A novel GSK-3 beta–C/EBP alpha–miR-122–insulin-like growth factor 1 receptor regulatory circuitry in human hepatocellular carcinoma†
Potential conflict of interest: Nothing to report.
Abstract
miR-122 is a highly abundant, hepatocyte-specific microRNA. The biomedical significance and regulatory mechanisms of miR-122 remain obscure. We explored the role of miR-122 in tumorigenesis in the context of gene regulatory network. The miR-122 promoter and its transactivator were identified by way of luciferase reporter system, electrophoretic mobility shift, and chromatin immunoprecipitation assays. The miR-122 regulatory circuitry and its implication in hepatocarcinogenesis were identified using livers of different development stages, human hepatocellular carcinoma (HCC) tissues and cell lines, and aflatoxin B1 (AFB1)-transformed cells. We characterized the −5.3 to −4.8 kb region upstream of miR-122 precursor as miR-122 promoter. Further investigation revealed that deletion of predicted CCAAT/enhancer-binding protein alpha (C/EBPα) binding sites C/EBPα knockdown significantly reduced miR-122 promoter activity and endogenous miR-122 expression; and C/EBPα directly interacted with the miR-122 promoter in vitro and in vivo. These data suggest that C/EBPα is a transactivator for miR-122 transcription. We further demonstrated that miR-122 suppressed insulin-like growth factor 1 receptor (IGF-1R) translation and sustained glycogen synthase kinase-3 beta (GSK-3β) activity. The activated GSK-3β not only repressed cell proliferation, but also activated C/EBPα, which maintained miR-122 levels and thereby enforced IGF-1R suppression. Interestingly, down-regulation of miR-122 and C/EBPα, and up-regulation of IGF-1R were frequently observed in HCC tissues, and decreased miR-122 levels were associated with worse survival of HCC patients. Moreover, AFB1 exposure resulted in decreased activity in GSK-3β, C/EBPα, and miR-122 and increased levels of IGF-1R, whereas restoration of miR-122 suppressed the tumorigenicity of HCC and AFB1-transformed cells. Conclusion: We have identified a novel GSK-3β–C/EBPα–miR-122–IGF-1R regulatory circuitry whose dysfunction may contribute to the development of HCC. Our findings provide new insight into miR-122's function and the mechanisms of hepatocarcinogenesis. (Hepatology 2010;52:1702-1712)
MicroRNAs (miRNAs) repress the expression of protein-coding genes through binding to the 3′ untranslated region (UTR) of target messenger RNAs (mRNAs).1 An understanding of the roles of miRNA in tumorigenesis is emerging. Aberrant expression of miRNAs has been observed in both benign and malignant tumors. miRNA expression signatures can reflect the developmental lineage of human tumors and the clinical behaviors of cancers. Deregulation of miRNAs may confer cells with malignant phenotypes, including uncontrolled cell proliferation, resistance to apoptosis, and the capability of invasion and metastasis.2-4 However, most of the published studies that explore the implication of miRNA in tumorigenesis have focused on identifying individual targets of miRNA rather than elucidating miRNA function in the context of gene regulatory network.
As in other solid tumors, multiple genetic and epigenetic changes in protein-coding genes have been described in hepatocellular carcinoma (HCC).5 However, the evidence accumulated so far cannot explain the full complexity of HCC. Recent studies suggest that dysfunction of miRNAs also contributes to HCC development.6-10 miR-122 is a hepatocyte-specific miRNA that facilitates the replication of hepatitis C virus11 and regulates the metabolism of lipids12 and the expression of hepatic circadian genes.13 Recently, few targets of miR-122, including cyclin G1, serum response factor, insulin-like growth factor 1 receptor (IGF-1R), a disintegrin and metalloprotease family-10 and -17, have been experimentally validated.14-16 Frequent miR-122 down-regulation is observed in HCC tissues.7, 14-16 To date, neither the mechanisms underlying miR-122 deregulation nor the regulatory networks of miR-122 have been investigated. In an attempt to explore the role of miR-122 in tumorigenesis in the context of gene regulatory network, we identify the promoter region of miR-122, reveal that CCAAT/enhancer-binding protein alpha (C/EBPα) directly transactivates miR-122 transcription, and demonstrate that decreased C/EBPα activity represents an important mechanism responsible for miR-122 deregulation in HCC. We further disclose a novel glycogen synthase kinase-3 beta (GSK-3β)-C/EBPα-miR-122-IGF-1R regulatory circuitry whose dysfunction is implicated in HCC development.
Abbreviations
AFB1, aflatoxin B1; C/EBPα, CCAAT/enhancer-binding protein alpha; ChIP, chromatin immunoprecipitation; EGFP, enhanced green fluorescent protein; EMSA, electrophoretic mobility shift assay; GSK-3β, glycogen synthase kinase-3 beta; HCC, hepatocellular carcinoma; IGF-1R, insulin-like growth factor 1 receptor; IgG, immunoglobulin G; miRNA, microRNA; mRNA, messenger RNA; NC, negative control; pre–miR-122, miR-122 precursor; qPCR, quantitative real-time polymerase chain reaction; RT-PCR, reverse-transcription polymerase chain reaction; siRNA, small interfering RNA; TSS, transcription start site; UTR, untranslated region.
Patients and Methods
Databases used for bioinformatic analysis and details about reagents and experimental procedures can be found in the Supporting Information Materials and Methods.
Patients and Human Specimens.
