In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression†
Potential conflict of interest: Nothing to report.
Abstract
Interleukin-22 (IL-22), which acts as either a proinflammatory or anti-inflammatory cytokine in various disease models, is markedly up-regulated in chronic liver diseases, including hepatitis B and C. In this report, we demonstrate a strong correlation between IL-22 expression in the liver with active, inflammatory human liver disease. To clarify the role of IL-22 up-regulation in the pathogenesis of liver diseases, liver-specific IL-22 transgenic (IL-22TG) mice, under the control of albumin promoter, were developed. Despite elevated IL-22 serum levels ranging from 4,000 to 7,000 pg/mL, IL-22TG mice developed normally without obvious adverse phenotypes or evidence of chronic inflammation (except for slightly thicker epidermis and minor inflammation of the skin) compared with wild-type mice. Interestingly, IL-22TG mice were completely resistant to concanavalin A–induced T cell hepatitis with minimal effect on liver inflammation and had accelerated liver regeneration after partial hepatectomy. Although they did not spontaneously develop liver tumors, IL-22TG mice were more susceptible to diethylnitrosamine-induced liver cancer. Microarray analyses revealed that a variety of antioxidant, mitogenic, acute phase genes were up-regulated in the livers of IL-22TG mice compared with those from wild-type mice. Conclusion: These findings indicate that localized production of IL-22 in the liver promotes hepatocyte survival and proliferation but primes the liver to be more susceptible to tumor development without significantly affecting liver inflammation. (HEPATOLOGY 2011;)
Interleukin-22 (IL-22) was originally identified as an IL-10–related T cell–derived inducible factor belonging to the IL-10 family.1 It is now known that IL-22 is mainly produced by Th17, Th22, γδT, natural killer, and natural killer T cells.2-4 IL-22 mainly targets epithelial cells, including hepatocytes, playing an important role in controlling bacterial infection, homeostasis, and tissue repair.2-8 IL-22 exerts its functions by binding to the heterodimer IL-10R2/IL-22R1 complex, followed by activation of signal transducer and activator of transcription 3 (STAT3) as well as other signaling pathways (albeit to a lesser extent), including STAT1 and STAT5.2-4 IL-10R2 is ubiquitously expressed on a variety of cell types, whereas IL-22R1 expression is restricted to epithelial cells in the skin, liver, pancreas, lung, and gut.2-4
IL-22 has been found to be up-regulated and implicated as a proinflammatory cytokine in the pathogenesis in various human diseases and in animal models, including psoriasis,9 rheumatoid arthritis,10 and Crohn's disease.11 In contrast, IL-22 has also been shown to prevent mice from liver injury,12-15 inflammatory bowel disease,16 and ulcerative colitis.17 To further clarify the biological significance of IL-22, Wolk et al.18 generated two types of IL-22 transgenic (IL-22TG) mice under the control of the EμLCK promoter expressed in lymphoid cells and the rat insulin II promoter expressed in pancreatic islet, respectively. Most of the IL-22 transgenic mice died within the first few days after birth. In addition, Savan et al.19 developed transgenic mice in which IL-22R1 was expressed on lymphocytes. These mice died of inflammation 6-8 weeks after birth and also showed high levels of circulating IL-22, thus demonstrating that aberrant expression of IL-22R1 was sufficient to drive IL-22 production by lymphocytes. In this study, we demonstrate that a high percentage of inflammatory cells in patients with chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) expressed IL-22 and that the number of IL-22–positive cells correlates positively with the grade of liver inflammation and serum levels of aspartate aminotransferase, thus implicating IL-22–expressing lymphocytes as mediators of pathogenesis in these diseases. At present, there are no small animal models available with chronic viral hepatitis and liver inflammation that are associated with elevation of IL-22 in the liver. As mentioned above, transgenic mice with IL-22 overexpression in lymphoid cells died within the first few days after birth.18 Thus, in order to define the role of elevated IL-22 in the pathogenesis of liver disease, we developed transgenic mice with overexpression of IL-22 in the liver under the control of the albumin promoter to imitate the situation in viral hepatitis patients with high levels of IL-22 in the liver. In contrast to EμLCK promoter–driven or the rat insulin II promoter–driven IL-22TG mice,18 liver-specific IL-22TG had no obvious adverse phenotypes and no overt inflammation, but were completely resistant to T cell hepatitis, had accelerated liver regeneration after partial hepatectomy, and showed increased sensitivity to diethylnitrosamine (DEN)-induced liver cancer.
