Visceral adiposity index is associated with histological findings and high viral load in patients with chronic hepatitis C due to genotype 1†
Potential conflict of interest: Nothing to report.
Abstract
Metabolic factors have been associated with liver damage in patients with genotype 1 chronic hepatitis C (G1 CHC). We tested visceral adiposity index (VAI), a new marker of adipose dysfunction in G1 CHC, patients to assess its association with host and viral factors and its link to both histological findings and sustained virological response (SVR). Two hundred thirty-six consecutive G1 CHC patients were evaluated by way of liver biopsy and anthropometric and metabolic measurements, including insulin resistance (IR), homeostasis model assessment (HOMA), and VAI using waist circumference, body mass index, triglycerides, and high-density lipoprotein cholesterol. All biopsies were scored by one pathologist for staging and grading and graded for steatosis, which was considered moderate to severe if ≥30%. Multiple linear regression analysis revealed that VAI score was independently associated with higher HOMA score (P = 0.009), log10 hepatitis C virus RNA levels (P = 0.01), necroinflammatory activity (P = 0.04), and steatosis (P = 0.04). Multiple logistic regression analysis revealed that IR (OR 3.879, 95% CI 1.727-8.713, P = 0.001), higher VAI score (OR 1.472, 95% CI 1.051-2.062, P = 0.02), and fibrosis (OR 2.255, 95% CI 1.349-3.768, P = 0.002) were linked to steatosis ≥30%. Logistic regression analysis revealed that older age (OR 1.030, 95% CI 1.002-1.059, P = 0.03), higher VAI score (OR 1.618, 95% CI 1.001-2.617, P = 0.04), and fibrosis (OR 2.608, 95% CI 1.565-4.345, P < 0.001) were independently associated with moderate to severe necroinflammatory activity. No independent associations were found between VAI score and both fibrosis and SVR. Conclusion: In G1 CHC patients, higher VAI score is independently associated with both steatosis and necroinflammatory activity and has a direct correlation with viral load. (HEPATOLOGY 2010.)
Metabolic factors, namely steatosis and insulin resistance (IR), are frequent findings in patients with genotype 1 chronic hepatitis C (G1 CHC).1-3 This fact could be of further interest in light of the clinical relevance of these metabolic risk factors, which seem able to interfere with the natural history of CHC. Different studies have shown that these metabolic features not only are independently associated with the severity of liver damage (necroinflammatory activity and fibrosis),3-6 but also are negative predictors of sustained virological response (SVR) after standard antiviral therapy.2, 5, 7
Recent studies have shown that visceral adipose tissue, originally considered a passive depot for energy storage, secretes a variety of substances that regulate metabolism, inflammation, and immunity, in turn participating in the pathogenesis of cardiovascular disease, IR, and diabetes.8, 9 In addition, visceral adiposity, when evaluated by way of magnetic resonance (the best estimate of visceral obesity), correlates with liver fat accumulation in healthy subjects10, 11 and with severity of both inflammation and fibrosis in nonalcoholic steatohepatitis.12 The association between visceral obesity and steatosis has also been found in other studies on nonalcoholic fatty liver disease and in CHC patients using waist circumference (WC) measurement, a surrogate marker of visceral adiposity.13-16 However, in most of these studies, the effect of visceral obesity on the histological features of the liver disease was not corrected for IR. In addition, the use of WC to indicate visceral obesity is not entirely accurate, because WC alone does not help in distinguishing between subcutaneous and visceral fat mass,17 the latter being the key factor in metabolic alteration development.
To overcome these problems, a recent study18 introduced the visceral adiposity index (VAI), a scoring system that uses both anthropometric (body mass index [BMI] and WC) and metabolic (triglycerides and high-density lipoprotein [HDL] cholesterol) parameters. The VAI, which is thought to be capable of indicating both fat distribution and function, has been proposed as a surrogate marker of adipose tissue dysfunction. It is also thought to be independently correlated with cardiometabolic risk. We aimed to assess the host and viral factors associated with VAI, as well as its association with histological features and with SVR in patients who have G1 CHC.
Abbreviations
ALT, alanine aminotransferase; BMI, body mass index; G1 CHC, genotype 1 chronic hepatitis C; HCV, hepatitis C virus; HDL, high-density lipoprotein; HOMA, homeostasis model assessment; IR, insulin resistance; PLT, platelet; SVR, sustained virological response; VAI, visceral adiposity index; WC, waist circumference.
