Clinical cofactors and hepatic fibrosis in hereditary hemochromatosis: The role of diabetes mellitus†‡
Potential conflict of interest: Nothing to report.
M.J.W. is supported by a National Health and Medical Research Council (NHMRC) Medical Postgraduate Scholarship and the Royal Brisbane and Women's Hospital Research Foundation. G.A.R. and L.W.P. are supported by the NHMRC. This work was supported, in part, by a kind donation from the Hemochromatosis Society of Australia. G.A.R. is also supported by a NHMRC Senior Research Fellowship (#552409).
Abstract
The risk of hepatic fibrosis and cirrhosis in hereditary hemochromatosis relates to the degree of iron loading, but iron alone does not explain the variability in disease penetrance. This study sought to identify clinical cofactors that increase the risk of progressive liver disease. We identified 291 patients from our database who were homozygous for the C282Y mutation in HFE and had undergone a liver biopsy with quantification of hepatic iron concentration (HIC) and fibrosis staging. Data were collected from a retrospective chart review, including age, gender, alcohol consumption, medical therapy, smoking history, metabolic risk factors, mobilizable iron, and laboratory results. Male gender, excess alcohol consumption, HIC, and the presence of diabetes were independently associated with increasing fibrosis stage in multivariate analysis. Of these, the presence of diabetes showed the strongest association (odds ratio, 7.32; P = 0.03). The presence of steatosis was associated with higher fibrosis scores, but this was of borderline statistical significance. Risk factors for hepatic steatosis were male gender, impaired glucose tolerance, and increased body mass index. Conclusion: The presence of diabetes was associated with more severe hepatic fibrosis independent of iron loading, male gender, and alcohol consumption. The mechanism for this association is unknown and deserves further evaluation; however, it is possible that diabetes produces an additional hepatic oxidative injury from hyperglycemia. Thus, management of such cofactors in patients with hemochromatosis is important to reduce the risk of liver injury and fibrosis. (Hepatology 2012;56:904–911)
Hereditary hemochromatosis, when found in those of northern European descent, is most commonly caused by an amino-acid substitution in the HFE protein.1 The prevalence of the relevant substitution, C282Y, is approximately 1 in 200 in susceptible ethnicities. The risk of hepatic iron loading with the development of fibrosis and cirrhosis has been recently clarified with the use of population-based studies. In a large, longitudinal study of C282Y homozygote subjects, it was shown that the risk of hepatic fibrosis and cirrhosis in males is at least 14% and 3%, respectively, with much lower rates observed in females.2
The variability in liver disease penetrance among C282Y homozygote patients has led to a search for cofactors influencing this progression. It has been established that males have a greater risk of hepatic fibrosis,2-5 and that this process is accelerated in those with viral hepatitis6 or exposure to alcohol.7-9 It has also been shown that steatosis is independently associated with fibrosis development.10 Contrary results were reported in a smaller cohort in which hepatic steatosis was associated with lower fibrosis scores, and in this group, the metabolic syndrome was not associated with disease progression.11
In contrast to the relatively stable incidence of genetic hemochromatosis, the prevalence of obesity and its metabolic consequences has increased across most of the world's populations over the last 30 years12 and this epidemic is currently affecting many coexistent diseases. Metabolic syndrome is a term describing the clustering of risk factors for cardiovascular disease and insulin resistance. Specific definitions of this syndrome vary, but generally include the presence of elevated body weight (particularly abdominal adiposity), dyslipidemia, hypertension, and insulin resistance.13, 14 Obesity and metabolic syndrome are risk factors for nonalcoholic fatty liver disease,15 and it is therefore expected that the incidence of hepatic steatosis is likely to also rise markedly.
