Gankyrin promotes tumor growth and metastasis through activation of IL-6/STAT3 signaling in human cholangiocarcinoma
Potential conflict of interest: Nothing to report.
Supported by the China National Natural Science Foundation (No. 81272705 and No. 81201878), Program for Innovative Research Team by Chinese Ministry of Education (IRT1122), Program for Innovative Research Team (in Science and Technology) in Higher Educational Institutions of Heilongjiang Province (2009td06), Heilongjiang Province Science Fund for Outstanding Youths (JC200616), Foundation of Harbin Science and Technology Bureau for Creative Young Talents (2010RFQXS069), and Foundation of Health Department of Heilongjiang Province (Grant No. 2009-043). The funders had no role in study design, data collection, or analysis, decision to publish, or preparation of the article.
Abstract
Although gankyrin is involved in the tumorigenicity and metastasis of some malignancies, the role of gankyrin in cholangiocarcinoma (CCA) is unclear. In this study we investigated the expression of gankyrin in human CCA tissues and cell lines. The effects of gankyrin on CCA tumor growth and metastasis were determined both in vivo and in vitro. The results showed that gankyrin was overexpressed in CCA tissues and cell lines. Gankyrin expression was associated with CCA histological differentiation, TNM stage, and metastasis. The multivariate Cox analysis revealed that gankyrin was an independent prognostic indicator for overall survival. Gankyrin overexpression promoted CCA cell proliferation, migration, and invasion, while gankyrin knockdown inhibited CCA tumor growth, metastasis, and induced Rb-dependent senescence and G1 phase cell cycle arrest. Gankyrin increased the phosphorylation of signal transducer and activator of transcription 3 (STAT3) and promoted the nuclear translocation of p-STAT3. Suppression of STAT3 signaling by small interfering RNA (siRNA) or STAT3 inhibitor interfered with gankyrin-mediated carcinogenesis and metastasis, while interleukin (IL)-6, a known upstream activator of STAT3, could restore the proliferation and migration of gankyrin-silenced CCA cells. The IL-6 level was decreased by gankyrin knockdown, while increased by gankyrin overexpression. Gankyrin regulated IL-6 expression by way of facilitating the phosphorylation of Rb; meanwhile, rIL-6 treatment increased the expression of gankyrin, suggesting that IL-6 was regulated by a positive feedback loop involving gankyrin in CCA. In the xenograft experiments, gankyrin overexpression accelerated tumor formation and increased tumor weight, whereas gankyrin knockdown showed the opposite effects. The in vivo spontaneous metastasis assay revealed that gankyrin promoted CCA metastasis through IL-6/STAT3 signaling pathway. Conclusion: Gankyrin is crucial for CCA carcinogenesis and metastasis by activating IL-6/STAT3 signaling pathway through down-regulating Rb protein. (Hepatology 2014;59:935–946)
Abbreviations
-
- AKT
-
- protein kinase B
-
- CCA
-
- cholangiocarcinoma
-
- CDK4
-
- cyclin-dependent kinase 4
-
- CRC
-
- colorectal cancer
-
- ERK
-
- extracellular signal-regulated kinase
-
- ESCC
-
- esophageal squamous cell carcinoma
-
- HCC
-
- hepatocellular carcinoma
-
- IHC
-
- immunohistochemical
-
- IL-6
-
- interleukin-6
-
- JNK
-
- c-Jun N-terminal kinases
-
- Lenti-shRNA
-
- lentivirus-mediated small hairpin RNA
-
- mRNA
-
- messenger RNA
-
- OS
-
- overall survival
-
- SA-β-gal
-
- senescence-associated β-galactosidase
-
- STAT3
-
- signal transducer and activator of transcription 3
-
- TBP
-
- TATA binding protein
-
- VEGF
-
- vascular endothelial growth factor
Cholangiocarcinoma (CCA) is the second most common primary hepatic malignancy after hepatocellular carcinoma (HCC).1 Epidemiologic studies showed that the incidence and mortality rates of CCA are increasing in several Western countries, including the U.S.2 Unfortunately, CCA is characterized by a poor prognosis and a very low 5-year survival rate.3 Currently, conventional radiotherapy and chemotherapy are not effective in prolonging long-term survival of CCA patients,4 thus the only curative treatment for CCA is surgical resection. However, most patients have advanced disease at the time of diagnosis and miss the optimal time for operation.5 Therefore, there is an urgent need to define the molecular mechanisms underlying CCA tumor growth and metastasis with a view to develop novel therapeutic strategies.