Patients and Methods
Patients.
We assessed 236 consecutive patients with G1 CHC who were recruited at the Gastrointestinal & Liver Unit at the University Hospital in Palermo. Patients were included if they had a histological diagnosis of CHC (any degree of fibrosis, including cirrhosis) on a liver biopsy performed within 6 months prior to enrollment. G1 CHC patients were characterized by the presence of anti–hepatitis C virus (HCV) and HCV RNA with persistently abnormal alanine aminotransferase (ALT) and by alcohol consumption of <20 g/day in the previous year or more, the latter evaluated by a specific questionnaire. Exclusion criteria were: (1) advanced cirrhosis (Child-Pugh class B and C); (2) hepatocellular carcinoma; (3) other causes of liver disease of mixed etiologies (excessive alcohol consumption, hepatitis B, autoimmune liver disease, Wilson's disease, hemochromatosis, α1-antitrypsin deficiency); (4) human immunodeficiency virus infection; (5) previous treatment with antiviral therapy or immunosuppressive drug and/or regular use of steatosis-inducing drugs (corticosteroids, valproic acid, tamoxifen, amiodarone); and (6) active intravenous drug addiction.
The study was performed in accordance with the principles of the Declaration of Helsinki and with local and national laws. Approval was obtained from the hospital's Institutional Review Board and Ethics Committee, and written informed consent was obtained from all patients.
Clinical and Laboratory Assessment.
Clinical and anthropometric data were collected at the time of liver biopsy. BMI was calculated, and patients were classified as normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), or obese (≥30 kg/m2). WC was measured at the midpoint between the lower border of the rib cage and the iliac crest. The diagnosis of arterial hypertension was based on the following criteria: systolic blood pressure ≥135 mm Hg and/or diastolic blood pressure ≥85 mm Hg (measured three times within 30 minutes in a sitting position and using a brachial sphygmomanometer) or use of blood pressure–lowering agents. The diagnosis of type 2 diabetes was based on the revised criteria of the American Diabetes Association, using a value of fasting blood glucose ≥126 mg/dL on at least two occasions.19 In patients with a previous diagnosis of type 2 diabetes, current therapy with insulin or oral hypoglycemic agents was documented. Metabolic syndrome was diagnosed according to Adult Treatment Panel III criteria.20
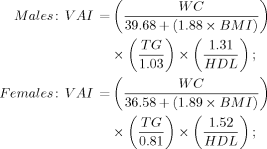
All patients were tested at the time of biopsy for HCV RNA by way of qualitative polymerase chain reaction (Cobas Amplicor HCV Test version 2.0; limit of detection, 50 IU/mL). HCV RNA–positive samples were quantified by way of Versant HCV RNA 3.0 bDNA (Bayer Co., Tarrytown, NY) expressed in IU/mL. Genotyping was performed with an INNO-LiPA HCV II assay (Bayer Co.).
Histology.
Slides were coded and read by one pathologist (D. C.) who was unaware of each patient's identity and history. A minimum biopsy specimen length of 15 mm or the presence of at least 10 complete portal tracts was required.23 Biopsies were classified according to the Scheuer numerical scoring system.24 The percentage of hepatocytes containing macrovesicular fat was determined for each ×10 field. An average percentage of steatosis was then determined for the entire specimen. Steatosis was assessed as the percentage of hepatocytes containing fat droplets (minimum 5%), and evaluated as a continuous variable. Steatosis was classified as absent to mild at <30%, or moderate to severe at ≥30%.
Antiviral Treatment Schedule and Outcomes.
Patients were treated with standard antiviral therapy with pegylated interferon α-2a (Pegasys, Roche, Basel, Switzerland) 180 μg/week plus ribavirin at a dosage of 1,000 or 1,200 mg/day according to body weight (< 75 kg, 1,000 mg/day; >75 kg, 1,200 mg/day) for 48 weeks. Patients were withdrawn from treatment if they did not achieve a virological response (defined as undetectable serum HCV RNA on polymerase chain reaction) within 24 weeks after the start of treatment. This endpoint was in accordance with the stopping rule as defined by the European Association for the Study of the Liver Consensus Conference on Hepatitis C.25
SVR was defined as negative serum HCV RNA on polymerase chain reaction 6 months after stopping antiviral therapy.
