Interleukin-10–mediated heme oxygenase 1–induced underlying mechanism in inflammatory down-regulation by norfloxacin in cirrhosis†‡
Potential conflict of interest: Nothing to report.
This work has been supported in part by grants CP05/0005 and PI08/1075 from Instituto de Salud Carlos III, Madrid, Spain, Excma. Diputación de Alicante, Spain, and Fundación FCVI-HGUA, Alicante, Spain.
Abstract
Patients with cirrhosis receiving norfloxacin show a restored inflammatory balance that likely prevents clinical complications derived from an excessive proinflammatory response to bacterial product challenges. This study sought to investigate associated inflammatory control mechanisms established in patients with cirrhosis receiving norfloxacin. A total of 62 patients with cirrhosis and ascites in different clinical conditions were considered. Blood samples were collected and intracellular and serum norfloxacin were measured. Inflammatory mediators were evaluated at messenger RNA and protein levels. Neutrophils from all patients were cultured with lipopolysaccharide (LPS) and anti–interleukin-10 (anti–IL-10) monoclonal antibody in different conditions. IL-10 and heme oxygenase-1 (HO-1) were up-regulated in patients receiving norfloxacin and correlated with norfloxacin in a concentration-dependent manner, whereas proinflammatory inducible nitric oxide synthase, cyclooxygenase-2, and nuclear factor-κB behaved inversely. Higher IL-10 levels correlated with lower white blood cell count and higher mean arterial pressure. No correlations were found between IL-10 and disease clinical scores or liver function markers in blood. Neutrophilic in vitro assays showed that the effect of LPS on proinflammatory mediator levels in the presence of norfloxacin was abrogated by significantly increasing IL-10 and HO-1 expression. After stimulation with LPS plus anti–IL-10, proinflammatory mediators were dramatically increased in patients receiving norfloxacin, and increasing intracellular norfloxacin concentrations did not decrease the expression levels of these proinflammatory molecules. Unblocking IL-10 restored proinflammatory mediator and HO-1 expression to previously observed levels in response to LPS stimulation. Conclusion: Although the described association does not necessarily mean causality, an IL-10–mediated HO-1–induced anti-inflammatory mechanism is present in patients with cirrhosis receiving norfloxacin, that is directly associated with cell-modulating events in these patients. (HEPATOLOGY 2011;)
Bacterial translocation (BT) is known as the process by which bacteria exit the intestinal lumen in certain diseases, such as decompensated cirrhosis, access mesenteric lymph nodes and, eventually, colonize other organs.1 This mechanism is involved in the pathogenesis of spontaneous bacterial peritonitis (SBP), one of the most representative and clinically relevant complications of cirrhosis.2, 3 To reduce the incidence of this complication, oral norfloxacin is administered, either as primary prophylaxis (400 mg twice a day for 1 week) to patients with upper gastrointestinal bleeding or as secondary prophylaxis (indefinitely, 400 mg daily) to those who have survived a previous episode of SBP. In this last condition, norfloxacin administration significantly reduces the incidence of bacterial infections4, 5 and, used as primary prophylaxis, also reduces noninfectious related clinical complications, such as hepatorenal syndrome, thus improving survival.6
BT in cirrhosis is related to an increased blood secretion of proinflammatory soluble mediators such as cytokines and nitric oxide (NO),7 which may be potentially harmful and can lead to severe clinical complications such as circulatory dysfunction and hepatorenal syndrome.6, 8 In fact, it has been recently shown that bacterial DNA translocation, a surrogate marker of BT, is associated with a marked worsening of the intrahepatic endothelial dysfunction in cirrhosis (Hepatology 2010, in press) and with an increased risk of death.9
Selective intestinal decontamination (SID) with norfloxacin as secondary prophylaxis of SBP not only removes bacterial products but also modulates patients' proinflammatory reaction, showing a direct cellular effect on neutrophil response to oxidative stress by reducing secretion of reactive oxygen species and increasing the apoptosis rate.10 Both processes affect modulation of nuclear factor-kappa B (NF-κB), which triggers proinflammatory gene transcription. Nevertheless, an effective inflammatory reaction also requires an adequate interaction with active anti-inflammatory mediators aimed at keeping a proper homeostasis.
The present work was designed to investigate the anti-inflammatory counteracting mechanisms occurring within patients with decompensated cirrhosis and how norfloxacin participates in the restoration of the inflammatory balance, completing norfloxacin's previously described immunomodulatory actions.
Abbreviations
AF, ascitic fluid; ASC, noninfected ascites; BT, bacterial translocation; COX-2, cyclooxygenase-2; ELISA, enzyme-linked immunosorbent assay; GM-CSF, granulocyte macrophage colony-stimulating factor; HO-1, heme oxygenase-1; IL-10, interleukin-10; iNOS, inducible form of nitric oxide synthase; LPS, lipopolysaccharide; mRNA, messenger RNA; NF-κB, nuclear factor kappa B; NOx, nitric oxide metabolites; PBS, phosphate-buffered saline; PMN, polymorphonuclear; SBP, spontaneous bacterial peritonitis; SID, selective intestinal decontamination; TGF-β, transforming growth factor beta.