Several studies have suggested that the presence of diabetes is a risk factor for fibrosis progression in nonalcoholic steatohepatitis (NASH),16, 17 and a mouse model supports these clinical studies.18 The role of metabolic cofactors in other forms of liver disease is less clear. Diabetes has been shown to be a risk factor for fibrosis progression in chronic hepatitis C virus (HCV) infection.19-21 In nondiabetic subjects with hepatitis C, insulin resistance can be correlated with fibrosis stage22 and occurs even in early stages of hepatic fibrosis.23 Although our data show that it is possible that hyperglycemia promotes the progression of liver fibrosis, it is also possible that diabetes may stem from increasing insulin resistance associated with increasing severity of liver fibrosis. An increased body mass index (BMI) and the presence of hyperglycemia are associated with hepatic fibrosis in alcoholic liver disease (ALD).24, 25 In e antigen–negative chronic hepatitis B infection, it has been shown that BMI and metabolic syndrome are associated with higher fibrosis staging and necroinflammatory activity independently of viral load.26, 27
Little is known about clinical factors influencing the highly variable progression to hepatic fibrosis in the iron-loaded liver of hemochromatosis, although it is well recognized that the development of cirrhosis heralds a critical point in a patient's prognosis.28 Because metabolic consequences of overnutrition have been shown to be important in several other forms of liver disease, we sought to explore the relationship between such clinical cofactors and the development of hepatic fibrosis in a well-characterized cohort of C282Y homozygous patients.
Abbreviations
ALD, alcoholic liver disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval; dw, dry weight; HCV, hepatitis C virus; HIC, hepatic iron concentration; NASH, nonalcoholic steatohepatitis; OR, odds ratio; SD, standard deviation.
Patients and Methods
Study Subjects and Design.
The subjects included in this study were identified from a large, prospectively collected clinical database of hemochromatosis patients seen at a single institution between 1964 and 2007. We identified all those with homozygosity for the C282Y mutation in HFE who had undergone liver biopsy with measurement of hepatic iron concentration (HIC) for standard clinical indications. A total of 291 patients met inclusion criteria, of whom 196 were male. Seventy percent of biopsies were performed to establish a diagnosis of genetic hemochromatosis preceding the availability of genetic testing. These subjects were then retrospectively genotyped. All patients gave informed consent with studies approved by the Human Research Ethics Committee of the Queensland Institute of Medical Research (Brisbane, Queensland Australia). Data were analyzed by M.J.W. and J.L.D., and all authors had access to this data.
Patients with viral hepatitis were excluded. Those less than 16 years of age at biopsy were excluded, because the development of the clinical features of hemochromatosis is unlikely at this age unless additional mutations in iron-homeostasis genes are present.
Clinical and Laboratory Data.
Retrospective chart review and clinic attendances were used to obtain clinical and laboratory details at the time of liver biopsy. Details about average daily alcohol consumption (g/day) were obtained by detailed questioning. Alcohol intake was coded as being excessive if average daily values were greater than 60 g for males or 40 g for females.7
Height and weight were recorded at clinic visits, and this information was used to calculate BMI. Overweight and obesity were defined in accord with the World Health Organization and National Institutes of Health.29 Smoking status was ascertained on history and defined as nonsmoker, current smoker, and ex-smoker.
Hypertension was determined to be present if the patient reported a history of this condition, was on current treatment with an antihypertensive agent, or had a blood-pressure measurement greater than 140 mmHg systolic or >90 mmHg diastolic.30 Dyslipidemia was defined by a reported history of hypercholesterolemia, treatment with a cholesterol-lowering medication, or a total cholesterol level >5.3 mmol/L.31 Diabetes was assigned if there was a patient-reported history of this condition, treatment with hypoglycaemic agents, a fasting blood-sugar level >7 mmol/L, or a random blood-sugar level >11.1 mmol/L.32
Serum concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and biochemical markers of iron status (e.g., serum ferritin and transferrin saturation) were measured by standard biochemical techniques.
Mobilizable iron was calculated by determining the total number of venesections to achieve iron depletion, as demonstrated by a serum ferritin <20 μg/L or transferrin saturation of <15% and assuming an iron content of 250 mg per venesection (450 mL).33
Liver Histology.
HIC was measured by atomic absorption spectrophotometry on fresh specimens and reported as micromoles per gram (dry weight; dw). Paraffin-embedded sections were stained with hematoxylin and eosin and Perls' Prussion blue. Scheuer's staging system34 was used to classify fibrosis as follows: F0, no fibrosis; F1, mild fibrosis with enlarged portal tracts; F2, moderate periportal or portal-portal septa, but intact architecture; F3, severe fibrosis with architectural distortion, but no cirrhosis; and F4, cirrhosis with architectural distortion. Iron loading was graded on a scale of 0-4.35 Steatosis was considered to be present if >5% of hepatocytes were affected and the severity was graded according to Brunt et al.36
Statistical Analysis.