Statistics.
Continuous variables were summarized as the mean ± SD, and categorical variables as frequency and percentage. A Student t test and chi-square test were used when appropriate. Multiple linear regression analysis was performed to identify independent predictors of VAI score as the continuous dependent variable. As candidate risk factors, we selected age, sex, BMI, WC, baseline ALT, platelet count levels, triglycerides, total and HDL cholesterol, VAI score, blood glucose, insulin, HOMA score, diabetes, arterial hypertension, log10 HCV RNA levels, steatosis, necroinflammatory activity score, and fibrosis.
Multiple logistic regression models were used to assess the relationship of steatosis, necroinflammatory activity, fibrosis, and SVR to the demographic, metabolic, and histological characteristics of patients. In the first model, the dependent variable was moderate to severe steatosis (1 = steatosis ≥30%; 0 = steatosis <30%). In the second model, the dependent variable was SVR (1 = present; 0 = absent). In the third model, the dependent variable was moderate to severe necroinflammatory activity (1 = grade 2-3; 0 = grade 1). In the fourth model, the dependent variable was severe fibrosis (1 = fibrosis 3-4; 0 = fibrosis 1-2). As candidate risk factors, we selected the same independent variables included in the linear model and added VAI score and log10 HCV RNA as additional independent variables.
Variables associated with the dependent variable on univariate analysis (probability threshold, P ≤ 0.10) were included in multivariate regression models. To avoid the effect of colinearity, diabetes, IR, HOMA score, blood glucose levels, and insulin levels, as well as waist circumference, BMI, HDL cholesterol, triglycerides, metabolic syndrome, VAI score, aspartate aminotransferase, and necroinflammatory activity were not included in the same multivariate model. Regression analyses were performed using Proc Logistic, Proc Reg, and a subroutine in SAS (SAS Institute, Inc., Cary, NC).26
Results
Patient Features and Histology.
The baseline features of the 236 patients are shown in Table 1. The majority of our patients were in the overweight to obese range, and nearly one-quarter of them were hypertensive. Diabetes was present in 11% of patients, and IR was present in 42.8%. Mean values for total cholesterol, HDL cholesterol, and triglycerides were within the normal range. Metabolic syndrome was diagnosed in 14.9% of patients. One patient in five had a fibrosis grade ≥3 by Scheuer score, with a high prevalence of moderate to severe necroinflammation (grade 2-3). Half of the cases had histological evidence of steatosis, though the grading was moderate to severe in only 40 cases (16.9%).
| Features | Values |
|---|---|
| Age, years | 51.7 ± 12.1 |
| Sex | |
| Male | 116 (49.2) |
| Female | 120 (50.8) |
| BMI, kg/m2 | 26.7 ± 4.7 |
| <25 | 86 (36.4) |
| 25-29.9 | 106 (44.9) |
| ≥30 | 44 (18.7) |
| Waist circumference, cm | 92.0 ± 11.8 |
| Arterial hypertension | |
| Absent | 180 (76.3) |
| Present | 56 (23.7) |
| Type 2 diabetes | |
| Absent | 210 (89) |
| Present | 26 (11) |
| Metabolic syndrome | |
| Absent | 201 (85.1) |
| Present | 35 (14.9) |
| ALT, IU/L | 90.8 ± 78.2 |
| Platelet count × 103/mmc | 209.7 ± 53.2 |
| Total cholesterol, mg/dL | 177.0 ± 35.1 |
| HDL cholesterol, mg/dL | 53.8 ± 17.1 |
| Triglycerides, mg/dL | 98.6 ± 45.3 |
| Blood glucose, mg/dL | 94.6 ± 30.6 |
| Insulin, μU/mL | 11.7 ± 5.9 |
| HOMA score | 2.77 ± 1.73 |
| IR (HOMA >2.7) | |
| Absent | 135 (57.2) |
| Present | 101 (42.8) |
| VAI score | 1.47 ± 0.99 |
| Log 10 HCV RNA | 5.5 ± 0.6 |
| HCV RNA ≥6 log 10 | |
| Absent | 169 (71.6) |
| Present | 67 (28.4) |
| Histology at biopsy | |
| Steatosis | |
| Continuous variable (percent of total cells) | 12.2 ± 17.3 |
| Categorical variable | |
| <5% | 116 (49.2) |
| ≥5% to <30% | 80 (33.9) |
| ≥30% | 40 (16.9) |
| Stage of fibrosis | |
| 1 | 66 (27.9) |
| 2 | 120 (50.8) |
| 3 | 46 (19.5) |
| 4 | 4 (1.7) |
| Grade of inflammation | |
| 1 | 48 (20.3) |
| 2 | 146 (61.9) |
| 3 | 42 (17.8) |
- Data are presented as the mean ± SD or n (%).
