The long and the short of interferon-gamma–inducible protein 10 in hepatitis C virus infection†
Potential conflict of interest: Nothing to report.
Casrouge A, Decalf J, Ahloulay M, Lababidi C, Mansour H, Vallet-Pichard A, et al. Evidence for an antagonist form of the chemokine CXCL10 in patients chronically infected with HCV. J Clin Invest 2011;121:308-317. (Reprinted with permission.)
Abstract
Chronic infection with hepatitis C virus (HCV) is a major public health problem, with nearly 170 million infected individuals worldwide. Current treatment for chronic infection is a combination of pegylated IFN-α2 and ribavirin (RBV); however, this treatment is effective in fewer than 50% of patients infected with HCV genotype 1 or 4. Recent studies identified the chemokine CXCL10 (also known as IP-10) as an important negative prognostic biomarker. Given that CXCL10 mediates chemoattraction of activated lymphocytes, it is counterintuitive that this chemokine correlates with therapeutic nonresponsiveness. Herein, we offer new insight into this paradox and provide evidence that CXCL10 in the plasma of patients chronically infected with HCV exists in an antagonist form, due to in situ amino-terminal truncation of the protein. We further demonstrated that dipeptidyl peptidase IV (DPP4; also known as CD26), possibly in combination with other proteases, mediates the generation of the antagonist form(s) of CXCL10. These data offer what we believe to be the first evidence for CXCL10 antagonism in human disease and identify a possible factor contributing to the inability of patients to clear HCV.
Comment
Chemokines play a critical role in the regulation of recruitment and positioning of leukocytes within the viral-infected liver.1-3 The chemokine system is complex, involving concurrent and sequential interactions between tissue-based chemokine gradients and chemokine receptors expressed on the surface of circulating leukocytes. Chemokine receptor-bearing effector cells recruited in this way ultimately determine both virological control and tissue injury, and thereby shape the course of disease.
In relation to chronic hepatitis C (HCV), multiple CC and CXC (C-C and C-X-C motif, respectively) chemokines have been shown to be highly up-regulated in the liver.4-11 In particular, expression of the interferon (IFN)-inducible chemokines that are ligands for CXC receptor 3 (CXCR3)—that is, CXCL10 (IFN-gamma–inducible protein 10 [IP-10]), CXCL9 (monokine induced by IFN gamma [MIG]), and CXCL11 (IFN-inducible T cell alpha chemoattractant [I-TAC])—are highly expressed and have been correlated with liver injury, notably lobular inflammation.5, 6, 9, 12 In the blood of patients who are acutely infected with HCV, CXCL10 peaks approximately 7 weeks after infection and correlates with HCV viral load and alanine aminotransferase elevation.9
CXCL10 was first cloned from the U937 myelo-monocytic cell line, from which the complementary DNA was shown to encode a precursor protein of 98 amino acids with a secreted protein of 77 amino acids.13 CXCL10 is highly up-regulated in hepatocytes in the HCV-infected liver,5 as well as sinusoidal endothelial cells.8 It is presumed that this up-regulation facilitates recruitment of HCV-specific as well as nonspecific T cells into the liver, thus potentiating liver inflammation. It is well recognized that the extent of hepatic inflammation in chronic HCV predicts subsequent fibrosis.14 Thus, this proinflammatory milieu drives hepatic stellate cell and myofibroblast activation and proliferation and progressive liver fibrosis.
Interestingly, higher serum levels of CXCL10 have been consistently shown to predict nonresponsiveness to IFN-alpha–based antiviral therapy for chronic HCV.2, 15-19 Intuitively, this seems something of a paradox, because it may be expected that greater immune activation within the liver would facilitate enhanced HCV clearance, especially because it is known that IFN-gamma also directly inhibits HCV replication.20 However, it should be recognized that there is also a strong correlation with enhanced interferon-stimulated gene expression, both in liver and peripheral blood leukocytes, and nonresponsiveness to IFN-alpha–based therapies.21, 22 Perhaps the high serum levels of CXCL10 reflect this phenomenon.