Patients and Methods
Patients
We conducted a prospective study in consecutively admitted patients with cirrhosis and ascites in any of the following clinical situations: patients with SBP (Group I), patients admitted for the treatment of uncomplicated ascites without bacterial infections (ASC), as determined by negative microbiological culture and no presence of bacterial DNA in blood and ascitic fluid (AF), who were not receiving long-term SID with norfloxacin as secondary prophylaxis of SBP (Group II) and patients undergoing SID with norfloxacin as secondary prophylaxis of SBP (Group III). No patient included in the study showed multinodular hepatocellular carcinoma, portal thrombosis, alcoholic hepatitis, previous liver transplantation, or previous transjugular intrahepatic portosystemic shunt. SBP was defined as the presence of >250 polymorphonuclear (PMN) cells/μL in AF. The ethics committee of the hospital approved the study protocol, and all patients gave informed consent to participate in the study.
Blood and AF were obtained from all patients at admission and were analyzed for routine biochemical and cytological studies. In patients with SBP, samples were obtained at the time of SBP diagnosis. Blood and AF cultures were performed in all cases, as described elsewhere.11 Aliquots of blood and AF were inoculated under aseptic conditions in sterile, rubber-sealed Vacutainer SST II tubes (BD Diagnostics, Belgium) that were never exposed to free air.
Detection of Bacterial DNA Fragments and Measurement of Endotoxin Levels in Serum
To detect the presence of bacterial DNA fragments in blood and AF, a broad-range polymerase chain reaction (PCR) was performed according to the methodology described elsewhere.12 A quantitative chromogenic limulus amoebocyte lysate test (BioWhittaker, Nottingham, UK) was followed to evaluate endotoxin levels in blood and AF samples as previously described.13 Samples and reagents were handled in an airflow chamber and processed with pyrogen-free material tested by the manufacturers.
Isolation and Culture of Human PMN Cells
PMN cells from peripheral blood samples were isolated with PolymorphPrep (Axis-Shield PoC, Oslo, Norway) according to manufacturer's instructions. After PMN cell isolation, cells were washed twice with freshly made phosphate-buffered saline (PBS) at 4°C. Cell viability was evaluated by trypan blue (Sigma, Madrid, Spain). Cells were resuspended in phenol-red–free Roswell Park Memorial Institute 1640 medium (Gibco BRL, Life Technologies, Paisley, UK) supplemented with 10% human type AB serum (BioWhittaker, Walkersville, MD), 100 IU/mL penicillin/streptomycin, and 2.5 mg/mL amphotericin B (Gibco BRL). Resuspended cells (2 × 106 cells/well) were cultured for 4 hours at 37°C and 5% CO2 in the following conditions: (1) without stimuli; (2) with 25 μg/mL lipopolysaccharide (LPS) (E. coli serotype 0111:B4; Sigma, Madrid, Spain); (3) with 25 μg/mL LPS in the presence of 1 mg/mL human anti–IL-10 monoclonal antibody (mAb) (rat immunoglobulin G1, clone 9D7; Thermo Scientific, Madrid, Spain); and (4) with 25 μg/mL LPS after a 4-hour culture with 25 μg/mL LPS in the presence of 1 mg/mL human anti–IL-10 mAb was washed out with PBS. Additional conditions testing an anti–IL-10 isotype-matched control mAb were performed as control (data not shown).
Cellular Lysates and Immunoblotting
Cells were lysed with a commercial lysis buffer (Tris-HCl, pH 7.5, 20 mmol/L, NaCl 150 mmol/L, Na2 ethylene diamine tetraacetic acid 1 mmol/L, ethylene glycol tetraacetic acid 1 mmol/L, Triton 1%, sodium pyrophosphate 2.5 mmol/L, β-glycerophosphate 1 mmol/L, Na3VO4 1 mmol/L, leupeptin 1 μg/mL; Cell Signaling Technology, Boston, MA), 1 mmol/L phenylmethylsulfonyl fluoride (PMSF). Protein concentration was obtained by Bradford assay. Protein extracts (15 μg protein/lane) were resolved under reducing conditions on 12% SDS-polyacrylamide gels and then transferred to Immobilon-P membranes (Millipore, Billerica, MA). Membranes were blocked with 5% milk in PBS with Tween-20 0.1% for 1 hour at room temperature then incubated with primary antibodies overnight at 4°C and finally for 1 hour at room temperature with the correspondent horseradish peroxidase–conjugated secondary antibody. The primary antibodies used against heme oxygenase-1 (HO-1), interleukin-10 (IL-10), cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), phosphorylated p65–NF-κB (Ser 536), and β-actin were purchased from Santa Cruz Biotechnology (Heidelberg, Germany). The activity of membrane-bound peroxidase was detected using the ECL chemiluminescent system from Amersham Pharmacia Biotech (Piscataway, NJ). Protein bands were scanned and quantified by densitometry using Scion Image software (Scion Corp., Frederick, MD). Band densities were related to total β-actin protein and are provided as Supporting Information.