Factors Associated with VAI Score.
The mean VAI score was 1.47. High ALT (P = 0.04), high insulin (P < 0.001), high HOMA score (P < 0.001), presence of arterial hypertension (P = 0.008), high log10 HCV RNA levels (P = 0.01), steatosis (P = 0.001), and severity of necroinflammatory activity (P = 0.04) were associated with higher VAI score in G1 CHC, though only higher HOMA score (P = 0.009), higher log10 HCV RNA levels (P = 0.01), necroinflammatory activity (P = 0.04), and steatosis (P = 0.04) were independent factors on multiple linear regression analysis (Table 2).
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | β Coefficient | SE | P Value | β Coefficient | SE | P Value |
| Mean age, years | 0.026 | 0.005 | 0.78 | — | — | — |
| Male sex | −0.070 | 0.129 | 0.28 | — | — | — |
| ALT, IU/L | 0.130 | 0.001 | 0.04 | — | — | — |
| Platelet count × 103/mmc | 0.035 | 0.001 | 0.58 | — | — | — |
| Blood glucose, mg/dL | 0.055 | 0.002 | 0.40 | — | — | — |
| Insulin, μU/mL | 0.326 | 0.010 | <0.001 | — | — | — |
| HOMA score | 0.284 | 0.036 | <0.001 | 0.182 | 0.039 | 0.009 |
| Type 2 diabetes | 0.019 | 0.206 | 0.77 | — | — | — |
| Arterial hypertension | 0.173 | 0.150 | 0.008 | 0.108 | 0.151 | 0.09 |
| Metabolic syndrome | 0.380 | 0.168 | <0.001 | — | — | — |
| Log 10 HCV RNA | 0.157 | 0.095 | 0.01 | 0.152 | 0.091 | 0.01 |
| Histology at biopsy | ||||||
| Steatosis | 0.224 | 0.004 | 0.001 | 0.135 | 0.004 | 2.09 |
| Stage of fibrosis | −0.005 | 0.083 | 0.94 | — | — | — |
| Grade of inflammation | 0.127 | 0.104 | 0.04 | 0.143 | 0.007 | 0.04 |
Factors Associated with log10 HCV RNA.
Considering the independent link between VAI score and log10 HCV RNA, we investigated the factors associated with log10 HCV RNA. Low BMI (P = 0.04), high triglycerides (P = 0.02), high VAI score (P = 0.01), and severity of necroinflammatory activity (P = 0.07) were associated with higher log10 HCV RNA in G1 CHC, though only a higher VAI score (P = 0.02) was an independent factor on multiple linear regression analysis (Supporting Information). Figure 1 shows the distribution of VAI scores in terms of log10 HCV RNA.
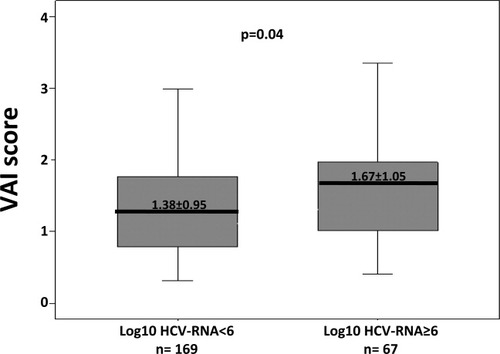
Distribution of VAI scores according to log10 HCV RNA at the threshold of 6 logarithms in patients with G1 CHC.
Factors Associated with Histological Features.