Gankyrin (also known as PSMD10), a small protein with seven ankyrin-repeat domains, was originally identified as a regulatory subunit of the 26S proteasome complex.6 Observations showed that gankyrin was expressed in almost all eukaryotic cells, especially in cancers, such as HCC,7-12 colorectal cancer (CRC),13 pancreatic cancer,14 esophageal squamous cell carcinoma (ESCC),15 and breast cancer.16 Gankyrin has been reported to negatively regulate several tumor suppressors, such as Rb and p53.17, 18 Gankyrin could bind with cyclin-dependent kinase 4 (CDK4) and facilitate the phosphorylation and degradation of Rb protein, thus leading to cell cycle progression.18 Although gankyrin has been proven to be involved in tumorigenesis and progression of different cancers, whether it contributes to tumor growth, or has other functions in CCA, still remains unclear.
In this study, we report gankyrin as an essential regulator of CCA tumor growth and metastasis by activating the interleukin-6 (IL-6)/signal transducer and activator of transcription 3 (STAT3) signaling pathway by way of negatively regulating Rb protein. These data strongly suggested that gankyrin might be an important oncogene in cholangiocarcinogenesis and metastasis.
Materials and Methods
Patients, Cell Lines, and Animals
In this study, 85 CCA and 21 noncancerous tissues were included as described in the Supporting Information. Four CCA cell lines, one human intrahepatic biliary epithelial cell line (HIBEpiC), and the nude mice were used as described in the Supporting Information.
Lentivirus-Mediated Small Hairpin RNA (Lenti-shRNA) Against Gankyrin
The Lenti-shRNA vector system (pGCSIL-GFP) was constructed, packed, and purified by GeneChem (Shanghai, China), and was manipulated according to the protocol provided by the manufacturer.
Taqman Real-Time Polymerase Chain Reaction (PCR) and Western Blot Analysis
TaqMan real-time PCR was performed as described.19 Western blot analysis was performed as previously described.19 The films were scanned and the optical density of each band was determined.
Wound-Healing Assay
A confluent monolayer of CCA cells was cultured overnight and a scratch was introduced with a pipette tip. Then images were captured at 0 hours and 24 hours using a light microscope.
Chromatin Immunoprecipitation (ChIP)
ChIP assays were performed using a commercially available ChIP assay kit (Simple ChIP Cell Signaling Technology) following the manufacturer's instructions as described in the Supporting Information.
Overexpression of Gankyrin
The pCMV-Tag2-h Gankyrin plasmid and pCMV-Tag2B empty vector were generous gifts from Prof. Fujita Jun (Department of Clinical Molecular Biology, Kyoto University, Japan). Transfection of the plasmids was performed using Lipofectamine 2000 according to the manufacturer's protocol.
Cell Growth and Soft Agar Assays
For the cell growth assays, CCA cells were seeded at a density of 0.5 × 104 per well. The number of viable cells was determined at different timepoints. For the soft agar assay, cells were plated in the medium containing 0.3% agar. The dishes were examined microscopically for colony formation after a 2-week incubation period.
Migration and Invasion Assays
The transwell migration and invasion assays were performed in the BD Falcon 24-multiwell insert system (BD Biosciences, San Jose, CA) as described in the Supporting Information.
Senescence-Associated β-Galactosidase (SA-β-Gal) Staining
SA-β-gal activity was determined using an SA-β-gal staining kit (Cell Signaling Technology) according to the manufacturer's instruction.
Subcutaneous CCA Experiments
Male BALB/c (5-6 weeks old) mice (n = 10/group) were inoculated subcutaneously in the flank with 5 × 106 TFK-1 cells or 3 × 106 HuCC-T1 cells suspended in phosphate-buffered saline (PBS). The mice were observed over 4 weeks for tumor formation as described in the Supporting Information.
Immunohistochemical (IHC) Analysis
Expression of gankyrin, Ki-67, CD31, and p-STAT3 in tumor tissues was evaluated using the IHC method as described in the Supporting Information.19, 20
IL-6 Silence by shRNA Plasmid
Silencing of IL-6 was achieved by way of transfection of the following specific shRNA vectors: IL-6-specific shRNA (sc-156148), and scramble shRNA control (sc-108060).