Norfloxacin
Norfloxacin in plasma samples was analyzed using a high-performance liquid chromatography method, as previously described.10 Briefly, chromatographic separation was performed using a reverse-phase Eclipse XDB-C18 column (5 μm pore size; 4.6 mm × 150 mm). The mobile phase was an acetonitrile tetrabutyl ammonium hydroxyphosphate buffer eluted at a flow rate of 1 mL/minute. Effluent was monitored at excitation and emission wavelengths of 278 and 456 nm, respectively. The limit of detection was 0.06 μg/mL (signal-to-noise ratio, 3:1). Norfloxacin was obtained from Sigma Chemical Co. (Madrid, Spain). Norfloxacin concentration in serum was expressed in milligrams per milliliter and in cells in milligrams per milliliter per 107 cells.
Measurement of Cytokine Levels
Enzyme-linked immunosorbent assays (ELISAs) for the quantitative measurement of IL-4, IL-10, IL-13, transforming growth factor-β (TGF-β), and granulocyte macrophage colony-stimulating factor (GM-CSF) levels were carried out in basal serum samples of patients, via Human Quantikine kits (R&D Systems, Minneapolis, MN), according to manufacturer's instructions. All samples were tested in triplicate and read in a microplate reader. Lower limits of detection of all cytokine assays were between 10 and 20 pg/mL. Standard curves were generated for each plate, and the average zero standard optical densities were subtracted from the rest of standards, controls, and samples to obtain a corrected concentration for all cytokines.
Measurement of NO Production
The sum of the NO metabolites (NOx) nitrite (NO2−) and nitrate (NO3−) is widely used as an index of NO generation14 and expressed as NOx levels per milliliter, which corresponds to 106 cells in this study. NOx levels in basal serum, and in supernatants from cultured PMNs, were calculated by measuring conversion of NO3− to NO2− by the enzyme nitrate reductase using an ELISA assay (R&D Systems). All samples were tested in duplicate and values were corrected by running samples with culture media without cells to assess background NOx levels.
Gene Expression and Protein Quantification
Genomic DNA was isolated from 5 × 106 cells by handling QIAmp DNA Blood Minikit (Qiagen, Hilden, Germany). Total cellular RNA was isolated from 5 × 106 cells by handling QIAmp RNA Blood Minikit (Qiagen). Quantitec SYBR Green (QIAgen) was used to perform gene expression in a IQ5 Real-Time PCR (Bio-Rad Laboratories, Hercules, CA). IL-10 gene expression was evaluated using 5′-CTGGGGCTCTGG GATAGCTGACC-3′ as forward primer and 5′-GCGT GGTCAGGCTTGGAATGGAA-3′ as reverse primer. Other primers used were: for HO-1, 5′-CAGCATGC CCCAGGATTTGTCAGA-3′ as forward and 5′-TCA CATAGCGCTGCATGGCTGG-3′ as reverse; for iNOS, 5′-CCACCTTTGATGAGGGGACTGGGC-3′ as forward and 5′- GGGGTAGGCTTGTCCCTG GGT-3′ as reverse; for COX-2, 5′-TAACCCCGCCAA AAGGGGTCCT-3′ as forward and 5′-GCATGCAGG TAGCCAGGCTTGA-3′ as reverse; and for NF-κB, 5′-TGCCAACAGCAGATGGCCCA-3′ as forward and 5′-CACCAGGCTGTGGGCATGCA-3′ as reverse. Protein levels were obtained by running commercially available ELISA assays (Quantikine human iNOS and Human total HO-1 from R&D Systems; COX-2 ELISA kit from EMD Biosciences, Darmstadt, Germany; and NF-κB ELISA kit from Oxford Biomedical Research, Oxford, MI) according to the manufacturer instructions.
Statistical Analysis
Continuous variables are reported as mean ± standard deviation and categorical variables as frequency or percentages. The Kolmogorov-Smirnov test was used to assess the normality of the distribution of continuous variables. Statistical differences of basal characteristics between groups were analyzed using the chi-square test for categorical data and the analysis of variance (ANOVA) test for quantitative data showing normal distribution or the Mann-Whitney U test for quantitative data showing non-normal distribution. Bivariate correlations between continuous variables were calculated using the Pearson test. Multiple comparisons were analyzed using the ANOVA test with Bonferroni correction. All reported P values are two-sided, and P values lower than 0.05 are considered to indicate significance. All calculations were performed using the SPSS 16.0 software (SPSS, Inc., Chicago, IL).