The univariate and multivariate comparisons of variables between patients with and without moderate to severe steatosis (≥30%) are reported in Table 3. Multivariate logistic regression analysis revealed that the following features were independently linked to moderate to severe steatosis (steatosis ≥30%): IR (OR 3.879, 95% CI 1.727-8.713, P = 0.001), higher VAI score (OR 1.472, 95% CI 1.051-2.062, P = 0.02), and fibrosis (OR 2.255, 95% CI 1.349-3.768, P = 0.002). Replacing in the model VAI with WC and triglycerides, the latter remained significantly associated with steatosis ≥30% (OR 1.008, 95% CI 1.000-1.016, P = 0.04) but not WC (OR 1.035, 95% CI 0.999-1.073, P = 0.06). In addition, when replacing VAI as the continuous variable with VAI as the categorical variable at the threshold of 1.3 (the best cutoff by receiver operating characteristic curve: sensitivity 67.5%, specificity 64.5%; area under the curve 0.659) on multivariate analysis, we obtained similar results (OR 2.791, 95% CI 1.261-6.175, P = 0.01). By contrast, VAI at the threshold of 1, which is considered normal,18 was not significantly associated with steatosis ≥30% on multivariate analysis (OR 1.681, 95% CI 0.691-4.098, P = 0.25). Figure 2 shows the distribution of VAI scores in terms of steatosis.
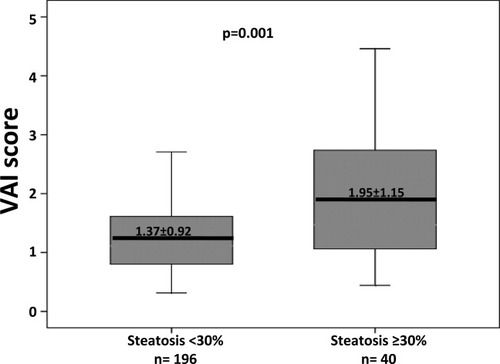
Distribution of VAI scores according to the presence or absence of moderate to severe steatosis (steatosis ≥30%) in patients with G1 CHC.
| Variable | Steatosis < 30% (n = 196) | Steatosis ≥ 30% (n = 40) | Univariate Analysis, P Value | Multivariate Analysis | |
|---|---|---|---|---|---|
| OR (95% CI) | P Value | ||||
| Age, years | 50.6 ± 12.6 | 56.6 ± 7.6 | 0.005 | 1.037 (0.999-1.075) | 0.05 |
| Sex, male/female | 101/95 | 15/25 | 0.10 | — | — |
| BMI, kg/m2 | 26.2 ± 4.2 | 29.2 ± 6.2 | 0.007 | — | — |
| Waist circumference, cm | 90.6 ± 11.3 | 98.5 ± 12.4 | 0.001 | — | — |
| ALT, IU/L | 89.1 ± 77.1 | 99.2 ± 83.9 | 0.45 | — | — |
| Platelet count × 103/mmc | 209.1 ± 50.4 | 212.4± 65.9 | 0.72 | — | — |
| Total cholesterol, mg/dL | 175.6 ± 33.6 | 183.6 ± 41.7 | 0.19 | — | — |
| HDL cholesterol, mg/dL | 54.0 ± 17.2 | 52.5 ± 16.6 | 0.60 | — | — |
| Triglycerides, mg/dL | 94.5 ± 42.8 | 118.4 ± 52.4 | 0.002 | — | — |
| Blood glucose, mg/dL | 93.4 ± 31.8 | 100.7 ± 23.1 | 0.16 | — | — |
| Insulin, μU/mL | 10.8 ± 5.2 | 15.8 ± 7.5 | <0.001 | — | — |
| HOMA score | 2.53 ± 1.50 | 3.94 ± 2.25 | <0.001 | 1.482 (1.136-1.835) | <0.001 |
| Arterial hypertension | 0.15 | — | — | ||
| Absent | 157 | 28 | |||
| Present | 39 | 12 | |||
| Type 2 diabetes | 0.37 | — | — | ||
| Absent | 176 | 34 | |||
| Present | 20 | 6 | |||
| Metabolic syndrome | 173/23 | 28/12 | 0.003 | — | — |
| VAI score | 1.37 ± 0.92 | 1.95 ± 1.15 | 0.001 | 1.439 (1.027-2.016) | 0.03 |
| Log 10 HCV RNA | 5.6 ± 0.6 | 5.5 ± 0.5 | 0.80 | — | — |
| Histology at biopsy | |||||
| Stage of fibrosis, 1/2/3/4 | 60/105/29/2 | 6/15/17/2 | <0.001 | 2.436 (1.433-4.141) | 0.001 |
| Grade of inflammation, 1/2/3 | 44/120/32 | 4/26/10 | 0.04 | 1.241 (0.635-2.425) | 0.52 |
- Data are presented as the mean ± SD or as the number of cases.