Normal liver tissues were obtained from patients undergoing resection of hepatic hemangiomas, and paired HCC and adjacent nontumor liver tissues were obtained from patients undergoing HCC resection at the Cancer Center at Sun Yat-sen University. All tissues were obtained from the Cancer Center's Bank of Tumor Resources. Both tumor and nontumor tissues were histologically confirmed. No local or systemic treatment had been conducted before surgical procedure. After surgical resection, no other anticancer therapy was administered before relapse. Patient characteristics are provided in Supporting Information Table 1. Informed consent was obtained from each patient, and the study was approved by the Institute Research Ethics Committee at the Sun Yat-sen University Cancer Center.
Cell Lines.
The cell lines used were HEK293T, the HCC cell lines HepG2 and Huh-7, the immortalized human fetal liver cell line L02 and its aflatoxin B1 (AFB1)-transformed subline L02-T,17 and the miR-122 stably expressing cell line HepG2-miR-122 and its control line HepG2-Mock.
Animal Studies.
All mouse experiments were approved by the Institutional Animal Care and Use Committee at School of Life Sciences at Sun Yat-sen University. Experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996) and were performed according to the institutional ethical guidelines for animal experiments. BALB/c nude mice were employed for tumorigenicity analysis. Livers from fetus, postnatal, and adult C57BL/6 mice were collected.
Oligonucleotides and Plasmids.
We used the following miRNA and small interfering RNA (siRNA) oligonucleotides (Genepharma, Shanghai, China): miR-122 mimics; miR-122 harboring a mutated seed region; siIGF-1R, siC/EBPα, and siGSK-3β that targeted human IGF-1R (3192-3212 nt, GeneBank accession no. NM_000875.3), C/EBPα (819-839 nt, GeneBank accession no. NM_004364.2), and GSK-3β (1816-1836 nt, GeneBank accession no. NM_001146 156.1) transcripts, respectively; and a negative control (NC) RNA duplex for both miRNA and small interfering RNA (siRNA). The sequence-specific miR-122 inhibitor (anti–miR-122) and its control (anti–miR-NC) were obtained from Dharmacon (Lafayette, CO). All oligonucleotide sequences are listed in Supporting Information Table 2.
We used the following plasmids: firefly luciferase reporter plasmids for verifying miR-122–targeted 3′-UTR and the miR-122 promoter region; pSi-shC/EBPα that expressed siRNA targeting 222−240 nt of C/EBPα (GeneBank accession no. NM_004364.2); pc3-gab-C/EBPα, pc3-gab-C/EBPα-T222A/T226A, and p3XFLAG-GSK-3β-S9A that expressed wild-type C/EBPα, T222A/T226A mutant C/EBPα, and S9A mutant GSK-3β, respectively; and retrovirus vector (pRetroX–miR-122) for expressing the miR-122 precursor (pre–miR-122). The pSi-shC/EBPα, pc3-gab-C/EBPα, and pc3-gab-C/EBPα-T222A/T226A vectors also expressed enhanced green fluorescent protein (EGFP).
Cell Transfection and Infection.
RNA oligonucleotides were transfected using Lipofectamine RNAiMAX. A final concentration of 50 nM duplex or 200 nM miRNA inhibitor was used unless indicated. RNA transfection efficiency is approximately 70%-80%10 and the overexpression of miRNA mimic persists for at least 4 days.8 Lipofectamine 2000 was used for transfection of plasmid alone or together with RNA oligonucleotides; GenJet In Vitro DNA Transfection Reagent (SignaGen Laboratories, MD) was used for transfection of Huh-7 with pSi-shC/EBPα or pSi-Vector. For infection, target cells were infected 3 to 4 times with retroviral supernatant supplemented with 4 μg/mL polybrene (Millipore, Billerica, MA).
Luciferase Reporter Assay.
Luciferase reporter assays were applied to characterize the miR-122 promoter and miR-122–targeted 3′-UTR. pRL-TK or pRL-CMV, both of which express Renilla luciferase, was cotransfected to correct the differences in both transfection and harvest efficiencies. The activity of the firefly luciferase reporter, which carried miR-122 promoter or miR-122-targeted 3′-UTR, was normalized to the Renilla luciferase activity.
Rapid Amplification of Complementary DNA Ends.
The miR-122 primary transcript from normal liver tissue was characterized using the 5′-Full RACE Kit (TaKaRa, Japan). Total RNA was treated with calf intestinal phosphatase and tobacco acid pyrophosphatase, followed by ligation to an RNA adaptor and gene-specific primed reverse-transcription polymerase chain reaction (RT-PCR) to amplify the 5′-end of the transcript.
Electrophoretic Mobility Shift Assay.
Electrophoretic mobility shift assay (EMSA) was conducted using a Gel Shift Assay System (Promega, Madison, WI). The probes, corresponding to the predicted C/EBPα binding sequences on the miR-122 promoter (Supporting Information Table 2), were end-labeled with γ-32P adenosine triphosphate, then incubated with Huh-7 nuclear extract. For competition assay, nuclear extract was preincubated with 100-fold molar excess of unlabeled oligonucleotides prior to adding labeled probe. For antibody-supershift assay, nuclear extract was preincubated with anti-C/EBPα antibody or normal immunoglobulin G (IgG) before adding to the binding reaction.
Chromatin Immunoprecipitation Assay.
The formaldehyde cross-linked chromatin complexes were immunoprecipitated using anti-C/EBPα or normal IgG (as a negative control), then collected with Protein A/G PLUS-Agarose. The DNA–protein cross-link was reversed by heating, after which the DNA was subjected to PCR.
Analysis of Cell Growth.
Bromodeoxyuridine incorporation and anchorage-independent growth assays were used to examine the capability of cells to proliferate and to grow in soft agar.