Results
Characteristics of Patients
A total series of 62 patients was included in the study. Of those, 22 patients had SBP, either with a positive (n = 9) or negative (n = 13) culture. No clinical or analytical statistically significant differences were observed between culture-positive and culture-negative patients with SBP. Bacterial DNA was identified in all 22 patients with SBP, regardless of their microbiological culture. Identified bacterial species were Escherichia coli (n = 12), Staphylococcus aureus (n = 4), Streptococcus spp. (n = 3), Klebsiella pneumoniae (n = 2), and Enterococcus faecalis (n = 1). No differences were observed in the proportion of gram-negative and gram-positive sequencing-identified microorganisms between culture-negative and culture-positive patients with SBP. Among patients with culture-positive SBP (n = 9), the culture-isolated microorganisms corresponded to those identified by nucleotide sequencing in all cases, except one identified as Staphylococcus aureus by sequencing but as Streptococcus pneumoniae by microbiological culture. Mean amplified bacterial DNA concentration was 32.1 ± 8.6 ng/μL and mean serum endotoxin levels were 1.46 ± 0.65 endotoxin units (UE)/mL. Twenty patients with cirrhosis and ASC, as determined by positive microbiological culture or bacterial DNA presence in blood and AF, who were not receiving SID with norfloxacin constituted Group II. Serum endotoxin levels within this group were 0.35 ± 0.06 UE/mL (P < 0.05 compared with SBP group). Finally, 20 patients with cirrhosis and ascites who were undergoing SID with norfloxacin as secondary prophylaxis of SBP were also included. The period of norfloxacin administration was shorter than 14 months in all patients. Bacterial DNA was not found in any sample in this group, and serum mean endotoxin levels were 0.32 ± 0.05 UE/mL (P < 0.05 compared with SBP patients).
Patients' clinical and analytical characteristics are shown in detail in Table 1. Mean age of included patients was 58 years, and 61% of them were male. Total white blood cells and PMN cells in AF were statistically increased in the overall series of SBP versus the rest of the patients. Sixteen of 22 patients with SBP, four of 20 patients with ASC, and all patients undergoing SID with norfloxacin had had previous episodes of ascites. Three patients with SBP, two patients with ASC and two patients undergoing SID had had previous episodes of encephalopathy. A 6-month period of follow-up was studied in all patients. Four patients with SBP, two patients with ASC, and three patients undergoing SID died during the follow-up. Causes of death included: liver insufficiency (n = 2), upper gastrointestinal bleeding (n = 1), renal failure (n = 1) in the group of patients with SBP; liver insufficiency (n = 1) and renal failure (n = 1) in the group of patients with ASC; and graft dysfunction after liver transplantation (n = 1), liver insufficiency (n = 1), and myocardial infarction (n = 1) in patients undergoing SID.
| Characteristic | Group I (n = 22), SBP | Group II (n = 20), Noninfected Ascites | Group III (n = 20), SID |
|---|---|---|---|
| Age (years) | 57.1 ± 11.4 | 59.2 ± 11.2 | 57.4 ± 10.4 |
| Male sex, n(%) | 11 (50%) | 13 (65%) | 14 (70%) |
| Ethiology | |||
| Alcohol | 10 | 14 | 12 |
| HCV | 6 | 3 | 2 |
| Alcohol + HCV | 4 | 3 | 4 |
| Others | 2 | 0 | 2 |
| Previous episodes of ascites, n (%) | 16 (72.7%) | 4 (20%) | 20 (100%) |
| Previous episodes of encephalopathy, n (%) | 3 (13.6%) | 1 (5%) | 2 (10%) |
| Child-Pugh A/B/C (n) | 0/14/8 | 0/14/6 | 0/12/8 |
| Child-Pugh score | 9.0 ± 1.5 | 9.2 ± 1.6 | 9.6 ± 1.9 |
| MELD Mean score | 16.8 ± 9.5 | 13.3 ± 4.2 | 14.9 ± 6.6 |
| Mean arterial pressure (mm Hg) | 75.2 ± 10.6 | 84.5 ± 14.3 | 81.9 ± 11.5 |
| Bilirubin (mg/dL) | 3.8 ± 3.2 | 3.3 ± 2.2 | 4.4 ± 4.7 |
| Albumin (g/dL) | 3.1 ± 1.1 | 2.8 ± 0.5 | 3.2 ± 0.5 |
| Quick (%) | 52.2 ± 21.5 | 60 ± 15.5 | 49.4 ± 12.0 |
| International normalized ratio | 1.8 ± 0.6 | 1.6 ± 0.5 | 1.7 ± 0.5 |
| Serum Creatinine (mg/L) | 1.2 ± 1.0 | 0.9 ± 0.5 | 1.0 ± 0.4 |
| Serum sodium (mEq/L) | 131.3 ± 8.8 | 135.2 ± 3.5 | 134.1 ± 3.6 |
| Serum potassium (mEq/L) | 4.4 ± 0.6 | 4.6 ± 0.4 | 4.1 ± 0.7 |
| AST (IU/L) | 67.2 ± 49.5 | 65.6 ± 48.2 | 66.5 ± 57.6 |
| AST (IU/L) | 38.2 ± 20.9 | 36.3 ± 29.6 | 36.2 ± 31.8 |
| Platelets/mm3 | 155000,0 ± 90153.0 | 124290 ± 62300 | 101285.7 ± 49468.6 |
| Total Blood WBC/mm3 | 8626.2 ± 5235.0 | 5644.6 ± 2485.1 | 3682.8 ± 2930.9 |
| Total Blood PMN/mm3 | 7018.1 ± 4732.4 | 4830.6 ± 2956.4 | 3200.8 ± 1862.5 |
| Total AF protein (g/dL) | 1.7 ± 1.2 | 1.7 ± 0.8 | 1.4 ± 0.5 |
| Total AF WBC/mm3 | 3653.1 ± 5416.2 | 204 ± 186.2* | 143.6 ± 156.5* |
| Total AF PMN/mm3 | 3396.6 ± 5230.9 | 45.8 ± 33.5* | 62.0 ± 14.9* |
- Values are expressed as mean standard ± standard deviation.