In Vivo Spontaneous Metastasis Assay
Male nude mice (BALB/c), 6-8 weeks of age, were used in the experiments (n = 10/group). HuCCT-1 cells (3 × 106 cells in 200 μL) that were stably transfected with either Lenti-shRNA negative control or Lenti-shRNA gankyrin and TFK-1 cells (3 × 106 cells in 300 μL) stably overexpressing gankyrin or vector were injected into the intraperitoneal cavity as previously described.21
Gelatin Zymography
The activity of matrix metalloproteinases (MMPs) in tumor tissue extracts was examined by electrophoresis on 10% sodium dodecyl sulfate polymerase acrylamide gel electrophoresis (SDS-PAGE) containing gelatin as described in the Supporting Information.
Statistical Analysis
Statistical analysis was performed with the GraphPad Prism software package (v. 4.02; San Diego, CA) or SPSS 16.0 software (Chicago, IL), and P < 0.05 was considered statistically significant.
A detailed description of Patients and Methods can be found in the online Supporting Information.
Results
Gankyrin Is Highly Expressed in Human CCAs and Predicts a Poor Prognosis
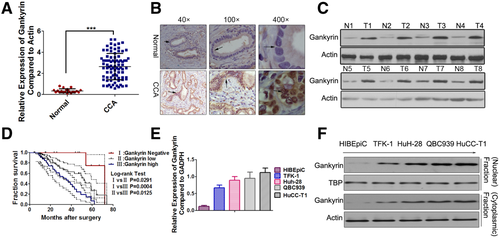
Real-time PCR results indicated that the messenger RNA (mRNA) level of gankyrin was significantly increased in CCA samples compared with that in the nonmalignant samples (Fig. 1A). IHC results showed that gankyrin exhibited strong staining in CCA tissues with a total positive rate of 87% (74/85), which was significantly higher than 9% (2/21) in nonmalignant tissues. Based on the IHC results, the CCA patients were divided into three groups (Supporting Fig. S1): the gankyrin-negative group, gankyrin-high group, and gankyrin-low group. Accordingly, the average percent of neoplastic ducts which stained positively for gankyrin in the gankyrin-high group (82.8 ± 4.3%) was higher than that in the gankyrin-low group (42.6 ± 3.1%). Gankyrin staining was located both in the nucleus and cytoplasm of the tumor cells, and the nuclear staining was relatively stronger than the corresponding cytoplasmic staining (Fig. 1B). The gankyrin protein level in the CCA tissues was confirmed to be significantly higher than that in the noncancerous tissues by western blot (Fig. 1C). Significant overall survival (OS) differences were observed between the patients with high-gankyrin and those with negative or low-gankyrin as evaluated by Kaplan-Meier methods (Fig. 1D). There were statistical associations between gankyrin and the clinicopathological characteristics, such as histological differentiation, TNM stage, etc. (Table SI). To strengthen the independent prognostic significance of gankyrin, we analyzed the OS using a Cox regression analysis. The multivariate analysis revealed that although patients' age, gender, histological differentiation, and metastasis had no prognostic significance, significant prognostic influences of TNM stage and gankyrin expression were found for OS (Table SII). A higher gankyrin mRNA level was detected in CCA cell lines than that in the HIBEpiC cells (Fig. 1E). Besides, gankyrin protein was expressed at a significantly higher level in CCA cell lines compared with HIBEpiC cells both at the nuclear and cytoplasmic levels (Fig. 1F). Importantly, different basal gankyrin levels were found to be associated with the invasive capabilities of the CCA cell lines (HuCC-T1〉HuH-28≈QBC939〉TFK-1) as shown in Fig. S2, suggesting gankyrin might predict CCA metastasis.
Knockdown of Gankyrin Significantly Suppresses CCA Cell Growth, Migration, and Invasion
To investigate the effects of gankyrin suppression on the malignant growth potential, we introduced Lenti-shRNA targeting gankyrin into CCA cells (Fig. S3A,B). Compared with the control shRNA, gankyrin expression was notably decreased by Lenti-shRNA1 (KD-1) and moderately decreased by other three shRNAs (KD-2, 3, and 4) as shown in Fig. S3C. Thus, KD-1 was chosen for further experiments. Cell growth assays showed that CCA-KD-1 cells grew much slower than control cells, and the statistical analysis showed a significant difference from the fourth day (Fig. 2A). When grown in soft agar, the number of CCA-KD-1 cell colonies apparently reduced, and the average size of these colonies was much smaller than that of the control (Fig. 2B).