Continuous variables were calculated as mean ± standard deviation (SD) if normally distributed and median (range) if not normally distributed. Comparisons between groups were performed using two-sample t tests for normally distributed variables, Mann-Whitney's U tests or logarithmic transformations for non-normally distributed variables, and Pearson's chi-squared test for categorical variables. P values of <0.05 were considered significant. Multivariate ordinal logistic regression analysis, using fibrosis stage as the outcome variable, was used to assess factors independently associated with fibrosis progression. Odds ratios (ORs) with 95% confidence intervals (CIs) are presented. Stata/IC software (version 10.1; StataCorp LP, College Station, TX) was used for all analyses.
Results
Patient Population.
Male subjects made up 67.4% (n = 196) of the study population. The stage of fibrosis was F0 in 163 (56.0%), F1 in 40 (13.8%), F2 in 32 (11.0%), F3 in 17 (5.8%), and F4 in 39 (13.4%). The clinical, demographic, and laboratory data of the 291 patients without (stage 0) and with (stage 1-4) hepatic fibrosis are summarized in Table 1. Male gender, age, iron loading, excess alcohol consumption, diabetes, steatosis, and cigarette smoking all showed associations with hepatic fibrosis in univariate analysis.
| Parameter | N | All Subjects | No Fibrosis (N = 163) | Fibrosis (N = 128) | P Value |
|---|---|---|---|---|---|
| Male, N (%) | 291 | 196 (67.4) | 90 (55.2) | 106 (82.8) | <0.001 |
| Age, years, (mean, SD) | 291 | 42.5 (13.8) | 41.1 (14.5) | 44.4 (12.6) | 0.037 |
| HIC, μmol/g dw (range) | 291 | 151 (12-847) | 127 (12-547) | 199.5 (16-847) | <0.001 |
| Serum ferritin, μg/L (range) | 284 | 913 (33-5500) | 599.5 (33-3,276) | 1,580 (96-5,500) | <0.001 |
| Transferrin sat., % (range) | 280 | 84 (12-100) | 79 (12-100) | 88 (27-100) | <0.001 |
| ALT, IU (range) | 155 | 52 (5-366) | 35 (5-151) | 66 (8-366) | <0.001 |
| AST, IU (range) | 150 | 32 (11-327) | 27 (11-70) | 46 (15-327) | <0.001 |
| Iron removed, g (range) | 212 | 6 (0-36.5) | 4.5 (0-20) | 8.375 (0.5-37.5) | <0.001 |
| BMI, kg/m2 (mean, SD) | 161 | 26.5 (4.52) | 26.3 (4.24) | 26.8 (4.83) | 0.496 |
| Smoking (%) | 149 | 59 (39.6) | 24 (31.2) | 35 (48.61) | 0.03 |
| Diabetes (yes, %) | 288 | 13 (4.5) | 2 (1.2) | 11 (8.7) | 0.002 |
| Hypertension (yes, %) | 221 | 71 (32.1) | 35 (29.7) | 36 (35.0) | 0.401 |
| Dyslipidemia (yes, %) | 194 | 65 (33.5) | 40 (37.0) | 25 (29.07) | 0.243 |
| Alcohol, g (range) | 277 | 10 (0, 200) | 5 (0, 200) | 20 (0, 200) | <0.001 |
| Alcohol excess (yes, %) | 277 | 48 (17.33) | 14 (8.97) | 24 (28.10) | <0.001 |
| Steatosis (yes, %) | 237 | 106 (44.73) | 46 (35.66) | 60 (55.56) | 0.002 |
- Alcohol excess is defined as >40 g/day for women and >60 g/day for men.
Cigarette exposure showed an association with liver fibrosis on univariate analysis, but this effect was not observed in a multivariate model. It can be shown that smoking is associated with male sex (OR, 2.16; 95% CI: 1.003, 4.652; P = 0.049) and alcohol consumption (OR, 4.9; 95% CI: 1.765, 13.497; P = 0.002), with both being well-recognized risk factors for hepatic fibrosis. From this data analysis, cigarette exposure served as a marker for other risk factors, rather than a direct promoter of fibrogenesis.
Risk Factors for Increasing Fibrosis Stage.