- * P ± 0.05 compared with SBP group. ALT, alanine aminotransferase; AST, aspartate aminotransferase; MELD, Model for End-Stage Liver Disease; PMN, polymorphonucleocyte; WBC, white blood cell.
IL-10 Skewed the Anti-Inflammatory Balance in Patients With Cirrhosis
Serum levels of different anti-inflammatory cytokines were evaluated in all patients included (Table 2). IL-10 was significantly increased in patients undergoing SID, being the only factor differentially expressed among the three groups of patients. Besides, a positive correlation was established between this cytokine and norfloxacin serum levels (Fig. 1A). On the contrary, IL-4, IL-13, TGF-β, and GM-CSF serum levels were not statistically different between groups and no correlation with norfloxacin serum levels was found for any of these cytokines. HO-1 protein levels in patients with SID were significantly increased compared to patients with SBP and patients with noninfected AF as well (Table 2). Similar to IL-10, a positive correlation between HO-1 and norfloxacin was found in serum of patients with SID (Fig. 1B). IL-10 and HO-1 protein (Fig. 1C) and messenger RNA (mRNA) levels (Fig. 1D) were also represented and confirmed a correlation between these two molecules for the three groups of patients.
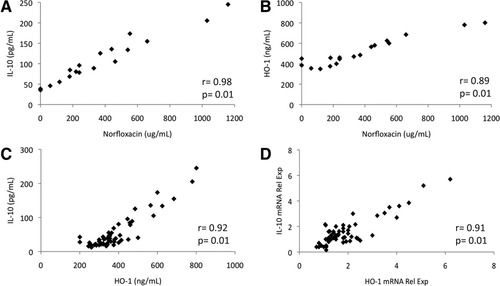
Correlations between serum norfloxacin and serum (A) IL-10 or (B) HO-1 levels in patients with SID. Correlations between IL-10 and HO-1 at (C) gene and (D) protein levels. “Rel Exp”, relative mRNA expression.
| Mediator | Group I (n = 22), SBP | Group II (n = 20), Noninfected Ascites | Group III (n = 20), SID |
|---|---|---|---|
| IL-4 (pg/mL) | 33.4 ± 5.4 | 34.3 ± 12.3 | 25.6 ± 12.6 |
| IL-10 (pg/mL) | 26.9 ± 9.1 | 30.0 ± 11.2 | 108.3 ± 58.1$ |
| IL-13 (pg/mL) | 42.0 ± 16.6 | 40.2 ± 15.8 | 43.8 ± 17.3 |
| TGF-β (pg/mL) | 30.8 ± 11.9 | 32.5 ± 13.4 | 36.5 ± 7.7 |
| GM-CSF (pg/mL) | 37.7 ± 14.9 | 38.9 ± 14.0 | 47.9 ± 18.1 |
| HO-1 (ng/mL) | 302.1 ± 40.1 | 339.4 ± 69.7 | 514.9 ± 138.8$ |
| COX-2 (ng/mL) | 107.0 ± 19.0 | 60.0 ± 9.3* | 41.6 ± 13.6$ |
| iNOS (U/mL) | 15.4 ± 3.4 | 12.2 ± 2.1* | 8.5 ± 2.4$ |
| NOx (nmol/L) | 33.9 ± 5.9 | 25.2 ± 4.6* | 17.5 ± 6.37$ |
| Act NF-κB (pg/mL) | 57.3 ± 11.9 | 37.3 ± 6.5* | 10.3 ± 4.1$ |
- Values are expressed as mean ± standard deviation.
- * P ± 0.05 compared with SBP group;
- $ P ± 0.05 compared with the rest of the groups. Act NF-κB, active NF-κB; NOx, nitric oxide metabolites; TGF-β, transforming growth factor beta.