Abbreviations
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ConA, concanavalin A; DEN, diethylnitrosamine; HBV, hepatitis B virus; HCV, hepatitis C virus; IL-22, interleukin-22; PCR, polymerase chain reaction; PHx, partial hepatectomy; pSTAT, phosphorylated signal transducer and activator of transcription; SAA, serum amyloid A; STAT, signal transducer and activator of transcription; TG, transgenic; WT, wild-type.
Materials and Methods
Human Liver Samples.
Most human cirrhotic liver samples were obtained from recipient livers after transplantation; some liver samples from patients with chronic HBV or HCV were obtained by way of biopsy (Supporting Information Table 1). Evaluation of severity of disease followed the Scheuer criterion. The degree of inflammatory infiltration was defined as grade (G), and the degree of fibrosis was defined as stage (S). Normal healthy liver samples were obtained from normal healthy donors for liver transplantation. The study protocol involved in human samples was approved by the local ethics committee, and all patients provided written informed consent.
Generation of IL-22TG Mice.
Liver-specific IL-22TG on the C57BL/6 background were generated by microinjection of the recombinant IL-22 expression vector containing the full-length murine IL-22 complementary DNA driven under a liver-specific albumin promoter. The detailed generation and genotyping are described in the Supporting Information. All animal experiments were approved by the National Institute on Alcohol Abuse and Alcoholism animal care and use committee.
DEN-Induced Hepatocellular Carcinoma Model.
Mice were injected with 10 μg/g or 20 μg/g body weight of DEN (Sigma, St. Louis, MO) at age of 15 days and sacrificed at 9 months old to examine incidence, size, and number of the tumors in the liver.
Statistical Analysis.
Data are expressed as the mean ± SD. To compare values obtained from three or more groups, one-factor analysis of variance was used, followed by Tukey's post hoc test. To compare values obtained from two groups, a Student t test was performed. The correlations between variables were assessed using the Spearman rank order test. P < 0.05 was considered statistically significant.
Additional Materials and Methods are detailed in the Supporting Information.
Results
Up-regulation of Hepatic IL-22 Expression in Patients with Chronic HBV or HCV.
Immunohistochemistry analyses with anti–IL-22 antibody were performed on 37 human liver tissue samples from patients with hepatolithiasis (5 cases), HBV (22 cases), and HCV (10 cases). No obvious IL-22–positive staining was observed in the livers from healthy donors or from patients with hepatolithiasis. In contrast, the majority of inflammatory cells in HBV patients who had cirrhosis with active hepatitis (inflammation grade 4 [G4]) stained positively for IL-22, whereas the number of IL-22+ lymphocytes was lower in those with lower inflammatory scores (G0-G3) (Fig. 1A,B and Supporting Information Table 1). A significant number of IL-22+ lymphocytes were also observed in HCV cirrhotic livers. All of these HCV cirrhotic liver samples had G2 inflammation, except for one that had G3. The number of IL-22+ lymphocytes in these HCV patients with G2-G3 was lower than that in HBV patients with a higher grade of inflammation (G4) (Fig. 1B). Furthermore, in both HBV and HCV cirrhotic livers, most of the IL-22+ lymphocytes were located in the inflamed regions but were also observed in liver sinusoids in some patients (Fig. 1A).