- Abbreviations: CI, confidence interval; OR, odds ratio.
The univariate and multivariate comparisons of variables between patients with and without moderate to severe necroinflammatory activity are reported in Table 4. Multivariate logistic regression analysis revealed that the following features were independently linked to moderate to severe necroinflammatory activity: older age (OR 1.030, 95% CI 1.002-1.059, P = 0.03), higher VAI score (OR 1.618, 95% CI 1.001-2.617, P = 0.04), and fibrosis (OR 2.608, 95% CI 1.565-4.345, P < 0.001). When replacing VAI with triglycerides, the latter remained significantly associated with moderate to severe necroiflammatory activity (OR 1.010, 95% CI 1.001-1.020, P = 0.03). In addition, when replacing VAI as the continuous variable with VAI as the categorical variable at the threshold of 1, VAI score was not significantly associated with moderate to severe necroinflammatory activity on multivariate analysis (OR 1.713, 95% CI 0.855-3.431, P = 0.12). By contrast, VAI at the above-mentioned threshold of 1.3 remained independently associated with moderate to severe necroinflammatory activity on multivariate analysis (OR 2.619, 95% CI 1.199-5.722, P = 0.01). Figure 3 shows the distribution of VAI scores in terms of necroinflammatory activity.
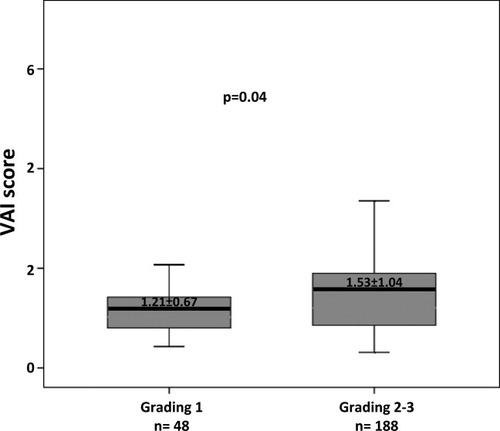
Distribution of VAI scores according to the presence or absence of moderate to severe necroinflammatory activity in patients with G1 CHC.
| Variable | Grading 1 (n = 48) | Grading 2-3 (n = 188) | Univariate Analysis, P Value | Multivariate Analysis | |
|---|---|---|---|---|---|
| OR (95% CI) | P Value | ||||
| Age, years | 46.7 ± 12.0 | 52.9 ± 11.8 | 0.001 | 1.030 (1.002-1.059) | 0.03 |
| Sex, male/female | 22/26 | 94/94 | 0.60 | — | — |
| BMI, kg/m2 | 26.6 ± 5.2 | 26.8 ± 4.6 | 0.78 | — | — |
| Waist circumference, cm | 91.7 ± 11.7 | 92.0 ± 11.9 | 0.85 | — | — |
| ALT, IU/L | 60.8 ± 37.0 | 98.5 ± 84.0 | 0.003 | — | — |
| Platelet count × 103/mmc | 220.5 ± 54.3 | 206.6 ± 52.6 | 0.10 | — | — |
| Total cholesterol, mg/dL | 179.9 ± 33.4 | 176.3 ± 35.6 | 0.52 | — | — |
| HDL cholesterol, mg/dL | 56.6 ± 17.3 | 53.1 ± 17.0 | 0.20 | — | — |
| Triglycerides, mg/dL | 87.1 ± 32.0 | 101.5 ± 47.8 | 0.04 | — | — |
| Blood glucose, mg/dL | 88.7 ± 12.7 | 96.4 ± 33.5 | 0.11 | — | — |
| Insulin, μU/mL | 10.8 ± 6.5 | 11.9 ± 5.8 | 0.26 | — | — |
| HOMA score | 2.47 ± 1.78 | 2.84 ± 1.72 | 0.18 | — | — |
| Arterial hypertension | 0.19 | — | — | ||
| Absent | 40 | 140 | |||
| Present | 8 | 48 | |||
| Type 2 diabetes | 0.20 | — | — | ||
| Absent | 45 | 164 | |||
| Present | 3 | 24 | |||
| Metabolic syndrome | 0.15 | — | — | ||
| Absent | 44 | 157 | |||
| Present | 4 | 31 | |||
| VAI score | 1.21 ± 0.67 | 1.53 ± 1.04 | 0.04 | 1.618 (1.001 – 2.617) | 0.04 |
| Log 10 HCV RNA | 5.5 ± 0.6 | 5.6 ± 0.6 | 0.38 | — | — |
| Histology at biopsy | |||||
| Steatosis | 7.8 ± 13.6 | 13.3 ± 17.9 | 0.04 | 1.007 (0.982-1.033) | 0.57 |
| Stage of fibrosis, 1/2/3/4 | 27/17/4/0 | 39/103/42/4 | <0.001 | 2.608 (1.565-4.345) | |
- Data are presented as the mean ± SD or as the number of cases.