Analysis of Gene Expression.
Northern blotting, semiquantitative RT-PCR, and quantitative real-time RT-PCR (qPCR) were performed to evaluate RNA levels. Immunoblotting and immunohistochemical staining were used to detect protein level.
Bioinformatic Tools and Statistical Analysis.
Data are presented as the mean ± SEM of at least three independent experiments. Analyses on the differences between groups were conducted using GraphPad Prism version 4.0 (GraphPad Software, Inc., San Diego, CA). Unless indicated, the differences between groups were analyzed using a Student t test when only two groups or assessed using one-way analysis of variance when more than two groups were compared. Two-factor analysis was performed using two-way analysis of variance with a posttest for subsequent comparisons of individual factors.
Overall survival was calculated from the date of tumor resection to the time of death. Kaplan−Meier survival curves and Cox proportional hazard regression analysis, which were applied to identify prognostic factors, were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL). All statistical tests were two-sided. P values <0.05 were considered statistically significant.
Results
Characterization of the miR-122 Promoter Region.
Although down-regulation of miR-122 is a frequent event in HCC tissues, the underlying mechanism has not yet been elucidated. miR-122 is located on chromosome 18q21.3, an intergenic region that does not undergo frequent allelic losses in HCC. Computational analysis did not predict any CpG island within 20 kb upstream of pre–miR-122. Therefore, deregulated transcription or processing rather than deletion and hypermethylation may account for miR-122 down-regulation. To examine this hypothesis, we first mapped the promoter region of the miR-122 transcript. Phylogenetic conservation analysis within the 20-kb genomic sequence upstream of pre–miR-122 identified a highly conserved region between −5 and −4 kb (denoted as −5/−4k, with the first base of pre–miR-122 assigned as 1, chr18: 54269286) (Fig. 1A). Promoter prediction further revealed a 570-bp region located at −5.4 to −4.8 kb (Fig. 1A).
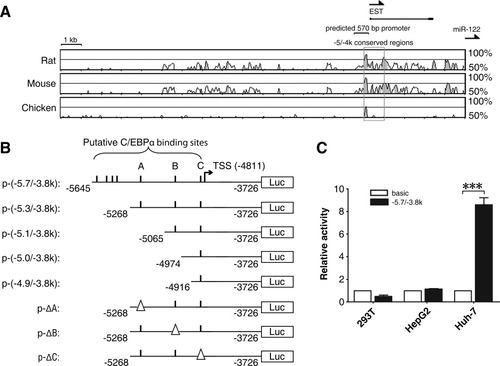
Identification of the miR-122 promoter. (A) Phylogenetic conservation analysis of the 20-kb genomic sequence upstream of pre–miR-122. Vista software was used to generate pairwise alignments for the sequences of human and other indicated species. Transcription of miR-122 appears toward the right. A potential promoter was predicted using the FirstEF program. Expressed sequence tag information was taken from the UCSC Genome Browser. Annotation of this region is shown on the top. (B) Schematic diagram for firefly luciferase reporter constructs containing the indicated genomic sequences upstream of pre–miR-122. Putative C/EBPα binding sites are depicted as short vertical lines. Deletion of the C/EBPα binding site is depicted as a triangle (Δ). (C) Activity of p-(−5.7/−3.8k) in different cell lines. pRL-CMV were cotransfected with pGL3-basic or p-(−5.7/−3.8k) reporter. No error bar is shown for the pGL3-basic transfectant (basic) because its normalized luciferase activity was set to 1. ***P < 0.001.
Based on these pieces of evidence, a luciferase reporter construct p-(−5.7/−3.8k) that contained the −5645 to −3726 bp region (chr18: 54263641-54265560) in the pGL3-basic vector (Fig. 1B) was generated. Increased luciferase activity was clearly observed in Huh-7 cells, yet no enhancement was observed in HepG2 or 293T cells transfected with p-(−5.7/−3.8k) (Fig. 1C). This result was consistent with miR-122 levels in these cell lines—that is, miR-122 was detectable in Huh-7 cells but not in HepG2 or 293T cells (Fig. 2A). However, no promoter activity was detected in the −3726 to −1 bp region (Supporting Information Fig. 1A). These results define an exclusive transcription motif within −5.7 to −3.8 kb. Next, rapid amplification of complementary DNA ends was employed to characterize the 5′ end of the primary miR-122 transcript. A transcription start site (TSS) was mapped to the −4811 bp site (chr18: 54264475) upstream of pre–miR-122, which was consistent with the 5′ termini of expressed sequence tag track in the UCSC Genome Browser (Fig. 1A,B, Supporting Information Fig. 1B,C). Thus, the miR-122 promoter region was narrowed down to −5.7 to −4.8 kb.
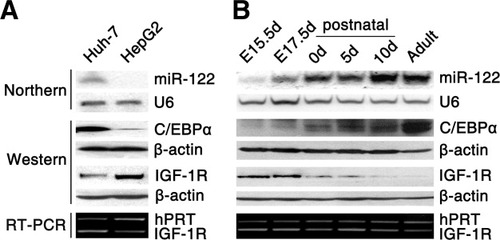
Detection of miR-122, C/EBPα, and IGF-1R expression. Expression patterns of miR-122, C/EBPα, and IGF-1R (A) in HCC cell lines and (B) during liver development are shown. miR-122 expression was analyzed by way of Northern blot analysis. The same membrane was hybridized sequentially with miR-122 and U6 probes at 37°C. Hybridization with an miR-122 probe revealed a single band migrating between xylene cyanol FF and bromophenol blue, which are the loading dyes that respectively comigrate with ∼30 bp and ∼10 bp RNA fragments in a 15% denaturing polyacrylamide gel. In (B), analyses were performed on livers collected from fetuses (E15.5d, E17.5d), postnatal (birth day as day 0) and adult (8 weeks) mice. U6, β-actin, and hypoxanthine-guanosine phosphoribosyl transferase (hPRT) were used as internal controls.