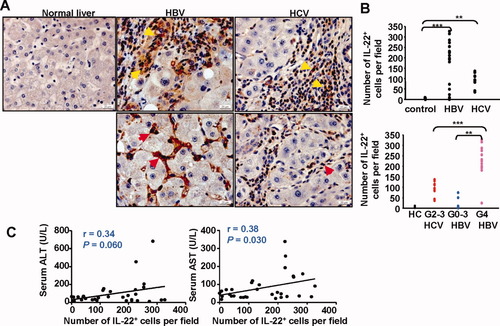
IL-22+ cells accumulate in the livers of patients with chronic HBV or HCV. (A) Immunohistochemical staining for IL-22 in normal healthy livers and HBV or HCV cirrhotic livers. Positive staining with IL-22 antibody is shown as a dark red color. IL-22+ immune cells are located mainly in inflammatory regions (upper panel, yellow arrows) but are also observed in sinusoids (lower panel, red arrows) in some patients. (B) The number of IL-22+ lymphocytes per field (×100) correlates positively with the grade (G) of inflammation. Each dot represents one patient. **P < 0.01, ***P < 0.001. (C) Correlation between the number of IL-22+ cells per field (×100) in the livers and serum ALT or AST from viral hepatitis patients.
IL-22 has been shown to protect against liver injury in various models,12-14 Therefore, we examined the correlation between the number of IL-22+ cells and liver damage. As shown in Fig. 1C, the number of IL-22+ cells had a positive correlation with serum levels of aspartate aminotransferase (AST) and also had a trend to a positive correlation with serum levels of alanine aminotransferase (ALT).
Interestingly, a positive correlation between serum IL-22 and liver injury was also observed in a mouse model of T cell hepatitis. As shown in Supporting Information Fig. 1, serum or hepatic IL-22 levels had a positive correlation with serum ALT and AST in this T cell hepatitis model induced by different doses of concanavalin A (ConA).
Generation of Liver-Specific IL-22TG Mice.
To understand the biological significance of IL-22 up-regulation in liver diseases, we generated liver-specific IL-22 transgenic mice (C57BL/6 background) in which IL-22 expression was under the control of the albumin promoter and enhancer. We obtained three founders (TG-8, TG-9, and TG-15) with serum levels of IL-22 reaching ≈6,000 pg/mL. All of the experiments described below were obtained from the TG-8 founder (referred to as IL-22TG). Many of these experiments were confirmed using TG-9 or TG-15, thus demonstrating that our findings are due to the transgene, not the unique founder line of mice.
Figure 2A shows that high levels of serum IL-22 were detected in the three founders of transgenic lines but not in wild-type (WT) mice. Serum levels of IL-22 were detected as early as 2 weeks in IL-22TG after birth and reached the peak level (≈6,000 pg/mL) at 1 month (Fig. 2B). Such levels of serum IL-22 were maintained for the lifetime of mice and did not change during the backcrossing with C57BL/6 mice.
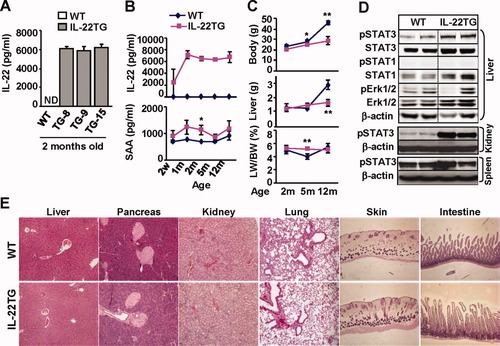
Characterization of IL-22TG mice. (A) Serum IL-22 levels from three founders of IL-22TG mice. (B) Serum IL-22 and SAA levels at various ages of IL-22TG mice. (C) Body and liver weight. *P < 0.05, **P < 0.01 compared with the corresponding WT groups. (D) Western blot analyses of various tissues from 2-month-old mice. (E) Hematoxylin and eosin staining of various organs from 2-month-old mice.
IL-22 is known to induce expression of acute phase proteins (e.g., serum amyloid A [SAA]) and multiple signaling pathways in hepatocytes.2, 20 Here we observed that IL-22TG mice had a trend to higher levels of serum SAA compared with WT mice, with a statistical difference being reached at age 2 months (Fig. 2B). In addition, microarray data revealed that hepatic RNA expression of SAA, as well as several other acute phase proteins, were elevated in IL-22TG mice versus WT mice (Table 1).