In multivariate logistic regression analysis using fibrosis stage as the outcome, well-established risk factors of male gender, excess alcohol consumption, and heavy iron loading were again shown to be significant (Table 2). Of the metabolic risk factors, there was a strong independent association between the presence of diabetes and the risk of fibrosis progression (OR, 7.32; P = 0.03). In this model incorporating both alcohol and impaired glucose tolerance, the effect of steatosis was of borderline statistical significance (P = 0.05). Age at biopsy, BMI, hypertension, and dyslipidemia did not associate with fibrosis progression.
| Predictor | OR | 95% CI | P Value |
|---|---|---|---|
| HIC* | 1.3 | 1.10, 1.54 | 0.002 |
| Male sex | 3.52 | 1.29, 9.56 | 0.014 |
| Diabetes | 7.32 | 1.21, 44.37 | 0.03 |
| Excess alcohol | 4.25 | 1.49, 12.20 | 0.007 |
| Steatosis | 2.48 | 1.00, 6.15 | 0.05 |
| Age† | 1.01 | 0.97, 1.04 | 0.624 |
| Hypertension | 1.07 | 0.43, 2.66 | 0.885 |
| Dyslipidemia | 0.88 | 0.40, 1.96 | 0.755 |
| BMI‡ | 0.97 | 0.89, 1.07 | 0.614 |
- * Per 50μmol/g dw.
- † Per year increase in age.
- ‡ Per kg/m2 increase in BMI.
Clinical Features of Diabetic Subjects.
Comparing diabetic subjects against those with healthy glucose levels, it is shown that there is no significant difference in gender distribution, age, or hepatic iron loading. Serum ferritin was higher at presentation in those with diabetes (median, 2,514 versus 864 μg/L), but transferrin saturation did not differ between groups.
Cigarette use and excessive alcohol consumption were both more likely in the diabetic cohort; however, BMI was not increased in those with diabetes. These finding are summarized in Table 3. Inclusion of all clinical and histological descriptors in a multivariate analysis showed that none independently predicted the presence of diabetes (not shown).
| Parameter | N | Nondiabetics | Diabetics | P Value |
|---|---|---|---|---|
| Male, N (%) | 288 | 184 (67) | 11 (84) | 0.182 |
| Age, years (mean, SD) | 288 | 42.2, 13.9 | 48.8, 11.4 | 0.10 |
| HIC, μmol/g dw (range) | 288 | 149 (12-675) | 288 (42-847) | 0.145 |
| Serum ferritin (range) | 281 | 864 (33-5,500) | 2,514 (210-4,880) | 0.002 |
| Transferrin sat. (range) | 277 | 84 (12-100) | 90 (40-100) | 0.17 |
| ALT, IU (range) | 151 | 50 (5-366) | 68 (22-81) | 0.542 |
| BMI, kg/m2 (mean, SD) | 160 | 26.50, 4.46 | 26.10, 5.23 | 0.810 |
| Cigarettes (%) | 149 | 53 (37.6) | 6 (75) | 0.035 |
| Dyslipidemia (%) | 194 | 63 (34.2) | 2 (20) | 0.500 |
| Alcohol excess (%) | 277 | 42 (15.9) | 6 (46.2) | 0.013 |
| Steatosis (%) | 234 | 97 (43.7) | 6 (50) | 0.668 |
| Steatohepatitis (%) | 157 | 16 (10.5) | 1 (20) | 0.502 |
| Cirrhosis (%) | 39 | 30 (77) | 9 (23) | <0.001 |
The presence of diabetes was more common in patients with advanced fibrosis (stage 3-4) than in those with lower stages; however, this still only represented a minority of those with severe fibrosis (Table 4).
| Fibrosis | |||
|---|---|---|---|
| F0-2 | F3-4 | Total | |
| Diabetes (%) | 4 (31) | 9 (69) | 13 (100) |
| No diabetes (%) | 228 (83) | 47 (17) | 275 (100) |
| Total | 232 | 56 | 288 |
- Pearson's chi-square = 21.54; P < 0.001.
Risk Factors for Hepatic Steatosis.