- Abbreviations: CI, confidence interval; OR, odds ratio.
Older age, higher WC, high ALT and platelet (PLT) levels, steatosis, and necroinflammatory activity were associated with severe fibrosis (P < 0.10). VAI score as continuous variable, and as categorical variable at the thresholds of 1 and 1.3, was not associated with severe fibrosis. Multivariate logistic regression analysis showed that the following features were independently linked to severe fibrosis: high PLT levels (OR 1.003, 95% CI 1.001-1.005, P = 0.02), steatosis (OR 1.026, 95% CI 1.007-1.045, P = 0.008), and necroinflammatory activity (OR 2.106, 95% CI 1.180-3.761, P = 0.01).
Factors Associated with SVR.
Of 162 patients who underwent and completed the antiviral treatment program, SVR was achieved in 77 (47.5%). Older age, high WC, high triglycerides, high blood glucose, high HOMA, high IR, diabetes, high VAI score, and steatosis were associated with lack of SVR at a threshold of P < 0.10 (Table 5). Logistic regression analysis revealed that older age (OR 0.970, 95% CI 0.944-0.997, P = 0.02) and steatosis (OR 0.969, 95% CI 0.945-0.993, P = 0.01) were the only independent negative predictors of SVR.
| Variable | No SVR (n = 85) | SVR (n = 77) | Univariate Analysis, P Value | Multivariate Analysis | |
|---|---|---|---|---|---|
| OR (95% CI) | P Value | ||||
| Age, years | 54.0 ± 11.4 | 48.0 ± 13.2 | 0.002 | 0.970 (0.943-0.996) | 0.02 |
| Sex, male/female | 37/48 | 41/36 | 0.21 | — | — |
| BMI, kg/m2 | 27.3 ± 4.9 | 26.1 ± 4.6 | 0.12 | — | — |
| Waist circumference, cm | 93.4 ± 12.7 | 89.5 ± 10.9 | 0.03 | — | — |
| ALT, IU/L | 85.5 ± 74.8 | 95.8 ± 87.2 | 0.41 | — | — |
| Platelet count × 103/mmc | 209.4 ± 50.9 | 212.3 ± 49.8 | 0.71 | — | — |
| Total cholesterol, mg/dL | 170.5 ± 36.1 | 179.4 ± 33.5 | 0.10 | — | — |
| HDL cholesterol, mg/dL | 54.9 ± 15.5 | 52.8 ± 18.6 | 0.42 | — | — |
| Triglycerides, mg/dL | 100.8 ± 48.3 | 88.8 ± 34.0 | 0.07 | — | — |
| Blood glucose, mg/dL | 99.2 ± 37.6 | 88.7 ± 19.8 | 0.03 | — | — |
| Insulin, μU/mL | 12.5 ± 6.4 | 11.1 ± 5.7 | 0.16 | — | — |
| HOMA score | 3.02 ± 1.79 | 2.54 ± 1.69 | 0.08 | 0.978 (0.786-1.216) | 0.84 |
| Arterial hypertension | 59/26 | 62/15 | 0.10 | — | — |
| Absent | |||||
| Present | |||||
| Type 2 diabetes | 71/14 | 73/4 | 0.02 | — | — |
| Absent | |||||
| Present | |||||
| Metabolic syndrome | 67/18 | 73/4 | 0.03 | — | — |
| Absent | |||||
| Present | |||||
| VAI score | 1.50 ± 1.03 | 1.26 ± 0.70 | 0.09 | 0.877 (0.583-1.320) | 0.52 |
| Log 10 HCV RNA | 5.5 ± 0.6 | 5.4 ± 0.7 | 0.36 | — | — |
| Histology at biopsy | |||||
| Steatosis | 17.6 ± 21.1 | 7.4 ± 10.4 | <0.001 | 0.968 (0.944-0.992) | 0.01 |
| Stage of fibrosis, 1/2/3/4 | 22/42/18/3 | 24/41/12/0 | 0.14 | — | — |
| Grade of inflammation, 1/2/3 | 10/60/15 | 23/39/15 | 0.10 | — | — |
- Data are presented as the mean ± SD or as the number of cases.