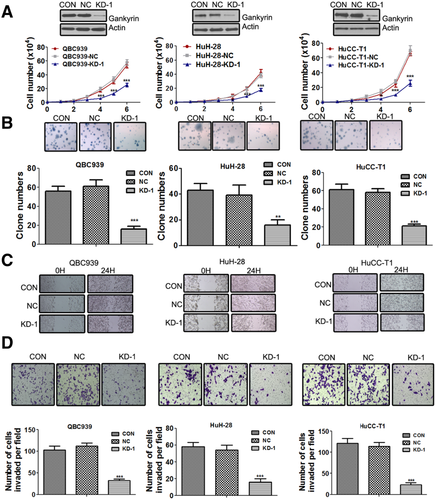
Gankyrin has been reported to be a major factor involved in cancer metastasis.16 Therefore, we examined the ability of gankyrin to modify CCA cell motility. In the wound-healing assay, control cells covered at least 80% of the gap at 24 hours. In contrast, the scratch was still largely uncovered at 24 hours in the CCA-KD-1 group (Fig. 2C; Fig. S4A). Meanwhile, the transwell migration assay showed that CCA-KD-1 cells migrated more slowly than control cells (Fig. S4B). Gankyrin knockdown could also significantly reduce the invasive capabilities of CCA cells through Matrigel membrane (Fig. 2D).
Gankyrin Knockdown Induces G1 Cell Cycle Arrest and Rb-Dependent Cell Senescence
The results demonstrated that gankyrin knockdown induced G1 phase cell cycle arrest in three CCA cell lines (Fig. 3A). Irreversible G1 cell cycle arrest is one of the major biomarkers of cellular senescence. Therefore, we postulated that the cell growth inhibition by gankyrin knockdown was due at least in part by inducing cellular senescence. To test this hypothesis, we examined whether gankyrin knockdown could induce cellular senescence by examining several biomarkers including morphological changes and SA-β-gal activity. Compared with HuCC-T1-NC or HuCC-T1-CON cells, HuCC-T1-KD-1 cells were much larger in size, with flattened shape, a feature of cell senescence. Accordingly, about 50% of KD-1 cells, but only <10% of control cells, were positive by SA-β-gal staining (Fig. 3B).
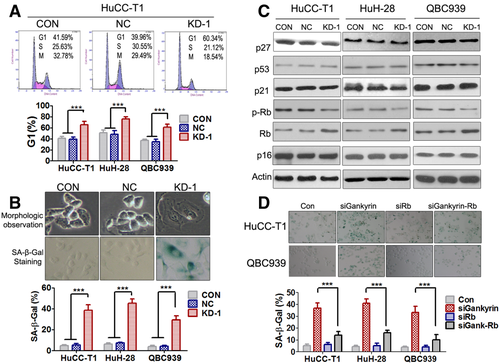
To investigate the intracellular mechanisms through which gankyrin knockdown induced senescence, several major senescence-triggering pathways, including p16/pRb, p27, and p53/p21 axes, were examined. Gankyrin knockdown inhibited the phosphorylation and degradation of Rb (Fig. 3C; Fig. S5A), but could not affect the p53/p21, p27, or p16 axes (Fig. 3C). To determine the role of Rb in cellular senescence, we performed SA-β-gal staining assay after simultaneous knockdown of Rb and gankyrin (Fig. S5B). The results indicated that Rb silence largely abrogated the senescence induced by gankyrin knockdown, suggesting Rb mediated the potential of gankyrin knockdown to induce cell senescence (Fig. 3D). The ChIP results showed that gankyrin knockdown reduced the binding of E2F-1 to the promoters of Rb-E2F dependent genes, such as Cyclin A and Cyclin E (Fig. S6), and indicated that gankyrin knockdown might cause G1 cell cycle arrest through Rb activation. However, there was no significant apoptosis after gankyrin knockdown in CCA cells (Fig. S7).