Regulation of miR-122 Transcription by C/EBPα.
Considering its enrichment in the liver, we inferred that miR-122 was likely transactivated by liver-enriched transcription factors. Prediction within the −5.7 to −4.8 kb region identified putative binding sites for three liver-enriched transcription factors. Among them, both HNF-3B and HNF-1 were up-regulated, whereas C/EBPα was down-regulated in HCC.18 Furthermore, C/EBPα was significantly down-regulated in our set of HCC tissues (Supporting Information Fig. 2A), and its expression pattern was consistent with that of miR-122 in HCC cell lines and during liver development (Fig. 2A,B). We thus evaluated the role of C/EBPα in miR-122 transcription. C/EBPα silencing (Supporting Information Fig. 2B) caused a significant reduction in the endogenous miR-122 expression (Fig. 3A) and in the promoter activity of p-(−5.7/−3.8k) (Fig. 3B), suggesting that C/EBPα is crucial for the transactivation of the miR-122 gene.
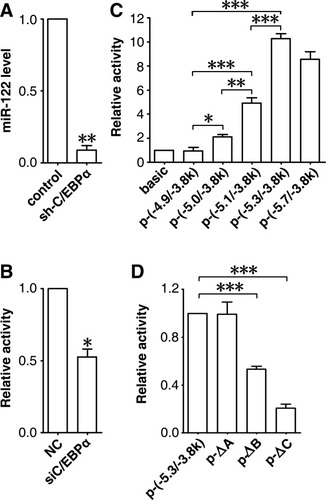
C/EBPα transactivates miR-122 transcription. (A) Knockdown of C/EBPα reduced the endogenous miR-122 level. Huh-7 cells were transfected with pSi-Vector (control) or pSi-shC/EBPα, which expressed EGFP only or together with siRNA targeting C/EBPα. Forty-eight hours after transfection, cells that successfully received the plasmid were sorted using fluorescence-activated cell sorting based on EGFP expression, then applied to qPCR analysis for endogenous miR-122 levels. No error bar is shown for pSi-Vector transfectants because its normalized miR-122 level was set to 1. (B) Silencing of C/EBPα reduced miR-122 promoter activity. Huh-7 cells were cotransfected with pRL-CMV, p-(−5.7/−3.8k), and NC or siC/EBPα. (C) Detailed delineation of the miR-122 promoter by way of 5′ deletion analysis. (D) Deletion of C/EBPα binding sites reduced miR-122 promoter activity. In (C) and (D), Huh-7 cells were cotransfected with pRL-CMV and the indicated luciferase reporters. For (B-D), no error bar is shown for NC (B), pGL3-basic (C), or p-(−5.3/−3.8k) transfectants (D) because their normalized luciferase activities were set to 1. *P < 0.05, **P < 0.01, ***P < 0.001.
Within the −5.7 to −4.8 kb region, seven C/EBPα consensus binding sites were predicted. To validate the role of these motifs, we performed 5′ deletion analysis (Fig. 1B). Compared with the activity of p-(−5.7/−3.8k), there was no significant change in that of p-(−5.3/−3.8k). However, a 50% decrease in p-(−5.1/−3.8k) activity was observed, and further deletion on the sequences proximal to the TSS caused a much more pronounced reduction in promoter activity (Fig. 3C). As expected, C/EBPα knockdown also remarkably reduced p-(−5.3/−3.8k) activity (Supporting Information Fig. 2C). Therefore, we suggest that the −5.3 to −4.8 kb region contains both TSS and transcription-related elements and is the promoter for the miR-122 gene.
Within this region, three C/EBPα consensus binding sites were predicted (referred to sites A, B, and C, respectively, in Fig. 1B and Supporting Information Fig. 1C). Deletion analysis on the predicted C/EBPα consensus binding sites revealed that removal of either site B (p-ΔB) or C (p-ΔC), but not A (p-ΔA), severely attenuated p-(−5.3/−3.8k) activity (Figs. 1B, 3D). Moreover, both EMSA (Fig. 4A) and antibody-supershift assays (Fig. 4B) revealed an interaction between C/EBPα and the sites B and C in vitro. Analysis using chromatin immunoprecipitation (ChIP) further confirmed the existence of a direct interaction between C/EBPα and the miR-122 promoter in vivo (Fig. 4C).