It has been known for more than a decade that dipeptidyl peptidase-IV (DPP4; also known as CD26) readily cuts two amino acids from the N-terminus of CXCL10.23 The in vitro half-life of CXCL10 in the presence of DPP4 is less than 2 minutes.24 CXCL10 is made by macrophages, hepatocytes, fibroblasts, and endothelial cells, whereas DPP4 is on the surface of endothelial cells and activated lymphocytes.25 DPP4 is normally found on the biliary canalicular surface of hepatocytes, where it would not be expected to contact CXCL10. However, some hepatocytes in the chronically injured liver lose their polarity and thus place DPP4 onto their entire cell surface.26 This would be expected to expose surrounding cells and extracellular fluid to the hepatocyte-derived protease. DPP4 is also normally present in serum,27 and levels increase in human serum, gut, and liver in chronic HCV infection,28 and in rat serum during liver regeneration.29 In addition, patients with chronic HCV have lower circulating levels of the active form of glucagon-like peptide-1,28 which is also inactivated by DPP4. Thus, it seems that functional DPP4 is highly active in chronic HCV infection.
The recent article from Casrouge et al.30 offers a significant leap in our understanding of the human health implications of this prior knowledge, and links elevated CXCL10 levels with increased DPP4 activity into a thought-provoking new paradigm. The new findings from Casrouge et al. are, first, that the DPP4-cleaved form of CXCL10 is no longer a chemoattractant, but rather an antagonist of signaling via its receptor, CXCR3; second, the cleaved (short) form of CXCL10 is more abundant in sera from patients with chronic HCV; and third, that DPP4 is the only protease to significantly convert CXCL10 to this short form. Finally, the authors have measured the amount of the antagonist (short) form of CXCL10 present in the serum using a novel immunoassay, and found that the CXCL10 short form is a better predictor of nonresponse to antiviral therapy. Thus, this finding turns the original paradox on its head (Fig. 1). Previously, we struggled with the paradigm that a molecule associated with enhanced immune activation was associated with failure to clear the virus. Now, this makes sense; the previously measured increased levels of CXCL10 are largely those of a chemokine antagonist likely to inhibit T cell recruitment, thereby potentially decreasing the likelihood of viral clearance.
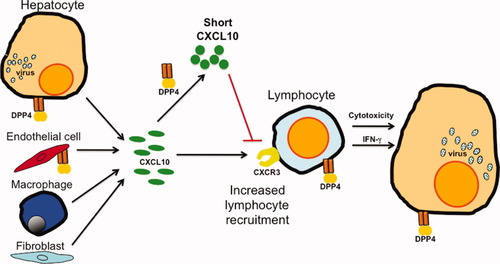
A new CXCL10 paradigm in hepatitis C. CXCL10 and DPP4 levels rise following HCV infection. Casrouge and colleagues30 found that DPP4 converts the active form of CXCL10 into a shorter form that is an antagonist (red line) rather than an agonist of the cognate receptor CXCR3. The short CXCL10 predominates and probably impedes CXCR3-mediated lymphocyte recruitment to the infected liver. This may logically explain the association between increased CXCL10 levels and poor antiviral response to IFN-based therapy. The cell types that make CXCL10 or DPP4 are depicted. DPP4 is shed from cell surfaces. Cells and structures are not shown to scale.
These data suggest that DPP4 inhibition, and hence maintenance of the full-length, agonist form of CXCL10, will be associated with enhanced recruitment of effector lymphocytes into the liver, and hence improved responsiveness to IFN-alpha–based antiviral therapies. Fortunately, DPP4 inhibition via sitagliptin, saxagliptin, and vildagliptin is already a successful treatment for Type 2 diabetes, with worldwide sales now exceeding US$3 billion per year.31 Thus, the door to clinical use of DPP4 inhibitors for patients with chronic HCV infection appears wide open.
Two issues, however, remain unresolved. First, there is some concern of the potential for a proinflammatory effect; notably, reports of pancreatitis associated with DPP4 inhibitor therapy32 suggest that worsened hepatitis may also be a plausible consequence. However, it should be noted that this examination of the US Food and Drug Administration adverse report database drew opposite conclusions to analyses of pooled data from clinical trials of sitagliptin33 and vildagliptin,34 both of which found no increased risk of pancreatitis in patients on DPP4 inhibitor therapy. Moreover, anti-inflammatory effects of a selective DPP4 inhibitor have been seen in a murine diabetes model.35
The second issue is that several other chemokines known to be overexpressed in the HCV-infected liver, including CCL5, CXCL9, and CXCL11, are also degraded by DPP4. The functional capabilities of the cleaved forms of these chemokines are incompletely characterized,36 and neither their expression nor their proteolytic breakdown in vivo have been studied in chronic HCV.
In conclusion, the article by Casrouge et al. reminds us that simple measurement of serum levels of inflammatory mediators can lead to misleading interpretations. Functionality is the key to understanding what has seemed to be a paradox in understanding of the immunopathogenesis of chronic HCV.
The complexities of chemokine regulation of leukocyte trafficking into the inflamed liver just became much more complicated!