| Gene Name | UniGene ID | Gene Symbol | Fold Change (IL-22TG versus Wild-Type) |
|---|---|---|---|
| Antioxidant genes | |||
| Metallothionein 1 | Mm.192991 | Mt1 | 20.4 |
| Metallothionein 2 | Mm.147226 | Mt2 | 37.1 |
| Mitogenic and proliferative genes | |||
| Cyclin B1 | Mm.380027 | Ccnb1 | 1.5 |
| Cyclin D1 | Mm.273049 | Ccnd1 | 1.8 |
| Growth arrest–specific gene 5 | Mm. 270065 | Gas5 | 1.5 |
| Growth arrest–specific gene 6 | Mm. 3982 | Gas6 | 1.6 |
| Neuropilin 1 | Mm.271745 | Nrp1 | 2.4 |
| Acute phase genes | |||
| Serum amyloid A1 | Mm.148800 | Saa1 | 3.2 |
| Serum amyloid A2 | Mm.200941 | Saa2 | 5.3 |
| Serum amyloid A3 | Mm.14277 | Saa3 | 1.7 |
| Serum amyloid A4 | Mm.3489 | Saa4 | 2.1 |
| Serum amyloid P-component | Mm.330510 | Apcs | 2.5 |
| Fibrinogen-like protein 1 | Mm.277955 | Fgl1 | 3.0 |
| Orosomucoid 2 | Mm.14173 | Orm2 | 9.2 |
| Orosomucoid 3 | Mm.381399 | Orm3 | 2.8 |
| CD14 | Mm.3460 | Cd14 | 1.6 |
| LPS binding protein | Mm.218846 | LBP | 2.0 |
| Antimicrobial genes | |||
| Lipocalin 2 | Mm. 9537 | Lcn2 | 25.1 |
| Proteinase 3 | Mm. 2364 | Prtn3 | 7.4 |
| Cytokine signaling–related genes | |||
| Suppressor of cytokine signaling 3 | Mm.3468 | Socs3 | 4.0 |
| Suppressor of cytokine signaling 2 | Mm.474283 | Socs2 | 2.8 |
| Signal transducer and activator of transcription 3 | Mm.249934 | Stat3 | 1.6 |
| Janus kinase 3 | Mm. 249645 | Jak3 | 1.6 |
All IL-22TG mice grew normally without obvious adverse phenotypes except a lower body weight after 5 months of age compared with WT mice (Fig. 2C). Food intake was similar in both IL-22TG and WT mice (data not shown). In addition, at 2 months of age, both IL-22TG and WT mice had a similar liver weight and liver/body weight ratio; at 5 months of age, IL-22TG mice had similar liver weights but a higher liver/body weight ratio compared with WT mice. In contrast, at 12 months of age, IL-22TG mice had a lower liver weight but similar liver/body weight ratio compared with WT mice.
Western blot analyses revealed that phosphorylated STAT3 (pSTAT3) but not pSTAT1 or extracellular signal-regulated kinase 1/2 activation was elevated in the livers of IL-22TG mice versus WT mice (Fig. 2D). Activation of pSTAT3 was also detected in the kidney but not the spleen from IL-22TG mice (Fig. 2D), indicating that the circulating IL-22 had effects beyond the tissue in which it is being produced. The lack of effects in the spleen was not surprising, as normal mouse lymphocytes/leukocytes lack IL-22R1.4
Histology analyses showed that all of the organs from IL-22TG mice had a normal histology except for slightly thicker epidermis and minor inflammation in the skin compared with WT mouse skin (Fig. 2E, Supporting Information Fig. 2a). No obvious inflammation or necrosis was observed in the organs obtained from IL-22TG mice. Furthermore, flow cytometric analyses of leukocytes from the liver and other organs revealed no significant difference in the total number of leukocytes and the percentages of cell populations when comparing WT and IL-22TG mice (Supporting Table 2). Finally, expression of IL-22R1 and IL-10R2 in the liver was comparable in WT and IL-22TG mice as demonstrated by reverse-transcription polymerase chain reaction (PCR) and real-time PCR (Supporting Information Fig. 2b).
IL-22TG Mice Are Protected from ConA-Induced T Cell Hepatitis.