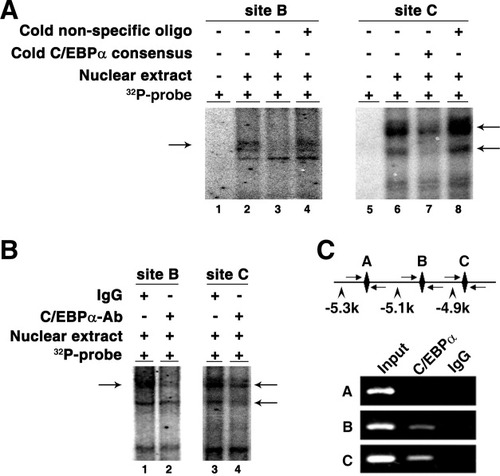
C/EBPα interacts with the miR-122 promoter sequence in vitro and in vivo. (A) EMSA verified the interaction of nuclear proteins with site B and C sequences of the miR-122 promoter. The analysis was performed in the presence (+) or absence (−) of Huh-7 nuclear extracts. Each binding reaction included a γ-32P–labeled probe that comprised site B or C sequences, as indicated. The radiolabeled intensity of the DNA–protein complexes (indicated by arrows) reduced markedly when excess (100×) unlabeled C/EBPα consensus binding sequence (lanes 3 and 7) was added, but remained unchanged with addition of a nonspecific scrambled oligonucleotide that had the same base composition as that of the probe (lanes 4 and 8). (B) Antibody-supershift assay identified C/EBPα as a potential nuclear protein interacting with site B and C sequences. The radiolabeled intensity of the DNA–protein complexes (indicated by arrows) reduced significantly when anti-C/EBPα antibody was added (lanes 2 and 4), but stayed unchanged with addition of control IgG (lanes 1 and 3). (C) C/EBPα directly interacted with the miR-122 promoter in vivo. A scheme of the amplicons is shown at the top. C/EBPα binding sites are depicted as diamonds and three primer sets as arrows. The chromatin complexes precipitated by anti-C/EBPα antibody or control IgG were amplified using three primer sets that encompassed the predicted C/EBPα binding sites A, B, and C, respectively. A specific strong band of the expected size was detected in the input DNA using either of the three primer sets, whereas only the fragments containing C/EBPα binding sites B or C (but not A) were detected in C/EBPα antibody–precipitated DNA. Rabbit IgG–precipitated DNA yielded no bands with either of the primer sets.
Taken together, C/EBPα may transactivate miR-122 transcription by directly binding to its promoter.
Identification of a Novel GSK-3β–C/EBPα–miR-122–IGF-1R Regulatory Circuitry and its Implication in Hepatocarcinogenesis.
The transactivation activity of C/EBPα is elicited through phosphorylation at Thr222/226 by GSK-3β.19, 20 We therefore evaluated the role of GSK-3β in miR-122 expression. Treatment with LiCl, an inhibitor of GSK-3β kinase, increased the level of phosphorylated Ser9–GSK-3β (inactive form of GSK-3β) and decreased the phosphorylation of Thr222/226-C/EBPα, without altering the total amount of GSK-3β or C/EBPα proteins (Fig. 5A). Both endogenous miR-122 levels and p-(−5.3/−3.8k) activity (Fig. 5B, left and middle panels) significantly decreased in LiCl-exposed cells. Consistently, knockdown of GSK-3β expression by siRNA (Supporting Information Fig. 3) also decreased endogenous miR-122 levels and p-(−5.3/−3.8k) activity (Fig. 5C). Furthermore, the decline of miR-122 that resulted from GSK-3β inactivation was abolished when C/EBPα was silenced (Fig. 5B, right panel). Cotransfection of the constitutively active form of GSK-3β with wild-type but not T222A/T226A mutant C/EBPα enhanced endogenous miR-122 levels (Fig. 5D). Collectively, we suggest that GSK-3β regulates miR-122 expression and that C/EBPα is a critical mediator of this effect.
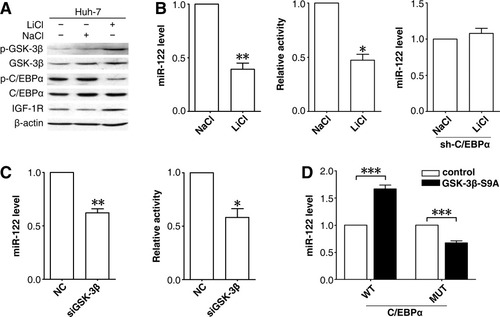
GSK-3β regulates C/EBPα activity and miR-122 expression. (A) Inhibition of GSK-3β activity impaired C/EBPα phosphorylation and enhanced IGF-1R levels. (B) Abrogation of GSK-3β activity repressed endogenous miR-122 expression and miR-122 promoter activity. In (A) and (B, left panel), Huh-7 cells were treated with 25 mM LiCl or NaCl (control) for 48 hours before (A) immunoblotting or (B, left panel) qPCR. In (B, middle panel), Huh-7 cells were cotransfected with p-(−5.3/−3.8k) and pRL-CMV for 24 hours, then treated with 25 mM LiCl or NaCl for 24 hours before luciferase assay. In (B, right panel), Huh-7 cells were transfected with pSi-shC/EBPα for 24 hours, then treated with 25 mM LiCl or NaCl for 48 hours. Cells that successfully received the plasmid were sorted using fluorescence-activated cell sorting based on EGFP expression, then applied to qPCR. (C) Knockdown of GSK-3β expression by siRNA reduced endogenous miR-122 expression and miR-122 promoter activity. Huh-7 cells were transfected with control RNA duplex (NC) or siGSK-3β for 48 hours, then applied to qPCR (left panel), or transfected with indicated duplex for 24 hours, followed by cotransfection with p-(−5.3/−3.8k) and pRL-TK for 48 hours and then luciferase assay (right panel). In (B) and (C), no error bar is shown for NaCl-treated or NC-transfected cells because their normalized miR-122 level or luciferase activity was set to 1. *P < 0.05, **P < 0.01. (D) Ectopic expression of GSK-3β with wild-type but not T222A/T226A mutant C/EBPα elevated endogenous miR-122 levels. The control plasmid (p3XFLAG-CMV) or constitutively active GSK-3β–expressing vector (p3XFLAG-GSK-3β-S9A) was cotransfected with pc3-gab–C/EBPα or pc3-gab–C/EBPα–T222A/T226A that expressed both EGFP and wild-type (WT) or mutant (MUT) C/EBPα. Huh-7 cells that successfully received the plasmids were sorted based on EGFP expression using fluorescence-activated cell sorting 48 hours posttransfection, followed by qPCR analysis. No error bar is shown for the p3XFLAG-CMV transfectant (control) because its normalized miR-122 level was set to 1. ***P < 0.001.