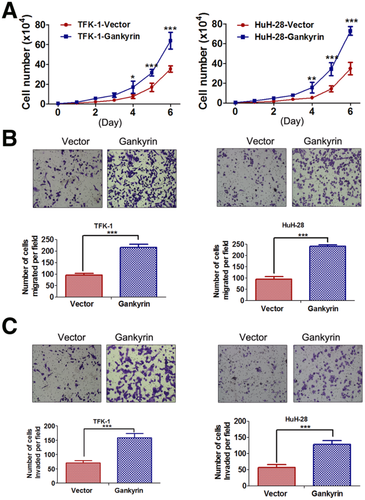
Overexpression of Gankyrin Promotes CCA Cell Growth, Migration, and Invasion
To determine the influence of gankyrin overexpression on biological behaviors of CCA cells, pCMV-Tag2-gankyrin plasmid was transfected into TFK-1 and HuH-28 cells. After selection, stable clones were gained and the western blot results confirmed remarkable gankyrin overexpression (Fig. S8). The growth curve assays indicated that CCA-gankyrin cells proliferated much faster than control cells, and the difference showed statistical significance from the fourth day (Fig. 4A). Accordingly, gankyrin overexpression could significantly promote the migration and invasion of TFK-1 and HuH-28 cells (Fig. 4B,C).
Gankyrin Regulates STAT3 Phosphorylation and Nuclear Translocation of p-STAT3
Given the evident effects of gankyrin on CCA cell growth and metastasis, signaling pathways involved in tumor growth and metastasis that might be activated by gankyrin were analyzed by examining the expression of phosphorylated forms of STAT3 (Fig. 5A,B), protein kinase B (AKT), extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinases (JNK) using western blot assay (Fig. S9A,B). The results indicated that only p-STAT3 level was observed to be significantly changed by gankyrin. Gankyrin knockdown decreased the phosphorylation of STAT3 (Fig. 5A; Fig. S9C), whereas gankyrin overexpression increased it (Fig. 5B; Fig. S9D). The STAT3 signaling pathway has been reported to play an important role in tumorigenesis and metastasis of several cancers, including CCA.22-24 IL-6, a major STAT3 activator, could restore the proliferation and invasiveness of shRNA gankyrin CCA cells (Fig. 5C). Meanwhile, suppression of STAT3 by siRNA (Fig. S10A; Fig. 5D) or STAT3 inhibitor (Fig. S10B) profoundly blocked gankyrin-enhanced TFK-1 cell proliferation and invasion. These results suggested that STAT3 signaling played a crucial role in mediating gankyrin's function. Interestingly, the IHC analysis illustrated that the expression of gankyrin in CCA tissues was closely associated with p-STAT3 expression (Fig. S11). Of the 85 CCA tissue samples, 67 had simultaneous positive expression of gankyrin and p-STAT3 (Table S1). The cellular localization of STAT3 assays showed that TFK-1-vector cells displayed a great distribution of p-STAT3 in the cytoplasm, while TFK-1-gankyrin cells displayed highly red fluorescent nuclei, revealing that gankyrin could induce the nuclear translocation of p-STAT3 in CCA cells (Fig. 5E).