Next we compared the liver injury in WT and IL-22TG mice in a model of ConA-induced T cell hepatitis. As illustrated in Fig. 3A,B, IL-22TG mice were completely protected from the liver injury induced by ConA injection. Whereas WT and IL-22TG mice had comparable levels of multiple cytokines and chemokines, including tumor necrosis factor α, IL-10, monocyte chemoattractant protein 1, and interferon-γ, serum levels of IL-6 were higher in WT mice than in IL-22TG mice (Fig. 2C). This increase in IL-6 may be due to massive liver necrosis observed in WT mice after ConA injection, because necrotic hepatocytes are known to stimulate Kupffer cells to produce IL-6.21
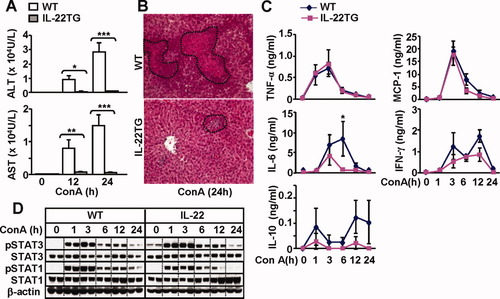
IL-22TG mice are resistant to ConA-induced T cell hepatitis. Mice were treated with ConA (10 μg/g) and then euthanized at different time points. (A) Serum ALT and AST levels. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with corresponding WT groups. (B) Hematoxylin and eosin staining of liver tissue from mice treated with ConA for 24 hours. (C) Serum cytokines. *P < 0.05 compared with corresponding WT controls. (D) Western blot analyses.
To further understand the mechanisms underlying the resistance of IL-22TG mice to ConA-induced liver injury, we examined hepatic STAT3 and STAT1 activation, because these transcription factors play important roles in protecting against and promoting ConA-induced liver injury, respectively.22 As illustrated in Fig. 3D, ConA injection induced activation of both STAT3 and STAT1 in WT mice. In contrast, IL-22TG mice had higher basal levels of pSTAT3, and activation of STAT3 was slightly enhanced whereas STAT1 activation was lower in IL-22TG mice compared with WT mice.
IL-22TG Mice Have Accelerated Liver Regeneration After Partial Hepatectomy.
Liver regeneration induced by partial hepatectomy (PHx) was also examined in IL-22TG mice. As illustrated in Fig. 4A, the liver/body weight ratio was similar before PHx (0) but was significantly higher in IL-22TG mice than in WT mice 32 hours, 48 hours, and 72 hours post-PHx, and returned to the same levels 96 hours after PHx in both groups. BrdU+ incorporation experiments demonstrated that IL-22TG mice had an accelerated peak of hepatocyte proliferation at 32 hours post-PHx, and this peak is significantly higher when compared with WT mice. In addition, the number of BrdU+ hepatocytes was higher at 28 hours but lower at 48 hours post-PHx in IL-22TG mice versus WT mice, respectively, and was comparable 72 hours and 96 hours after surgery in both groups (Fig. 4B,C). These findings indicate that IL-22TG mice have accelerated liver regeneration after PHx.
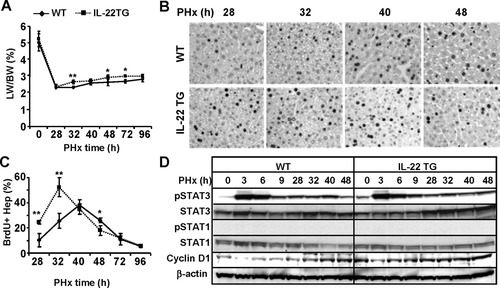
IL-22TG mice have accelerated liver regeneration after PHx. Mice were subject to PHx and injected with BrdU 2 hours before sacrifice. Liver tissues were collected for BrdU immunostaining. (A) Liver/body weight ratio. (B) Immunohistochemical staining with anti-BrdU antibody. BrdU+ cells appear as dark nuclei. (C) Number of BrdU+ hepatocytes. (D) Western blot analyses of liver tissues from partially hepatectomized WT and IL-22TG mice. *P < 0.05 and **P < 0.01 compared with corresponding WT mice.