On the other hand, the IL-10 pathway acting to counterbalance molecules such as iNOS, COX-2, and NF-κB was significantly higher in patients with ASC compared to patients with SID. Levels became significantly further up-regulated in patients with SBP (Table 2). In the group of patients undergoing SID, levels of these proinflammatory mediators inversely correlated with norfloxacin serum levels (Fig. 2). The sum of NOx in serum of patients with SID was significantly lower than in patients with noninfected AF. Values positively correlated with iNOS protein levels (r = 0.92, P = 0.01) when the total series of patients from all three groups were considered.
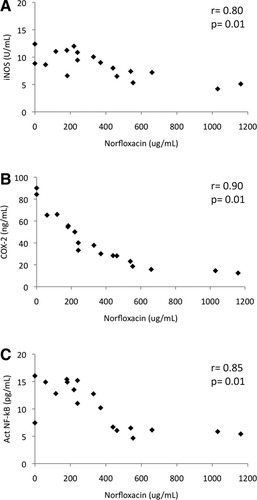
Correlations between serum norfloxacin and serum (A) iNOS, (B) COX-2, and (C) NF-κB levels in patients with SID.
Mean arterial pressure correlated with IL-10 serum levels (Fig. 3) and IL-10 mRNA levels (r = 0.74, P = 0.01) in the overall series of patients included in the study. Total blood white blood cells inversely correlated with IL-10 both at serum and mRNA levels (r = −0.54, P = 0.02 and r = −0.55, P = 0.02, respectively). A correlation was also observed between IL-10 and norfloxacin serum levels in patients with SID (r = 0.96, P = 0.00), as well as between IL-10 mRNA and intracellular norfloxacin levels (r = 0.89, P = 0.01). No correlations were found between IL-10 and disease clinical scores or liver function markers in blood.
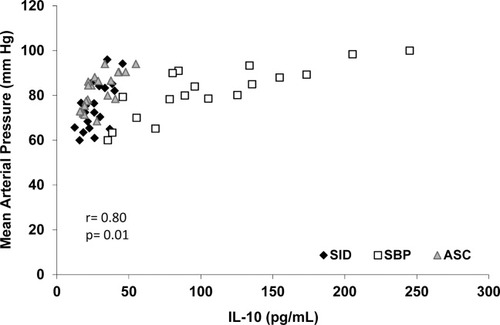
Correlation between serum IL-10 levels and mean arterial pressure in all patients included in the study distributed by groups.
HO-1–Dependent IL-10 Regulation of Proinflammatory Response in Patients With SID
Mean intracellular norfloxacin concentration in patients with SID was 4.1 ± 3.7 μg/mL/107 cells, ranging from 1.0-15.2 μg/mL/107 cells. Patients with SID were further distributed according to intracellular norfloxacin concentration into three subgroups (Nflx1: 0-2.5 μg/mL/107 cells, n = 6; Nflx2: 2.5-5 μg/mL/107 cells, n = 9; and Nflx3: <5 μg/mL/107 cells, n = 5) and, serving patients with noninfected AF and patients with SBP as controls, were subjected to neutrophilic in vitro assays to validate the associated proinflammatory regulatory mechanism present in patients under treatment with norfloxacin.
Figure 4 shows mRNA expression levels of IL-10, HO-1, COX-2, NF-κB, and iNOS in neutrophils of patients with SBP and noninfected AF, and of patients with SID distributed according to intracellular norfloxacin concentration after 4-hour resting culture or stimulated with LPS. As can be observed, in a nonstimulated situation, increasing intracellular amounts of norfloxacin significantly up-regulates IL-10 and HO-1 mRNA expression, compared with patients with noninfected AF and patients with SBP. On the other hand, proinflammatory mediators are down-regulated in patients with SID as intracellular norfloxacin concentrations rise, compared with patients with noninfected AF or patients with SBP. When LPS is added to culture, proinflammatory mediators respond by increasing their gene expression levels in patients with noninfected AF to levels similar to those present in patients with SBP. However, in the presence of norfloxacin, levels of these molecules are held to levels similar to those present in nonstimulated conditions, abrogating the effect of LPS and significantly increasing IL-10 and HO-1 expression as intracellular norfloxacin concentrations rise. Protein expression of all studied molecules in both conditions mimic those obtained for gene expression and can be followed in the corresponding blots along Fig. 4 (data on protein band densitometry is provided in Supporting Information Fig. 1).