In a search for the potential targets regulated by miR-122, we identified IGF-1R among the list of predicted targets. IGF-1R is a receptor tyrosine kinase that mediates growth-stimulatory signals. In response to growth stimulation, IGF-1R activates Akt through phosphorylation at Ser-473 and Thr-308. The activated Akt in turn phosphorylates the Ser-9 of GSK-3β and therefore inactivates the kinase activity of GSK-3β, which consequently attenuates the proteolysis of cyclin D1.21 We speculated that there might exist a novel GSK-3β–C/EBPα–miR-122–IGF-1R regulatory circuitry. To test this hypothesis, we first experimentally validated whether IGF-1R was the direct target of miR-122. Obviously, miR-122 inhibited the activity of firefly luciferase with the wild-type but not mutant 3′-UTR of IGF-1R. In contrast, mutant miR-122 that harbored a mutated seed region displayed no effect on the wild-type 3′-UTR of IGF-1R (Fig. 6A, Supporting Information Fig. 4). These results were further verified by silencing miR-122 expression with anti-miR-122 (Supporting Information Fig. 5A,B). Both gain-of-function and loss-of-function analyses also disclosed a suppressive effect of miR-122 on the expression of endogenous IGF-1R protein (Fig. 6B, Supporting Information Fig. 5C). More interestingly, a significant down-regulation of miR-122 and overexpression of IGF-1R was frequently observed in HCC tissues (Supporting Information Fig. 6, Supporting Information Table 3). Furthermore, an inverse association between the expression of miR-122 and that of IGF-1R was also found in HCC cells and during liver development (Figs. 2 and 6C). Therefore, miR-122 may repress IGF-1R expression by directly binding to its 3′-UTR.
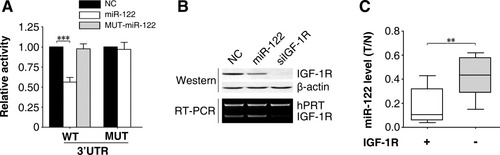
IGF-1R is a direct target of miR-122. (A) miR-122 inhibited the activity of luciferase reporter containing the wild-type 3′-UTR of IGF-1R. NC, wild-type, or mutant miR-122 duplexes were cotransfected with pRL-TK and a firefly luciferase reporter plasmid carrying either the wild-type (WT) or mutant (MUT) 3′-UTR of IGF-1R. No error bar is shown for NC transfectants because their normalized luciferase activities were set to 1. ***P < 0.001. (B) Expression of miR-122 reduced the protein but not mRNA level of IGF-1R. HepG2 cells were transfected with the indicated RNA duplex for 48 hours before immunoblotting and RT-PCR. β-Actin and hypoxanthine-guanosine phosphoribosyl transferase (hPRT) were used as internal controls. (C) miR-122 levels were inversely correlated with IGF-1R expression in HCC tissues. IGF-1R protein was detected by way of immunohistochemical staining, whereas miR-122 levels were detected using qPCR. The y axis indicates the fold change in miR-122 levels in HCC (T) compared with matched nontumor liver (N). The boxes represent the interquartile range (25th to 75th percentiles). The horizontal line inside the box indicates the median. Vertical whiskers extend to the maximum and minimum values. The cases are divided into two groups (x axis): with (+) and without (−) detectable IGF-1R. **P < 0.01.
We then evaluated the implication of miR-122 in the transduction of growth signals by way of the IGF-1R/Akt/GSK-3β/cyclin D1 pathway. In response to IGF-1, NC-transfected cells displayed significantly increased phosphorylation of Ser473-Akt and Ser9–GSK-3β and in turn enhanced cyclin D1 expression (Fig. 7A) and DNA replication (Supporting Information Fig. 7A), whereas introduction of either miR-122 or siIGF-1R dramatically abolished these effects. Consistently, inhibition of miR-122 led to elevated phosphorylation of Ser473-Akt and Ser9-GSK-3β, and increased levels of cyclin D1 (Fig. 7B) and DNA replication (Supporting Information Fig. 7B) upon IGF-1 stimulation. Further analysis revealed that inhibition of GSK-3β activity, using kinase inhibitors, rescued the reduction of cyclin D1 and DNA replication in miR-122 and siIGF-1R transfectants (Fig. 7C, Supporting Information Fig. 7C). These results were also confirmed by knockdown of GSK-3β expression (Fig. 7D). Interestingly, in consistent with the above observation that abrogation of GSK-3β activity caused a decrease in the transactivation activity of C/EBPα and the expression of endogenous miR-122, an increase in IGF-1R protein level in GSK-3β-inactivated cells was found (Fig. 5A,B).