In order to define the underlying mechanism for this enhanced liver regeneration in IL-22TG mice, western blot analyses for STAT3 and STAT1 activation were performed. Figure 4D shows that STAT3 but not STAT1 activation was detected after PHx and that such activation was comparable between WT and IL-22TG mice. However, IL-22TG mice had higher basal levels of pSTAT3 than WT mice. In addition, expression of cyclin D1 was higher in IL-22TG mice than in WT mice prior to treatment and at early time points after PHx (Fig. 4D). Thus, the increased levels of cyclin D1 may contribute to the accelerated liver regeneration in IL-22TG mice after PHx.
IL-22TG Mice Do Not Spontaneously Develop Liver Tumor but Exhibit Accelerated DEN-Induced Tumorigenesis.
IL-22 has been shown to promote hepatoma cell proliferation and survival12; however, no tumor was observed in all organs, including the liver, in IL-22TG mice up to 2 years of age (data not shown). Next, we examined whether IL-22TG mice were more susceptible to DEN-induced liver tumorigenesis. As illustrated in Fig. 5A,B, IL-22TG mice had a larger liver tumor size and a higher liver/body weight ratio than WT mice 9 months after DEN injection. Liver histology confirmed that the liver tumors from both WT and IL-22TG mice are hepatocarcinoma (Supporting Information Fig. 3a). The incidence of tumor development in IL-22TG mice was 100%, whereas the WT littermate group only had 60% and 80% tumor incidence after injection of 10 μg/g and 20 μg/g, respectively (Supporting Information Fig. 3b). In addition, the maximum size of tumors and the number of tumors were greater in IL-22TG mice compared with WT mice after DEN injection (Fig. 5C,D).
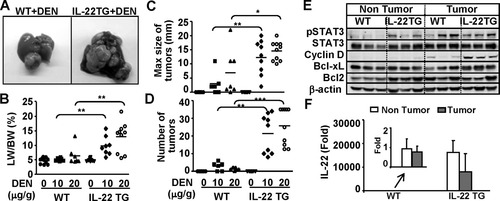
IL-22TG mice do not spontaneously develop liver tumors but are more susceptible to DEN-induced liver tumorigenesis. Mice were injected with 10 μg/g or 20 μg/g body weight DEN at 15 days of age and then sacrificed at 9 months of age. Liver tissue and tumors were collected. (A) Representative photographs of liver with tumors. (B) Liver/body weight ratio. (C) Size of tumors. (D) Number of tumors. (E) Western blot analysis. (F) Real-time PCR analysis of IL-22 expression in 9-month-old DEN-treated WT mice and IL-22TG mice. The value of nontumor tissues from DEN-treated WT mice was set as 1. In panels B, C, and D, *P < 0.05, **P < 0.01, and ***P < 0.001.
Western blot analysis demonstrated that pSTAT3 activation was detected in both nontumor and tumor regions in IL-22TG mice, but was detected only in the tumor region in WT mice. In addition, cyclin D1 and Bcl-xL expression were higher in the tumors from IL-22TG mice than in those from WT mice (Fig. 5E). Real-time PCR analyses revealed that IL-22 was detected at low levels in nontumor and tumor tissues from DEN-treated WT mice, but was detected at very high levels in nontumor and tumor tissues from DEN-treated IL-22TG mice (Fig. 5F). The levels of IL-22 in tumor tissues were slightly lower compared with those in nontumor tissues from DEN-treated IL-22 mice (Fig. 5F). Furthermore, the number of Ki67-positive cells was higher in the tumor tissues from IL-22TG mice compared with WT mice (Supporting Information Fig. 3c).
To rule out whether increased DEN-induced liver tumorigenesis in IL-22TG mice was due to an increase in DEN metabolism–associated liver injury and oxidative stress induced by a single dose of DEN injection at age 15 days, we measured serum ALT and AST, liver glutathione (an antioxidant), malondialdehyde (a marker of lipid peroxidation), and 8-hydroxy-2′-deoxyguanosine (a major product of DNA oxidation). As illustrated in Fig. 6A, DEN injection induced elevation of serum ALT and AST in both WT and IL-22TG mice, with lower serum ALT in the latter group 12 hours after injection. A single dose of DEN injection induced elevation of malondialdehyde and 8-hydroxy-2′-deoxyguanosine but reduction of glutathione in WT mice. Similar changes were observed in IL-22TG mice. These findings indicate that a single injection of DEN induced similar levels of lipid peroxidation and DNA oxidation in the liver of WT and IL-22TG mice at age 15 days, suggesting the increased DEN-induced liver tumorigenesis in IL-22TG is not caused by an increase in DEN-metabolism.