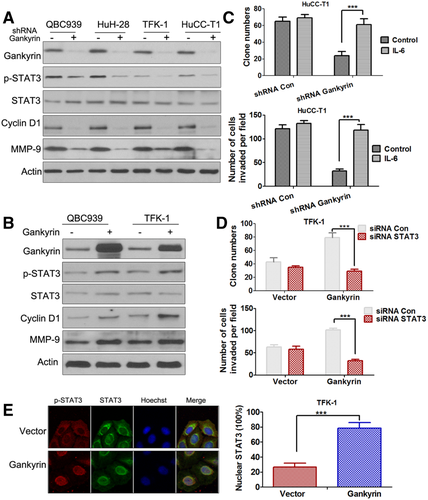
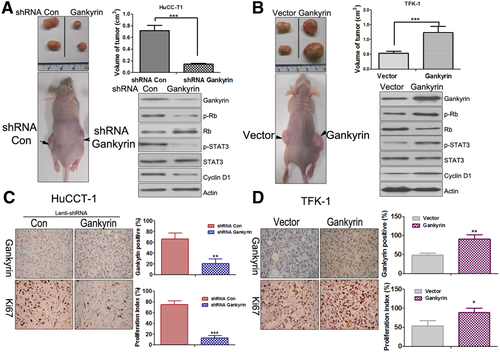
Gankyrin Accelerates Cholangiocarcinogenesis In Vivo
To determine whether gankyrin could affect tumorigenesis in vivo, the aforementioned HuCC-T1-KD-1 or TFK-1-gankyrin cells were injected subcutaneously at flank into nude mice. As shown in Fig. 6A, the tumor volume of the gankyrin knockdown group decreased to approximately one-fourth of the control group. Conversely, when gankyrin was overexpressed, the tumor volume increased ∼2-fold compared to the control group (Fig. 6B). An early tumor formation was noticed in the gankyrin overexpressed group (5.11 ± 1.21 days) in comparison to the vector group (10.19 ± 2.14 days). On the other hand, the time of tumor appearance was prolonged in the HuCC-T1-KD-1 group (14.35 ± 2.86 days) compared to the control group (8.98 ± 2.95 days). The expression of gankyrin, Rb, p-Rb, STAT3, p-STAT3, and CyclinD1 was also determined in the tumor tissues, which was similar to the results in vitro (Fig. 6A,B; Fig. S12A). The IHC results indicated that gankyrin, Ki-67, and CD31-stained vessels were significantly decreased in gankyrin knockdown xenograft tumors, while increased in gankyrin overexpressed tumors (Fig. 6C,D; Fig. S12B).
Gankyrin Increases IL-6 by Way of Negatively Regulating Rb Protein and Establishes a Positive Feedback Loop to Regulate IL-6 Signaling
We next sought to elucidate whether STAT3 activation induced by gankyrin was dependent on IL-6. The results showed that the mRNA level of IL-6 was significantly decreased in the gankyrin knockdown subcutaneous xenograft tumors compared with that in the control group (Fig. 7A, left panel). Conversely, the IL-6 level of subcutaneous xenograft tumors was dramatically increased in the gankyrin overexpressed group (Fig. 7A, right panel) compared with that in the control group. To show the direct evidence that gankyrin could regulate the IL-6 level, the protein in the conditioned medium of the cultured CCA (HuCC-T1 and TFK-1) cells was examined by enzyme-linked immunosorbent assay (ELISA) assay. IL-6 protein level was significantly decreased in CCA-KD-1 cells compared with that in control cells (Fig. 7B, left panel), whereas it was increased in CCA-gankyrin cells (Fig. 7B, right panel) compared with that in the vector control cells. Based on the facts that Rb could suppress IL-6 production and gankyrin could facilitate the phosphorylation and degradation of Rb,25-27 we hypothesized that gankyrin might affect IL-6 through the regulation of Rb. Interestingly, the results suggested that Rb knockdown could indeed increase IL-6 (Fig. 7C,D). Simultaneous knockdown of gankyrin and Rb (Fig. S13A; Fig. 7D) could rescue the IL-6 level by gankyrin knockdown alone, indicating that gankyrin might activate the IL-6 signaling through negatively regulating of Rb.
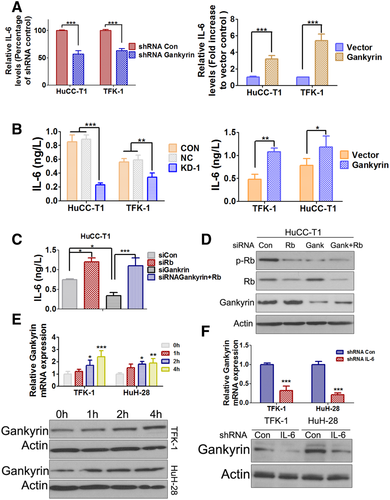
As IL-6 signaling shares a longstanding association with CCA carcinogenesis,28-30 we investigated whether IL-6 could regulate gankyrin. Recombinant IL-6 (rIL-6) treatment increased the mRNA and protein levels of gankyrin both in TFK-1 and HuH-28 cells (Fig. 7E). However, the effects were not particularly dramatic, probably because of the high endogenous IL-6 level of CCA cells. Therefore, we transfected IL-6 shRNA into CCA cells to establish stable IL-6 knockdown cell lines (Fig. S13B). The mRNA and protein levels of gankyrin were obviously decreased in the IL-6-shRNA TFK-1 and IL-6-shRNA HuH-28 cells compared with the control (Fig. 7F). Furthermore, the western blot results also indicated that rIL-6 could dramatically increase gankyrin expression in IL-6 shRNA CCA cells, suggesting IL-6 could indeed regulate gankyrin expression (Fig. S14).
