achieved by the triple-combination therapy of sorafenib/AMD3100/anti-PD-1 (P = 0.007 versus control). Data are presented as mean ± SEM (n = 6 per group), *P < 0.05, **P < 0.005.
PD-1 Blockade Combined With CXCR4 Inhibition and Sorafenib Delays HCC Growth
We next examined whether blocking PD-1 improves the efficacy of sorafenib and CXCR4 inhibition during persistent hypoxia. To this end, we assessed the efficacy of anti-PD-1 treatment alone or in combination with sorafenib plus or minus AMD3100 (Supporting Fig. S9A). Average body weight was similar across all treatment groups, and no other signs of toxicity were observed. Wound healing (after median laparotomy) was not impaired in any of the treatment groups. As expected, the sorafenib/AMD3100 combination significantly inhibited HCA-1 tumor growth. Moreover, anti-PD-1 treatment alone showed comparable efficacy. Interestingly, combining sorafenib with anti-PD-1 treatment did not show efficacy, while the triple-combination group (sorafenib/AMD3100/anti-PD-1) showed the most pronounced delay in tumor growth versus the control group (Fig. 3B, Supporting Fig. S10A). Furthermore, the number of lung metastases was significantly reduced by anti-PD-1 treatment alone and by sorafenib plus AMD3100, and it was further inhibited by addition of anti-PD-1 treatment (Fig. 3C). Neither sorafenib alone nor anti-PD-1 antibody with sorafenib affected spontaneous lung metastasis formation.
Next, we sought to further confirm the activity of anti-PD-1 therapy in a genetically engineered (Mst-mutant) model of HCC in mice with cirrhotic livers (Supporting Fig. S9B).13 Using ultrasound imaging in this model, we found that anti-PD-1 treatment may stabilize the growth of established HCCs. Moreover, we found that the combination of anti-PD-1 antibody with sorafenib and AMD3100—but not anti-PD-1 antibody with sorafenib alone—led to regression of established tumors (Fig. 3D, Supporting Fig. S10B).
PD-1 Blockade Combined With CXCR4 Inhibition and Sorafenib Increases Tumor Cell Death In Vivo
We next evaluated whether the efficacy seen with addition of anti-PD-1 treatment to sorafenib plus AMD3100 is due to increased tumor cell death. We found that tumors in the triple-combination group had extensive areas of tumor necrosis in the HCA-1 tumors, which were not detectable to a similar extent in any of the other treatment groups (Fig. 4A). We next quantified cell apoptosis and found that the addition of anti-PD-1 to sorafenib and AMD3100 dramatically increased the number of cleaved caspase-3–positive cells in tumors compared to the other groups (Fig. 4B). Similar findings were obtained after analyzing HCC tissues from Mst-mutant mice (Fig. 4C). Thus, adding anti-PD-1 antibody to sorafenib and AMD3100 treatment significantly decreases primary tumor growth by triggering tumor necrosis and apoptosis and further reduces the incidence of lung metastases.
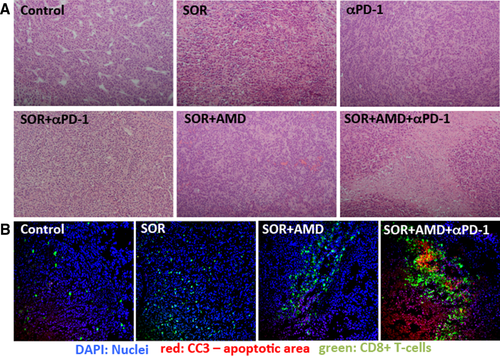
Anti-PD-1 treatment combined with sorafenib and AMD3100 enhances antitumor immune responses in HCC. (A) Representative hematoxylin and eosin staining shows rare areas of necrosis in untreated tumors and some necrotic areas in sorafenib- or anti-PD-1 antibody only–treated HCA-1 tumors. Sorafenib plus AMD3100 treatment resulted in further enhancement of tumor necrosis, and the triple-combination treatment resulted in extensive necrosis, with lymphocytic immune cell infiltration in the necrotic areas (10× magnification). (B) Representative immunofluorescence staining for CD8+ T cells (FITC, green) and cleaved caspase-3 (Cy5, red) in frozen HCA-1 tumor sections. Counterstaining of nuclei by 4′,6-diamidino-2-phenylindole (blue). Tumor-infiltrating cytotoxic CD8+ cells colocalized with areas of cell apoptosis only in the triple-combination treatment group. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole.