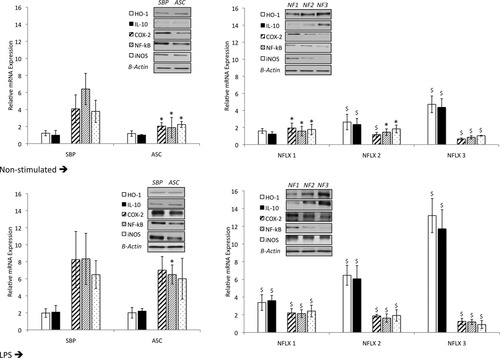
mRNA expression levels of IL-10, HO-1, COX-2, NF-κB, and iNOS in neutrophils of patients with SBP and noninfected AF, and of patients with SID distributed according to their intracellular amount of norfloxacin (see Materials and Methods) after 4-hour resting culture or stimulation with LPS. A representative immunoblot of IL-10, HO-1, iNOS, COX-2, and NF-κB protein expression in study groups is also represented. *P < 0.05 compared with SBP; $P < 0.05 compared with SBP, noninfected ascites and the previous norfloxacin condition. NFLX, norfloxacin.
In vitro experiments with anti–IL-10 mAb were conducted to validate the inflammation regulatory mechanism associated with norfloxacin in patients with SID (Fig. 5). After stimulation with LPS plus anti–IL-10 mAb, half of the cultured cells were directly tested and the other half was washed with PBS and recultured with LPS alone. In the first set, stimulation with LPS plus anti–IL-10 mAb induced higher levels of proinflammatory mediators than LPS-stimulated cells in all groups of patients. Especially relevant, all proinflammatory mediators were dramatically higher than in LPS-stimulated cells from patients with SID, reaching levels observed in cells from patients with noninfected AF. Besides, increasing intracellular norfloxacin concentrations did not decrease their expression levels, as happened with LPS-stimulated cells. Although anti–IL-10 did not affect IL-10 synthesis, proinflammatory control was clearly abolished, probably through an IL-10 downstream regulation of HO-1, which was severely decreased in anti–IL-10 presence. In the second set, levels of proinflammatory mediators were restored to those present in Fig. 4 and increasing amounts of norfloxacin were again associated with decreasing COX-2, iNOS, and NF-κB expression levels. Unblocking IL-10 also restored HO-1 expression to previously observed levels. Protein expression mimicked, again, gene expression and is represented in the corresponding blots along Fig. 5 (data on protein band densitometry is provided in Supporting Information Fig. 1). As a control, IL-10 levels were measured in all cell supernatants, to evaluate free IL-10 available for its receptor to signal down. Levels were almost undetectable for the first set of cultured cells and significantly increased in the second set (data not shown).
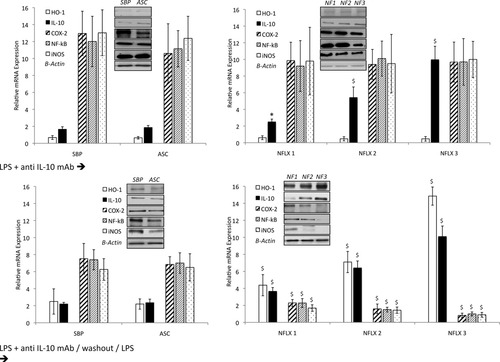
mRNA expression levels of IL-10, HO-1, COX-2, NF-κB, and iNOS in neutrophils of patients with SBP and noninfected AF, and of patients with SID distributed according to their intracellular amount of norfloxacin in cells differently stimulated with anti–IL-10 mAb (see Materials and Methods). A representative immunoblot of IL-10, HO-1, iNOS, COX-2, and NF-κB protein expression in the study groups is also represented. *P < 0.05 compared with SBP; $P < 0.05 compared with SBP, noninfected ascites and the previous NFLX condition. NFLX, norfloxacin; anti–IL-10 mAb, anti–IL-10 monoclonal antibody.
Discussion
The current study shows an IL-10–mediated HO-1–induced anti-inflammatory mechanism present in patients with cirrhosis receiving norfloxacin as secondary prophylaxis for SBP. Although the association between norfloxacin and this mechanism does not imply causality and would require further molecular studies, it directly associates norfloxacin with cell-modulating events in these patients.
Besides its known bactericidal effect on intestinal gram-negative bacterial flora, norfloxacin has been shown to induce several immunological effects at the cellular level, decreasing proinflammatory cytokines, controlling oxidative burst, and restoring the apoptotic rate in compromised PMN cells.10 However, molecular control of the proinflammatory response remained to be underlined in this setting. To validate our results, we compared patients with SID with a matched control group of patients with cirrhosis and ascites who are not taking norfloxacin and also with patients who have SBP, as a control group with an overt infection.
In our study, an initial screening of several anti-inflammatory mediators revealed IL-10 as a significantly increased cytokine in patients with SID compared with patients with noninfected AF that positively correlated with serum norfloxacin levels. IL-10 signaling induces the expression of HO-1, a stress-inducible protein with anti-inflammatory activity, through a p38 mitogen-activated protein kinase–dependent pathway.15, 16 Accordingly, we observed that HO-1 levels were also increased in patients with SID versus patients with noninfected AF and that significantly correlated with norfloxacin serum levels as well (Fig. 1). On the contrary, different proinflammatory mediators such as NF-κB, iNOS, and COX-2 showed a norfloxacin-dependent down-regulation in patients with SID compared with patients with noninfected AF and patients with SBP (Fig. 2).