Of the 237 subjects with information about hepatic steatosis available, 106 (44.7%) had fat present on histological assessment. It has previously been shown that male gender, alcohol consumption, and increased BMI increase the incidence of hepatic steatosis in C282Y homozygote patients.10 This is confirmed in the current study. Hypertension, a further component of metabolic syndrome, was shown to increase the risk of hepatic steatosis (OR, 3.28; P < 0.001) in univariate analysis. Interestingly, patients who were diagnosed with diabetes at the time of liver biopsy did not have higher rates of hepatic steatosis (OR, 1.28; P = 0.669). However, in those who developed impaired glucose tolerance at any stage in follow-up, this relationship was stronger and statistically robust (OR, 4.12; P = 0.004). Multivariate analysis confirmed that hepatic steatosis is more common in males with an elevated BMI and predisposition to impaired glucose tolerance (Table 5). Hypertension and alcohol consumption did not remain independently associated.
| Predictor | OR | 95% CI | P Value |
|---|---|---|---|
| BMI* | 1.27 | 1.14, 1.42 | <0.001 |
| Male sex | 3.65 | 1.27, 10.51 | 0.016 |
| Diabetes† | 5.98 | 1.33, 26.81 | 0.019 |
| Age‡ | 1.01 | 0.97, 1.04 | 0.657 |
| Excess alcohol | 0.75 | 0.25, 2.27 | 0.610 |
| Hypertension | 1.75 | 0.67, 4.55 | 0.248 |
- * Per 1 kg/m2.
- † Includes subjects diagnosed with diabetes at any stage during follow-up.
- ‡ Per year.
Exploring the Relationship Between BMI and Iron Status.
A significant negative correlation was present between BMI and HIC, such that heavier subjects tended to present with lower HIC values (rs, −0.22; P = 0.005) (Fig. 1). ALT values and age of subjects at biopsy did not show a significant difference across BMI categories, so this effect cannot be explained by earlier presentation and biopsy in overweight subjects. The proportion of subjects with steatosis present on biopsy increased from 46.6% in the overweight category to 79.4% in the obese category, compared to only 22.8% in lean subjects (Table 6). It is evident that there is an inverse relationship between steatosis grades and hepatic iron content (Fig. 2). Using mobilizable iron as a surrogate marker of body iron burden, the association is lost between this and both steatosis and BMI (Supporting Fig. 1).
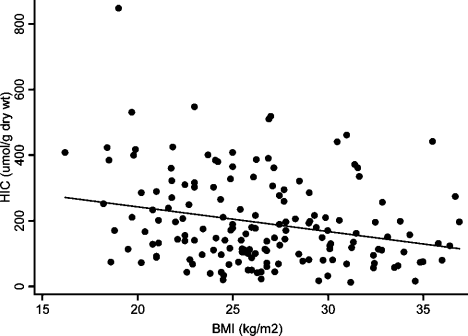
BMI versus HIC. BMI at the time of biopsy versus measured HIC (rs = −0.22; P = 0.005).
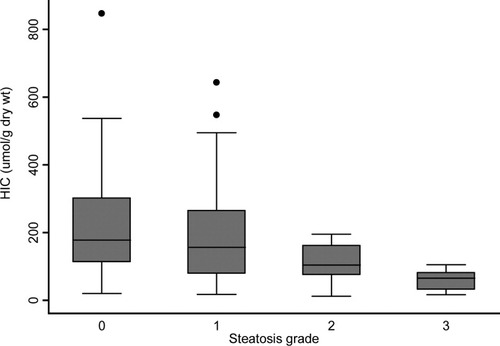
HIC versus grade of steatosis. In C282Y homozygous subjects, the value of HIC on liver biopsy specimens decreased versus increasing grade of steatosis. P < 0.001 (Kruskal-Wallis).
| BMI Categories | ||||
|---|---|---|---|---|
| Parameter | Normal | Overweight | Obese | P Value* |
| Age, years (median, range) | 41 (16-67) | 45 (21-73) | 43 (23-66) | 0.531 |
| ALT, U/L (median, range) | 51 (5-366) | 46 (13-204) | 57 (14-191) | 0.249 |
| Mobilizable iron, g (median, range) | 6 (1.25-37.5) | 5 (1-16.75) | 7 (2.25-22.5) | 0.116 |
| Steatosis present (%) | 22.8 | 46.6 | 79.4 | <0.001 |
- * Kruskall-Wallis' analysis of variance and Pearson's chi-squared test for percentages.
Discussion
Hereditary hemochromatosis is a common genetic condition with a high degree of phenotypic variability. Factors such as age, gender, diet, and blood loss are known to influence iron loading in C282Y homozygotes37; however, HIC does not account for all of the variability in the progression of liver disease. We used a large study population with detailed clinical, laboratory, and biopsy data to investigate cofactors influencing the development of hepatic fibrosis and cirrhosis. In this cohort, the established risk factors of male gender, heavy hepatic iron loading, and alcohol consumption were associated with increasing stages of hepatic fibrosis. In addition, there was an independent strong association between diabetes and fibrosis progression. These finding are pertinent, given the global increase in average BMI that has occurred over the last 30 years.12 It is expected that there will be more frequent interactions between metabolic risk factors and genetic diseases, such as HFE hemochromatosis.