Addition of Anti-PD-1 Treatment to Sorafenib and AMD3100 Increases Intratumoral Penetration and Activation of CD8+ T Lymphocytes in HCC
We first examined the pattern of lymphocyte distribution in treatment-naive human HCCs and found significantly reduced numbers of CD8+ T lymphocytes penetrating into the tumor proper compared to the tumor margin (Supporting Fig. S11). We next performed a similar analysis as well as flow-cytometric analyses of lymphocytic populations in the murine HCCs. We also evaluated whether adding anti-PD-1 treatment increases or activates antitumor T lymphocytes. We first assessed the percentages of CD8+ T lymphocytes (cytotoxic T cells) in HCC tissues from mice in different treatment groups by flow cytometry. We found no difference in the fraction of these cells normalized either to the total number of CD45+ tumor-infiltrated leukocytes or to the total number of cells across treatment groups in HCA-1 tumor grafts or HCCs induced in Mst-mutant mice (Supporting Fig. S12). We then examined the distribution of the CD8+ T lymphocytes in tumor sections by immunofluorescence staining. Sorafenib alone did not alter CD8+ T-lymphocyte number or distribution, and the combination of sorafenib and AMD3100 only slightly increased the number of CD8+ T lymphocytes localized deeper within the primary tumors. Addition of anti-PD-1 treatment to sorafenib and AMD3100 significantly increased cell intratumoral penetration by CD8+ T lymphocytes in both models (Fig. 5). The tumor-infiltrating CD8+ T lymphocytes colocalized with apoptotic cells within the HCCs (Fig. 4B).
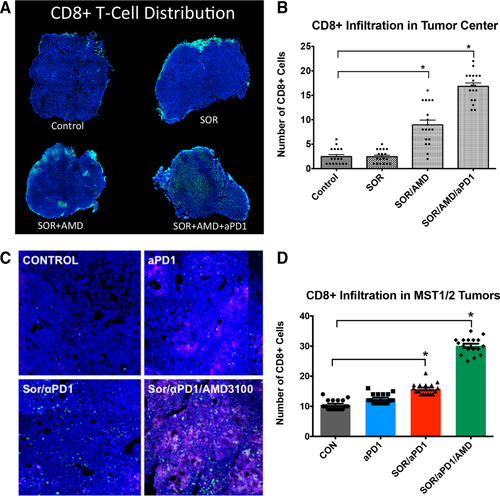
Anti-PD-1 treatment combined with sorafenib and AMD3100 increases intratumoral CD8+ T-lymphocyte distribution in HCC. (A-D) The number of cytotoxic T lymphocytes infiltrating the tumor proper is increased only in the sorafenib+AMD3100+αPD-1 treatment group. Representative confocal microscopy of immunofluorescence for CD8+ T lymphocytes (FITC, green) in HCC tissue sections (nuclei by 4′,6-diamidino-2-phenylindole, in blue) from HCA-1 grafted tumors (A) and representative regions of HCC tumors in the Mst genetically engineered mouse model. Red areas in the MST tumors indicate apoptotic regions of MST HCC tumors (staining for cleaved-caspase 3, Cy5 in red) (C). (B,D) Addition of anti-PD-1 antibody changed the distribution of CD8+ T lymphocytes in the tumors: triple-combination treatment resulted in significantly higher numbers of cytotoxic T lymphocytes in the tumor center. Data are mean ± SEM (n = 5-6 per group), *P < 0.05 versus control.
To test whether these tumor-infiltrating CD8+ T lymphocytes were activated after PD-1 blockade, we evaluated biomarkers of activation, i.e., IFN-γ, IL-2, and TNF-α expression levels. Enzyme-linked immunosorbent assay showed that addition of anti-PD-1 treatment to sorafenib and AMD3100 significantly increased the intratumoral levels of IFN-γ, IL-2, and TNF-α by 50%-130% compared to control (Fig. 6). In contrast, sorafenib or anti-PD-1 alone and the combination treatment of sorafenib plus AMD3100 or sorafenib plus anti-PD-1 did not significantly change IFN-γ, IL-2, and TNF-α expression levels (Fig. 6). Similarly, only triple-combination treatment significantly increased IFN-γ, IL-2, and TNF-α expression levels in genetically induced tumors in Mst-mutant mice (Supporting Fig. S13).
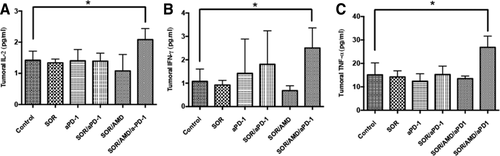
Addition of anti-PD-1 antibody to sorafenib and CXCR4 inhibition increases the expression of biomarkers of T-lymphocyte activation in HCC. (A-C) In HCA-1 tumors versus HCCs in other treatment groups PD-1 blockade combined with sorafenib and AMD3100 induced a significant increase of the T-lymphocyte activation biomarkers IL-2 (A), TNF-α (B), and IFN-γ (C). Data are presented as mean ± SEM (n = 5 per group), *P < 0.05.