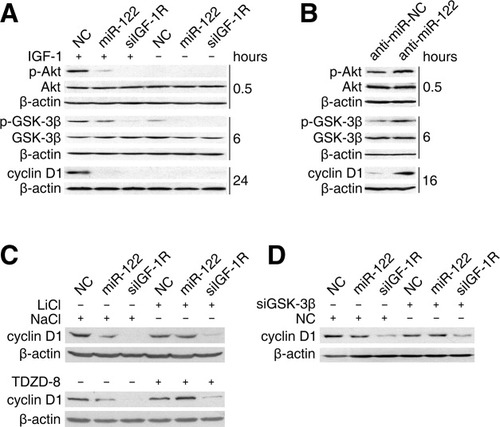
miR-122 blocks the transduction of extracellular growth signal by modulating the IGF-1R/Akt/GSK-3β/cyclin D1 pathway. (A) Expression of miR-122 blocked the transduction of growth signal. HepG2 cells transfected with indicated duplex were serum-starved for 24 hours, then stimulated with 2 nM IGF-1 (+) or maintained starved (−, nontreatment control) for the indicated times before immunoblotting. (B) Inhibition of miR-122 enhanced the transduction of growth signals. Huh-7 cells were transfected with anti–miR-NC or anti–miR-122, serum-starved for 24 hours, then stimulated with 5 nM IGF-1 for the indicated times before immunoblotting. (C) Inhibition of GSK-3β activity by kinase inhibitors rescued miR-122–induced repression of cyclin D1. (D) Knockdown of GSK-3β expression by siRNA rescued miR-122–mediated suppression of cyclin D1. In (C) and (D), HepG2 cells transfected with the indicated duplex were serum-deprived for 24 hours, then stimulated to enter S-phase by culturing in complete medium for 24 hours before immunoblotting. In (C), 10 mM of NaCl (control) or LiCl (top panel) or 10 μM of dimethyl sulfoxide (vehicle control) or TDZD-8 (bottom panel) was added to the medium during starvation and the stimulation process. β-actin was used as an internal control.
Taken together, we suggest the following regulatory circuitry: miR-122 suppresses IGF-1R expression and then attenuates IGF-1R/Akt signaling, which sustains GSK-3β activity and in turn represses cyclin D1 expression and cell proliferation. On the other hand, the activated GSK-3β maintains high levels of miR-122 via C/EBPα, which enforces IGF-1R suppression (Fig. 8A). In a pathological situation, disruption of this regulatory circuitry may result in uncontrolled cell proliferation and in turn hepatocarcinogenesis. In agreement with this hypothesis, a diminution of miR-122 and phosphorylated Thr222/226-C/EBPα and an enhancement of IGF-1R protein and phosphorylated Ser9–GSK-3β were observed in L02 cells after transformation by AFB1 (Fig. 8B,C), which is a major contributor to hepatocarcinogenesis. Furthermore, significant down-regulation of miR-122 was a frequent event in HCC tissues and was associated with higher TNM stage (Supporting Information Table 1) and shorter overall survival (Supporting Information Fig. 8, Supporting Information Table 4). More importantly, restoration of miR-122 dramatically inhibited the anchorage-independent growth of AFB1-transformed L02 cells (Fig. 8D,E), as well as the HCC cell line (Supporting Information Fig. 9A,B), and also suppressed tumor formation of HCC cells in vivo (Supporting Information Fig. 9C-E).
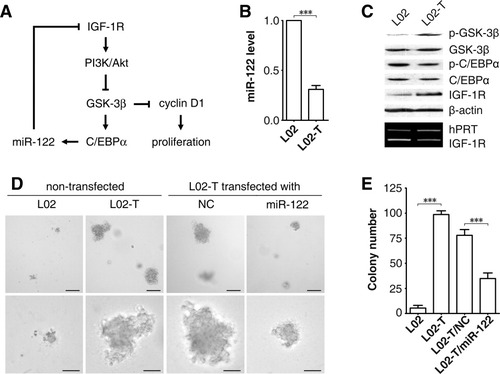
The regulatory circuitry of miR-122 is disrupted by AFB1. (A) Schematic overview of a novel miR-122 regulatory circuitry. (B) AFB1 exposure caused a decrease in miR-122 expression. Vehicle-treated cells (L02) and AFB1-transformed cells (L02-T) were subjected to qPCR. No error bar is shown for L02 cells because its normalized miR-122 level was set to 1. ***P < 0.001. (C) AFB1 exposure resulted in impaired activities of GSK-3β and C/EBPα, and elevated levels of IGF-1R protein. β-Actin and hypoxanthine-guanosine phosphoribosyl transferase (hPRT) were used as internal controls. (D) Restoration of miR-122 expression suppressed anchorage-independent growth of AFB1-transformed cells. L02 and L02-T cells without transfection and L02-T cells transfected with NC (control) or miR-122 duplex were subjected to soft agar assay. Scale bars, 100 μm (top) and 50 μm (bottom). (E) Quantitative plots for (D). ***P < 0.001.
Discussion
We identified a novel miR-122 regulatory circuit and implicated its importance in hepatocarcinogenesis. Our data provide new insights into the regulatory network of miR-122, suggest an important role of miR-122 in the molecular etiology of HCC, and provide a basis for the potential application of miR-122 in prognosis prediction and cancer therapy.
Although miRNA biology has advanced quickly, much of the effort has been dedicated to identifying the targets of miRNAs rather than understanding the regulation of miRNA genes. The nature of the miRNA promoter remains one of the unexplored questions, and their characterization should help to uncover the miRNA regulatory networks. We identified the promoter for the miR-122 gene and classified C/EBPα as a transactivator of miR-122 transcription because (1) C/EBPα knockdown significantly reduced activity and endogenous miR-122 expression; (2) two C/EBPα consensus binding sites were mapped in close proximity to the TSS of the miR-122 gene, and deletion of either site caused a dramatic decrease in miR-122 promoter activity; (3) C/EBPα directly interacted with the miR-122 promoter in vitro and in vivo; and (4) C/EBPα and miR-122 were both enriched in the liver, and they displayed similar expression patterns during liver development and in HCC cells.