From this starting point, patients with SID were distributed into three subgroups according to intracellular norfloxacin range levels to further investigate the inflammatory balance dependency on norfloxacin concentration. LPS treatment significantly increased mRNA expression levels of all proinflammatory mediators in neutrophils from patients with noninfected AF to those present in SBP whereas increasing amounts of intracellular norfloxacin limited proinflammatory molecule levels to those observed in resting cells. On the other hand, IL-10 and HO-1 mRNA levels were increased as intracellular norfloxacin rose in response to LPS, whereas levels in patients with noninfected AF and with SBP remained similar to those present in resting cells (Fig. 3).
The association between norfloxacin and IL-10 in the regulation of inflammation was confirmed by blocking secreted IL-10. Under these conditions, IL-10 failed to induce HO-1 expression. Accordingly, no regulation of proinflammatory cytokines was established by norfloxacin, regardless of its intracellular concentration, and the LPS effect was significantly increased compared with IL-10 unblocked conditions. Finally, anti–IL-10 washout restored mRNA expression levels in response to LPS (Fig. 4). According to these results, IL-10/IL-10 receptor (IL-10R) complex disruption with anti–IL-10 would prevent downstream signaling and HO-1 would not be induced. Therefore, there would be no modulation of proinflammatory mediators that would fail to decrease with increasing amounts of norfloxacin, reaching levels observed in patients with noninfected AF in response to LPS. Studies in other settings have demonstrated that blocking either IL-10 or IL-10R reverses the anti-inflammatory effects driven by IL-1017, 18 and are even associated with enhanced mortality at the time of polymicrobial sepsis in mice.19 In the context of liver disease, defective expression of IL-10 is associated with inflammation in alcoholic cirrhosis20 and IL-10–deficient mice are more sensitive to ethanol-induced liver injury.21
The role of HO-1 in such regulatory mechanisms has become relevant in recent years because its induction has been shown to prevent ethanol-induced inflammation in the intestine22 and liver23 and also in oxidative damage in hepatocytes.24 During endotoxemia, COX-2–deficient mice show an improved survival against LPS-induced inflammation by increasing IL-10 secretion due to alterations in HO-1 and iNOS gene expression levels25, 26 and also in endotoxemic mice, exogenous administration of recombinant IL-10 has been shown to reduce lethality.27-29 Along the same line of evidence, IL-10–derived HO-1 induction in patients with SID, and its correlation with norfloxacin intracellular levels, would decrease COX-2 and iNOS expression, drawing a fine-balanced mechanism of inflammatory response. The intimate link between norfloxacin and IL-10 induction remains to be identified and, therefore, causality cannot be proved. In fact, an alternative explanation could be a prolonged compensatory anti-inflammatory response during prophylaxis with norfloxacin due to changes in BT episodes or species. Effect of quinolones on signal transduction is poorly understood, although studies in human monocyte cell lines treated with moxifloxacin, a norfloxacin-like quinolone, have suggested a role in the inhibition of the IκB complex degradation.30 Precisely, IL-10 has been described to target the IκB complex, as well, during its inhibitory role on IL-8 in cystic fibrosis epithelial cells.31
The clinical consequences of controlling molecules involved in hemodynamic disturbances, such as COX-2, iNOS, and NOx, shown by IL-10 and probably triggered through norfloxacin, are difficult to observe. In our study, we found no association between the expression of these molecules and disease clinical or analytical markers. However, in our series of patients, both lower white blood cell count and higher mean arterial pressure correlated with higher IL-10 serum levels. This last finding suggests the implication of this molecule in ameliorating circulatory dysfunction. In fact, it has been reported that norfloxacin administration allows an improvement of the hemodynamic status in patients with cirrhosis.32 Because of the strong correlation we observe with IL-10 in this study, we provide a possible explanatory mechanism for norfloxacin to improve the hemodynamic status in patients with SID. Despite this possible physiological explanation for the positive effects related to long-term prophylaxis with norfloxacin, and the evidences previously mentioned,6 studies on norfloxacin's ability to keep an inflammatory control in the long term and the appropriate randomized clinical trials to evaluate the expansion of primary prophylaxis in high-risk patients with cirrhosis remain to be pursued. Also, the possibility of implementing IL-10–derived therapeutic strategies, according to its increasingly recognized function as a complex immunologic regulator,33 deserves consideration in future studies.
In summary, this study provides evidence of an IL-10–driven anti-inflammatory response in patients with cirrhosis undergoing SID with norfloxacin as secondary prophylaxis of SBP and a mechanism by which IL-10 would control the inflammatory activity in these patients. This mechanism is associated with norfloxacin in a concentration-dependent manner and suggests the direct implication of this quinolone on balancing the inflammatory reaction in patients with cirrhosis. Further studies on molecular interactions between norfloxacin and IL-10 engaging this physiological control need to be pursued.