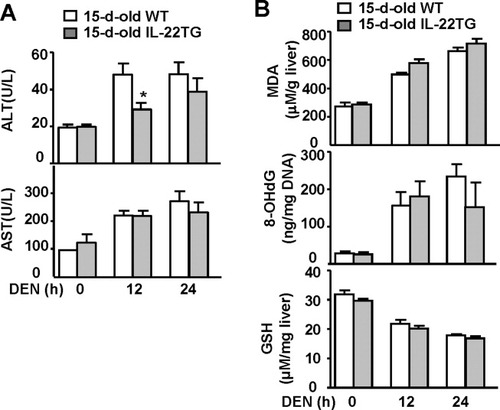
Liver injury, oxidative stress, and DNA damage in 15-day-old WT and IL-22TG mice after a single dose of DEN injection. WT and IL-22TG mice were injected intraperitoneally with DEN (10 μg/g) for 12 hours and 24 hours. (A) Sera were collected for measurement of ALT and AST. *P < 0.05. (B) Liver tissues were collected for measurement of malondialdehyde (MDA), 8-hydroxy-2′-deoxyguanosine (8-OHdG), and glutathione (GSH).
Up-regulation of Acute Phase Protein, Antioxidant, Mitogenic, and Antimicrobial Genes in Livers from IL-22TG Mice.
Microarray analyses revealed that many genes are induced in the livers from IL-22TG mice compared with WT mice (Table 1). Among them, the expression of two antioxidant genes, metallothionein 1 and 2, was up-regulated 20- and 37-fold in IL-22TG mice versus WT mice, respectively. Induction of these antioxidant genes in hepatocytes may be responsible for the hepatoprotection of IL-22. Several mitogenic and proliferative genes were also up-regulated from 1.5- to 2.4-fold in the livers of IL-22TG mice compared with those of WT mice, which is likely responsible for IL-22 promotion of liver regeneration and DEN-induced liver carcinongenesis.
IL-22 has been shown to stimulate hepatocytes to produce several acute phase proteins, including SAA, CD14, and LPS binding protein, and these genes were up-regulated in IL-22TG mice compared with WT mice (Table 1). In addition, several other acute phase genes, such as orosomucoid, fibrinogen-like protein, and serum amyloid P-component, were also up-regulated in the livers of IL-22TG mice compared with WT mice. It has been well documented that IL-22 plays an important role in protecting against bacterial infection by stimulating epithelial cells to produce antibacterial proteins.2, 3 In the current study, we show that expression of two antimicrobial genes—lipocalin 2 and proteinase 3—was highly induced in the livers of IL-22TG versus WT mice. Collectively, these findings suggest that targeting hepatocytes by IL-22 may also play an important role in the host defense against bacterial infection through induction of acute phase proteins and antimicrobial proteins.
Discussion
Although the hepatoprotection of IL-22 has been well documented,12-14 the current study from IL-22TG mice provides several novel findings and implications of IL-22 in the pathogenesis of human liver diseases. First, IL-22TG mice grew normally, suggesting that the therapeutic application of IL-22 in treating patients with liver injury may have minimal side effects. Second, the protective role of IL-22 in liver injury is due to its direct hepatoprotection and not due to modulation of the inflammatory response. Third, hepatic IL-22 is up-regulated in patients with chronic HBV and HCV, and likely promotes hepatocyte survival and may accelerate liver cancer promotion in these patients.