Intriguingly, deletion from −5268 to −5065 bp led to a 50% decrease in the promoter activity, but removal of the predicted C/EBPα binding site A, which was located within this region, had no effect, and ChIP revealed no binding of C/EBPα to site A. Furthermore, all analyses—including individual site deletion, EMSA, antibody-supershift, and ChIP—demonstrated that sites B and C were important for C/EBPα binding and miR-122 transcription. However, the promoter activity of p-(−4.9/−3.8k), which contained site C, was reduced to a level comparable to that of pGL3-basic. This potential discrepancy may be explained by the following: (1) multiple transcription factors, in addition to C/EBPα, may spatiotemporally regulate miR-122 expression; and (2) lack of sufficient proximal sequence may seriously disturb transcription of the p-(−4.9/−3.8k) construct, which only carries 110 bp upstream of TSS. It has been shown that the 250-bp flanking sequence upstream of TSS is required for the recruitment and stabilization of the transcription machinery.
It is noticeable that introduction of constitutively active GSK-3β resulted in a significant increase of miR-122 expression in wild-type C/EBPα transfectants but a decrease of miR-122 levels in T222A/T226A-C/EBPα transfectants. The transcriptional repressor Rev-erbα has been shown to regulate the circadian transcription of miR-122,13 and to be phosphorylated and stabilized by GSK-3β.22 We speculate that overexpression of active GSK-3β may phosphorylate both C/EBPα and Rev-erbα. The suppressive effect of Rev-erbα on the miR-122 transcription may be compromised by abundant wild-type C/EBPα but possibly becomes obvious when overexpressing T222A/T226A-C/EBPα, the mutant form that is resistant to GSK-3β-mediated phosphorylation.
Our data uncover a novel miR-122 regulatory circuitry that involves GSK-3β, C/EBPα, miR-122, and IGF-1R. It is well known that IGF-1R mediates the bioactivity of IGF-1 and that the liver is the major source of IGF-1.21 It is possible that once IGF-1R is expressed, autocrine interaction of IGF-1 with IGF-1R stimulates hepatocyte proliferation. Therefore, tight regulation of IGF-1R expression in the liver is especially crucial in preventing runaway proliferation of hepatocytes. To date, much effort has focused on exploring the transcriptional regulation of IGF-1R.21 We found that miR-122 expression started to increase in the liver late in gestation and reached a maximum in the adult liver, whereas IGF-1R protein sharply declined along with age after birth and was undetectable in quiescent hepatocytes; fetal and adult livers displayed similar mRNA levels of IGF-1R despite a large difference in their protein levels; and miR-122 directly suppressed the translation but not transcription of IGF-1R. Our data suggest that posttranscriptional regulation of IGF-1R by miR-122 is another key mechanism to maintain the level of IGF-1R protein below a certain threshold in quiescent hepatocytes, which ensures the spatial segregation of the IGF-1/IGF-1R axis and prevents hepatocytes from aberrant proliferation.
In pathological situations, disruption of any part of the miR-122 circuitry may lead to a cycle of progressively decreased activities of GSK-3β, C/EBPα, and miR-122, and enhanced activity of IGF-1R. This contention is supported by our observations in HCC tissues and cell models. We found that C/EBPα protein and miR-122 was significantly reduced in HCC tissues and GSK-3β inactivation impaired the transcription activity of C/EBPα and inhibited miR-122 expression. It has been shown that AFB1 can activate Akt, whereas HBV-encoding protein may suppress GSK-3β activity.23, 24 Therefore, decreased C/EBPα activity, which may result from reduced GSK-3β activity or decreased C/EBPα expression, can serve as an important mechanism to down-regulate miR-122 and interrupt the miR-122 circuitry during hepatocarcinogenesis. Considering the prevalence of C/EBPα down-regulation in HCC tissues and the significance of C/EBPα in mediating the effect of GSK-3β on miR-122 expression, it is understandable that some HCC cells display low miR-122 expression even in the presence of active GSK-3β.
We found that down-regulation of miR-122 was frequent in HCC tissues and was associated with poorer overall survival; miR-122 restoration dramatically repressed the tumorigenicity of HCC cells. Recently, other groups have shown that miR-122 expression increases the sensitivity of HCC cells to apoptosis15, 25 and reduces in vivo intrahepatic metastasis of hepatoma cells.16 These data emphasize the biomedical significance of miR-122, whose down-regulation may confer malignant phenotypes, including uncontrolled proliferation, resistance to apoptosis, and metastasis. The contribution of miR-122 down-regulation to tumorigenesis may be mediated, at least partly, by IGF-1R up-regulation. Mounting evidence reveals that IGF-1R plays an important role in cell proliferation, antiapoptosis, and cell motility.21, 26 Although IGF-1R overexpression has been observed in different types of tumors, the underlying mechanisms remain obscure.21, 27 Amplification of the IGF-1R locus has been reported.28 We found that cell models with an marked decrease in miR-122 (such as L02-T and HepG2 cells) displayed a significant increase in IGF-1R protein but not its mRNA level; thus, down-regulation of miR-122 may represent an effective approach by which liver cells overexpress IGF-1R.
Emerging evidence suggests that miRNAs and their target genes may form positive or negative regulatory loops.29-31 Here, we also identified an miR-122 regulatory circuit that involves its targets and its transcriptional activator. These findings argue that miRNAs, together with proteins, constitute functional networks that regulate cellular activities, and the integration of miRNAs may further enhance the robustness of gene regulation. More extensive exploration on miRNA-mediated regulation within the context of networks will provide a comprehensive view of how miRNAs exert their functions and how gene expression is regulated at the system level.