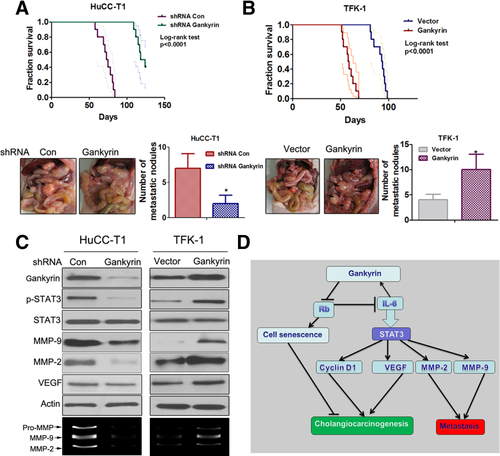
Gankyrin Promotes CCA Metastasis In Vivo
The role of gankyrin in the metastatic phenotype was examined in vivo by implanting CCA cells into the peritoneal cavity of nude mice and monitoring the lethality over a 130-day period (Fig. 8A,B). Mice bearing HuCC-T1-NC tumors invariably died within 90 days, while the HuCC-T1-KD-1 tumors produced no mortality until 110 days. A necropsy revealed that the control cells extensively colonized the visceral organs and formed multiple metastatic nodules (Fig. 8A), while the number of metastatic nodules was reduced in mice bearing HuCC-T1-KD-1 cells. Decreased expression of gankyrin, p-STAT3, VEGF, MMP-2, and MMP-9 in the HuCC-T1-KD-1 metastatic nodules was confirmed by western blot (Fig. 8C, left panels). The effects of gankyrin overexpression on metastasis were also determined in vivo. As shown in Fig. 8B, the mice bearing TFK-1-gankyrin tumors invariably died within 70 days, while TFK-1-vector cells produced no mortality around 90 days. The necropsy indicated that gankyrin remarkably increased the formation of metastatic nodules. The western blot results showed that the expression of gankyrin, p-STAT3, VEGF, MMP-2, and MMP-9 was significantly increased in metastatic nodules formed by TFK-1-gankyrin cells compared with the vector control (Fig. 8C, right panels). Gelatin zymography assays confirmed that the MMP-2 and MMP-9 activities were significantly decreased in HuCC-T1-KD-1 metastatic nodules but were increased in TFK-1-gankyrin metastatic nodules (Fig. 8C).
Discussion
Efforts to elucidate the molecular mechanism underlying tumorigenicity and metastasis of CCA are urgently warranted to identify new biomarkers for prediction and intervention.1-4 Previous studies have intensively investigated the function of gankyrin as a component of proteasome complex in explaining its oncogenic activity.7-15 A new study reported that gankyrin could eliminate tumor suppressor C/EBPα and promote tumor development in a carcinogen-induced liver cancer model.31 Interestingly, recent studies also indicated gankyrin knockdown decreased the expression of stemness factors such as Nanog and Oct-4, suggesting gankyrin could control stem cell behavior.12, 32 These findings indicated that targeting gankyrin might be a promising strategy for cancer prevention and treatment. In this study we demonstrated the significance and underlying mechanisms for gankyrin in CCA tumor growth and metastasis. Analyzing the associations of gankyrin with pathological characteristics revealed significant correlations of gankyrin with histological differentiation, TNM stage, etc. (Table SI). The Kaplan-Meier analysis illustrated that high levels of gankyrin correlated with low rates of survival of CCA patients after surgical resections, revealing that gankyrin might play an important role in CCA pathogenesis.