The fact that liver-specific IL-22TG mice had no obvious adverse phenotypes suggests that the therapeutic application of IL-22 in treating patients with acute liver injury and alcoholic hepatitis may have few side effects. IL-22TG mice driven by the EμLCK or insulin II promoter had severe adverse phenotypes (most mice died within a few days after birth).18 In contrast, the liver-specific IL-22TG mice described here develop normally and have no obvious adverse phenotypes. One possible explanation for the differences in the studies is that the IL-22TG driven by the EμLCK or insulin II promoter resulted in high levels of IL-22 expression before birth, whereas the IL-22TG driven by the albumin promoter only expressed IL-22 after birth (albumin expression by hepatocytes occurs after birth). Recently, Liang et al.20 reported that injection of 5 × 1010 particles of IL-22 adenovirus resulted in serum levels of 35,000-95,000 pg/mL IL-22 and causes hematological changes, loss of body weight, and thymic atrophy, whereas we have previously shown that injection of 2 × 108 particles of IL-22 adenovirus resulted in serum levels of 5,000 pg/mL IL-22 (similar to those in liver-specific IL-22TG mice) and did not induce obvious adverse phenotypes.14 These findings suggest that only very high doses of IL-22 may cause severe adverse phenotypes. However, it is unlikely that therapeutic application of IL-22 will reach such high concentrations of IL-22 (35,000-95,000 pg/mL) in the serum reported in the study by Liang et al.20 Regardless, monitoring IL-22 levels will be important in any therapeutic applications.
Although the hepatoprotection of IL-22 is well documented,12-14 the role of IL-22 in liver inflammation remains obscure. Liang et al20 reported that a single injection of IL-22 up-regulated expression of CXCL1 in the liver, followed by a transient increase in circulating neutrophils, suggesting IL-22 may promote liver inflammation. In contrast, blockage of IL-22 either through using a neutralizing antibody12 or genetic deletion13 exacerbated inflammation, whereas treatment with IL-2212 or overexpression of IL-22 (the current study) ameliorated ConA-induced liver inflammation, indicating IL-22 suppresses liver inflammation in this model. It is plausible that IL-22 plays dual roles in controlling liver inflammation: promoting liver inflammation by stimulating hepatocytes to produce acute phase proteins and chemokines, and inhibiting liver inflammation by preventing hepatocyte damage and subsequently reducing necrosis-associated liver inflammation. The final effect of IL-22 on liver inflammation is likely determined by the balance between the proinflammatory and anti-inflammatory effects of IL-22 and is dependent on the types of liver diseases and liver injury models.
A previous study reported that the expression of hepatic IL-22 messenger RNA was up-regulated in patients with viral hepatitis.23 Here we demonstrate that IL-22+ immune cells are accumulated in the livers of patients with viral hepatitis (Fig. 1); however, the types of inflammatory cells responsible for IL-22 production in viral hepatitis patients remain obscure. Because Th17, Th22, natural killer, and natural killer T cells, which are known to produce IL-22,2-4 are elevated in the livers in patients with viral hepatitis,24-26 these cells likely contribute to hepatic IL-22 expression in the patients. Further studies are needed to confirm this assessment.
The next obvious question is how IL-22 up-regulation might affect the progression of viral hepatitis disease progression. First, it has been reported that IL-22 does not inhibit HCV replication in vitro,23 suggesting that elevated IL-22 may not affect HCV replication directly. However, we cannot rule out the possibility that IL-22 may affect HCV or HBV replication indirectly through modulation of the antiviral effect of interferons, because STAT3, a major IL-22 downstream signal, is known to modulate interferon-mediated signaling pathways.27 Second, although the number of IL-22+ cells correlated positively with the serum levels of AST in patients with viral hepatitis (Fig. 1), and serum and hepatic IL-22 levels correlated positively with serum ALT and AST in mice with T cell hepatitis (Supporting Information Fig. 1), the studies from animal models suggest that IL-22 protects against liver injury and promotes hepatocyte proliferation (current study).12-14 Thus, elevation of IL-22 in patients with viral hepatitis likely plays a compensatory role in protecting against hepatocellular damage. Third, although IL-22TG mice did not spontaneously develop liver tumor, these mice had accelerated DEN-induced liver tumor formation. This observation suggests that high levels of IL-22 alone may not induce liver tumor but that IL-22 may synergistically promote hepatic carcinogenesis in patients with chronic viral hepatitis. It is plausible that liver cancer in viral hepatitis patients is often associated with inflammation that produces IL-22, which may act as a local paracrine factor to stimulate liver cancer cell proliferation. Thus, inhibition of IL-22 could be a potential therapeutic option for the treatment of liver cancer in patients with high levels of inflammation and IL-22 in the liver.