Previously, a large, long-term study of German patients demonstrated the significance of diabetes in hemochromatosis, showing a reduction in cumulative survival in diabetic patients.38 Those with diabetes were also found to have a higher risk of cirrhosis and evidence of heavier iron loading. This cohort was recruited from the mid-20th century and is likely to represent a group with very advanced disease. Over 50% had cirrhosis at presentation and almost 75% of these also had diabetes. Likewise, an early study into the etiology of diabetes in hemochromatosis used a cohort where >80% were cirrhotic and described a pattern of insulin resistance in such patients.39 The present study shows that diabetes and the severity of iron overload are independent factors in advanced liver disease in hemochromatosis. Over the last 30 years, improved detection and available genetic testing has allowed patients to be diagnosed at an earlier stage and with less-severe iron loading,40 and therefore other hepatic insults may become more important. There is relatively little other literature regarding the contribution of metabolic cofactors in hemochromatosis. Adams et al. considered a cohort of 86 patients of whom only 67 were C282Y homozygous.11 In this group, only iron loading (i.e., HIC) was found to be independently predictive of hepatic fibrosis, with other risk factors, including alcohol consumption, male gender, and steatosis, losing statistical significance. It is possible that this relates to a small sample size that lacked sufficient power to detect an effect. Adams et al. showed that in C282Y/H63D compound heterozygotes (N = 19), impaired glucose tolerance was associated with more advanced hepatic fibrosis. This observation fits with the notion of compound heterozygotes having a milder form of iron loading that would not be expected to lead to hepatic injury without cofactors.41 In our study, C282Y/H63D heterozygotes were excluded to ensure a well-defined population where the predominant condition related to iron loading. We identified diabetic patients as those with hyperglycemia on venous blood tests or a requirement for insulin/hypoglycaemic therapy before or at the time of liver biopsy. This produced a small number of confirmed cases with a strong effect. It is likely that there were other patients with impaired glucose tolerance or hyperinsulinemia who cannot be identified by a retrospective analysis, particularly given that some of this data come from a time when metabolic cofactors were less appreciated.
Our results show that the diabetic subjects were not more likely to be older males with heavier iron stores, which would argue against this condition being simply related to pancreatic iron deposition and a marker of advanced disease. The diabetic cohort did present with higher serum ferritin levels; however, this may represent an inflammatory acute-phase response, rather than iron loading per se, because transferrin saturation and HIC did not differ. Cigarette use and heavy alcohol consumption were more prevalent in the diabetic group and it is known that both of these factors may induce pancreatic injury independently. Diabetes related to impaired glucose tolerance and metabolic syndrome is usually observed with higher BMIs and steatosis. In our study, subjects with impaired glucose tolerance had a trend to higher BMIs, although this did not reach statistical significance. The catabolic effect of cirrhosis and loss of steatosis once cirrhosis is established may be a potential explanation. It is likely that insulin resistance and alcohol both contribute to the development of hepatic steatosis, and this confounding effect may explain why the presence of steatosis was of borderline statistical significance in the development of fibrosis in this model.
From these data, it appears difficult to characterize the factors leading to diabetes, and it is likely that more than one mechanism exists. Some subjects may be classical “bronzed diabetics” from pancreatic iron loading and others with metabolic syndrome and insulin resistance. Nonetheless, this study has shown that the presence of diabetes is a clear risk factor for more advanced hepatic fibrosis.
The link between diabetes and fibrosis has been recognized in other causes of hepatic injury. Studies involving subjects with hepatitis C, hepatitis B, NASH, and ALD have suggested that impaired glucose tolerance may be associated with the development of hepatic fibrosis.15-17, 24-27 It is known that hyperglycemia is a pathological state producing detrimental effects on many organs. In the liver, it may increase the risk of oxidative damage by the induction of mitochondrial dysfunction.42 The iron-loaded liver is already subject to oxidative stress with iron catalyzing the production of reactive oxygen species, leading to lipid peroxidation, protein, and DNA changes.43 This could lead to impaired hepatocyte replication, and the concept of a requirement for a “second hit” to stimulate the development of fibrogenesis has been proposed.44 Using this analogy, one might postulate that this second insult could come in the form of hepatic injury resulting from alcohol or diabetes.