The results of gankyrin knockdown inhibited CCA cell proliferation, migration, and invasion, while gankyrin overexpression showed the opposite effects and supported the important functions of gankyrin in CCA pathogenesis. Although gankyrin knockdown had no significant effects on cell apoptosis (Fig. S7), we found that G1 cell cycle arrest and cellular senescence were induced by gankyrin knockdown by way of activation of Rb. The senescence induced by gankyrin knockdown was largely dependent on Rb, as (1) Rb was significantly dephosphorylated and increased after gankyrin knockdown, and (2) simultaneous knockdown of gankyrin and Rb remarkably abrogated the cellular senescence induced by gankyrin knockdown alone. Gankyrin overexpression generated larger tumors in subcutaneous xenograft models and more metastatic nodules in orthotopic xenografts models. In contrast, gankyrin knockdown led to severe suppression of CCA tumor growth and metastasis in vivo. Our data indicated that gankyrin knockdown could decrease CD31-stained vessels and VEGF expression while gankyrin overexpression could increase them in the xenograft tumors. These data were consistent with the findings in several recent studies, which reported that gankyrin could promote VEGF production, suggesting that gankyrin might also play an important role in tumor angiogenesis.33 As a subunit of the 26S proteasome, gankyrin has been known to degrade p53 and promote tumorigenicity.34 However, gankyrin knockdown did not affect p53 expression in CCA cells, as shown in Fig. 3C. As reported, p53 mutation is one of the most frequently encountered genetic alterations and plays a central role in CCA carcinogenesis.35 Therefore, we hypothesized that the reason for this phenomenon might be the mutation of p53 in CCA, such as the R175H mutation in HuCC-T1 cells.35 Gankyrin could bind to MDM2 and, consequently, facilitate p53 degradation. However, although MDM2 could drive the degradation of both mutant and wild-type p53, the ability of MDM2 to function as a ubiquitin ligase was proved to be less important in the degradation of mutant p53, which led to p53 accumulation in tumor cells.34 Therefore, p53 mutation in CCA might explain the phenomenon that gankyrin knockdown had no significant effects on p53 expression in the CCA cell lines here.
Our in vitro and in vivo studies suggested that STAT3 signaling was responsible for gankyrin-mediated CCA tumor growth and metastasis. Gankyrin could increase the phosphorylation of STAT3, resulting in upregulation of Cyclin D1, VEGF, and increased MMP-2 and MMP-9 activities. We further showed that the STAT3 activation by gankyrin was dependent on IL-6. Gankyrin could regulate the IL-6 level through negatively regulating Rb. Interestingly, the finding that rIL-6 could also increase the expression of gankyrin outlined a positive feedback loop involving IL-6/STAT3 signaling and gankyrin. This finding was consistent with a recent study that demonstrated that gankyrin could activate IL-8 to promote hepatic metastasis of CRC.36 Bai et al. identified a transcriptome regulated by gankyrin, populating the IL-8 signaling pathway, which mediates the effect of gankyrin in cell proliferation and migration. Our study provided a novel interconnection of gankyrin with the IL-6/STAT3 pathway and added a piece to the puzzle of the IL-6 story, classically recognized to be pivotal in CCA pathogenesis.29, 30 Together, these findings highlight the importance of gankyrin in tumor metastasis and point out its interconnection with interleukins and its candidacy as a potential prognostic marker and therapeutic target to improve the clinical management of metastatic cancer.
In conclusion, our study showed that gankyrin was overexpressed in human CCA cell lines and patient samples, and gankyrin overexpression could promote CCA tumor growth and metastasis both in vitro and in vivo. These results were consistent with the roles of gankyrin involved in tumorigenesis and metastasis of other cancers.10-18 Our study elucidated a novel pathway in gankyrin-mediated cholangiocarcinogenesis and metastasis (Fig. 8D), which suggested new therapeutic targets, including gankyrin/Rb/IL-6/STAT3 signaling pathways, in CCA prevention and treatment. Further work should be performed to obtain more information about gankyrin, and to explore the clinical diagnosis value and develop new drug therapy for CCA.
Acknowledgment
The authors thank Tohoku University (Japan) for providing the HuCC-T1, TFK-1, and HuH-28 cell lines, thank Prof. Fujita, Jun (Department of Clinical Molecular Biology, Kyoto University, Japan) for providing the gankyrin and vector plasmids, Prof. S.G. Wang for providing the QBC939 cell line, Ms. R.N. Liu (Rutgers, State University of New Jersey) for reviewing the article, and Prof. B.D. Gai (Department of Surgery, Third Affiliated Hospital of Jilin University) for selfless assistance.