Discussion
Hepatocellular carcinoma is a highly vascularized tumor, and the antiangiogenic agent sorafenib showed increased survival in two large phase III trials in HCC patients.1, 2 Unfortunately, recent experience with various other antiangiogenic agents has shown that many HCC tumors are highly resistant to antiangiogenic therapy.30, 31 Several recent reports concluded that the increase in recruitment of myeloid-derived cell populations to tumors represents a critical step in the emergence of resistance to anti-VEGF therapy and facilitates tumor progression (reviewed in Duda et al.32). Indeed, we have previously shown in orthotopic (both grafted and genetically engineered) models of HCC in mice that increased intratumoral hypoxia after sorafenib treatment led to increased SDF1α expression, which promoted treatment resistance. We now show that the SDF1α/CXCR4 axis also promotes tumor vascularization, likely by recruitment of proangiogenic myeloid cells.33 In addition to proangiogenic and proinflammatory effects, hypoxia can trigger EMT in cancer cells, which may play an important role in tumor progression and particularly in metastasis.34 We show that the SDF1α/CXCR4 pathway can directly mediate transition to an EMT phenotype in HCC cells in a hypoxic microenvironment. Collectively, these data may explain the unaltered progression of the disease at distant sites in the face of sorafenib treatment. Indeed, CXCR4 blockade prevented EMT despite persistent hypoxia, reduced metastatic burden, and increased survival in mice with HCC. These findings may be relevant not only for sorafenib but also for any other hypoxia-inducing therapy in HCC.
We also found that, in addition to Gr-1+ myeloid cells, there is an increase in M2-type TAMs and Tregs in HCA-1 tumors after sorafenib treatment, indicating the induction of an immunosuppressive microenvironment in sorafenib-treated HCCs. In these HCCs CXCR4 blockade reduced the infiltration of these immunosuppressive cells despite persistent hypoxia but failed to promote antitumor cellular immune responses. Here, we provide proof-of-principle data that addition of the immune checkpoint inhibitor anti-PD-1 antibody to AMD3100 and sorafenib treatment is safe and could facilitate antitumor immune responses by increasing the infiltration and activation of CD8+ T lymphocytes inside the tumor. The triple-combination treatment inhibited both the growth of the primary tumor and the formation of lung metastases in orthotopic murine HCCs and regressed established tumors in a genetically engineered mouse model of HCC in mice with underlying liver cirrhosis. Although clinical translation of a triple therapy including sorafenib and two immune modulating agents seems challenging, our data highlight the clinical relevance of studying the role of the immune microenvironment in resistance to antiangiogenic treatment as well as for the future development of immunotherapy in HCC.
Immune checkpoint inhibition is a very promising approach across various tumor types, with multiple clinical trials ongoing, including a trial in HCC.18 A challenging aspect for the development of immunotherapies will be their integration in existing therapeutic regimens.18 Thus, our study may have important implications for the development of immunotherapy in HCC. In line with previous reports, we show that intratumoral CD8+ T-lymphocyte infiltration is limited in human HCC. Moreover, we show that the antivascular effects of sorafenib promote hypoxia-mediated immunosuppression, including increased PD-L1 expression in HCC. We also demonstrate that to enhance immunotherapy efficacy in HCC, approaches such as CXCR4 inhibition might be critical to prevent immunosuppression. Alternatively, dose titration of sorafenib or more selective anti-VEGF/VEGFR2 agents (such as antibodies) might help to inhibit angiogenesis without a pronounced increase in hypoxia and, thus, synergize with novel immunotherapies against HCC.
In summary, in addition to promoting tumor desmoplasia,13 the SDF1α/CXCR4 pathway mediates stroma polarization toward an immunosuppressive microenvironment and contributes to systemic disease progression after antiangiogenic treatment in HCC (Fig. 7). Blockade of both CXCR4 and PD-1 prevents suppression of immune cell function in HCC tumors, enhances immune cell tumor penetration and activation, and ultimately delays HCC progression. Further understanding of the complex interaction between antiangiogenic, antimetastatic, and immunotherapeutic agents is warranted to facilitate progress in the systemic treatment of advanced HCC.
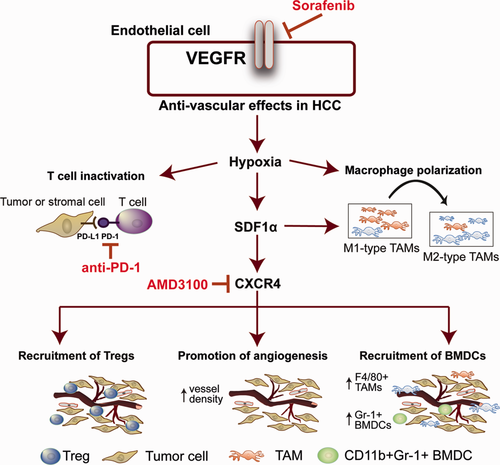
Sorafenib treatment induces SDF1α/CXCR4 axis–mediated immunosuppression in the HCC microenvironment.
Acknowledgment
We thank V. Chauhan, I. Chen, G. Lauwers, S. Pillai, and T. Vardam for useful discussions and A. Khachatryan, O. Pulluqi, S.C. Min, and C. Smith for their outstanding technical support.
References
Author names in bold designate shared co-first authors.