Several caveats must be placed on this explanation. First, such retrospective studies demonstrate associations rather than causality. It remains possible that the development of diabetes may occur as a consequence of iron loading of the pancreas in these patients. Diabetes could therefore serve to identify those with heavy iron loading and the hepatic risks associated. However, our results suggest that diabetic patients did not have a higher iron burden (as determined by HIC or iron removed at venesection), although serum ferritin levels were more likely to be abnormal and it is possible that this has produced heavy pancreatic deposition. It is known that iron toxicity can lead to beta-cell dysfunction and a reduced insulin response to glucose45, 46; however, this alone does not explain the link with hepatic injury. Finally, it remains a possibility that the presence of cirrhosis leads to the development of diabetes—so-called “hepatogenous diabetes.” From our data, the predisposition to diabetes is a risk factor for hepatic steatosis in conjunction with increased BMI and male gender. Steatosis has been shown to be a risk factor for hepatic fibrosis,10 although in advanced fibrosis, the steatosis may be lost. This suggests a pathway linking diabetes to the development of hepatic fibrosis, rather than vice versa. This may eventually be proven an oversimplification, because it is acknowledged that adipose tissue is metabolically active and interacting through adipokines with iron-related molecules in the face of metabolic derangements.47 Hemochromatosis is an inherited condition, and it is known that genetic polymorphisms in insulin-receptor–signaling pathways are associated with fibrosis progression and altered glucose homeostasis in NASH.48 Further studies will be required to determine whether coinheritance of such polymorphisms may help explain disease penetrance in hemochromatosis.
Smoking has not traditionally been considered a risk factor for liver injury, and relatively few studies have investigated the role of cigarette consumption on the progression of chronic liver disease. It is accepted that cigarette exposure may accelerate other chronic “fibrogenic” diseases, and there are potential explanations as to how this could occur in liver disease.49 Interestingly, a recent study has suggested that smoking may be associated with an increased likelihood of hepatic fibrosis in NASH and this effect appears to occur through a pathway of induced insulin resistance.50 In our cohort, although there did appear to be an association between cigarette usage and hepatic fibrosis in univariate analysis, this effect was not observed in the multivariate model. It can be shown that there is a clustering of lifestyle-related risk factors, including cigarette smoking, alcohol exposure, and elevated BMI. Though cigarette consumption was not shown to lead directly to more severe liver injury in hemochromatosis in our study, it marks an individual as having other potential risk factors.
Although it was not the primary aim of this study, the data obtained allow further clarification of the interaction between hepatic iron content and body mass. It is commonly noted in the general population that those with an increased BMI may have increased serum ferritin without evidence of iron overload. Conversely, it has previously been shown that in those with HFE-associated hemochromatosis, hepatic iron loading is inversely correlated with body mass, despite similar age at biopsy and serum alanine transaminase.10 From our data, it appears that this effect is the result of the increased proportion of liver biopsies containing steatosis in overweight/obese patients, such that the HIC (expressed as grams per weight of liver tissue) is artificially lowered by the presence of fat. The increased volume of liver tissue resulting from steatosis therefore reduces iron concentration, although not necessarily total hepatic iron content. When using mobilizable iron as a marker of total body iron load, the relationship between iron and BMI is lost. The presence of steatosis is associated with an elevated BMI, male gender, and the predisposition to impaired glucose tolerance. Therefore, in patients with fatty liver disease, hepatic iron concentration may not accurately reflect the total iron burden and may underestimate the risk of liver disease.
In summary, these findings further clarify factors modifying the progression of hepatic fibrosis and cirrhosis in C282Y hemochromatosis. In particular, this study has demonstrated an association between the presence of diabetes and higher fibrosis stage, which remains independent of hepatic iron loading, male gender, alcohol consumption, and steatosis. It could be argued that our data suggest that clinicians may then need to consider liver biopsy at a lower threshold of serum ferritin in such patients to ensure that the diagnosis of cirrhosis is not missed. It would also naturally suggest that one should not fail to address the control of metabolic risk factors in patients with hemochromatosis, because phlebotomy alone may not be sufficient in an era of obesity.