- Abbreviations: CI, confidence interval; OR, odds ratio.
Discussion
In a cohort of patients with G1 CHC, we found that VAI score, a new index of adipose tissue dysfunction, was independently associated with both steatosis and necroinflammation and was also related to high HCV viral load.
Data in the literature show that VAI score appears able to indirectly indicate both fat distribution and function in nonobese healthy patients and in primary care patients. Therefore, the peculiarity of this index lies in the fact that it may reflect other nonclassic cardiometabolic risk factors, such as altered production of adipocytokines/cytokines, increased lipolysis, and plasma-free fatty acids, which are not signified by BMI, WC, triglycerides, and HDL cholesterol separately.18
In this study, we found that moderate to severe necroinflammatory activity is independently associated not only with older age but also with VAI score. To the best of our knowledge, this is the first evidence of an independent link between adipose dysfunction and liver inflammation in CHC, speculating that this index may be able to reflect the ability of adipose tissue to generate proinflammatory mediators capable of participating in liver inflammatory response during HCV infection.
In the same group of patients, we also demonstrated that steatosis was independently associated with both IR and VAI score. Data show that IR due both to viral and host factors is the key factor in liver steatosis development in HCV patients,27, 28 and some studies have shown a link between obesity and steatosis in this group of patients.13-16 However, most of these studies did not correct the effect of obesity for the presence of IR. Accordingly, it is worth noting that in our study, both IR and high VAI score were independently associated with steatosis, leading us to speculate on the ability of adipose tissue to interfere with liver fatty accumulation not only by IR promotion, but also by exercising its well-known function as an endocrine organ able to modulate metabolic functions, including steatogenesis.
In this study, we found no association between severe fibrosis and VAI score; however, we confirmed that steatosis and necroinflammatory activity, two well-known risk factors for fibrosis,2-6, 29, 30 were independently associated with severe fibrosis. Therefore, we suggest that factors affecting the VAI score participate in the severity of liver fibrosis by promoting and amplifying both steatosis and liver inflammation.
From a clinical point of view, and in accordance with our results, we recommend that (1) the VAI be used as an indicator of adipose-related liver damage, (2) prospective studies evaluate VAI as a predictor of liver disease progression, and (3) the VAI be considered a new therapeutic outcome in the management of G1 CHC patients.
We confirmed the reported association between VAI score and IR18 and, to the best of our knowledge, are the first to have found a linear, independent association between VAI score and high HCV RNA viral load. These data are consistent with reports that have shown a direct association between viral load and BMI31 and between HCV RNA status and obesity.32 Although this study was not designed to clarify the pathogenetic link between adipose-related features, VAI score in particular, and viral load in G1 CHC, a few hypotheses would agree with the data in the literature. Experimental and clinical studies have shown a direct relationship between viral load and IR in CHC.27 We could not confirm this association in our study, probably due to the demographic, metabolic, and histological characteristics of the patients. However, it is possible to speculate that because HCV is able to induce hepatic and peripheral IR,33, 34 it could similarly interfere with adipose tissue function. HCV could interfere with adipocyte function indirectly, by favoring proinflammatory cytokine production35 and by prompting macrophage fat infiltration, and directly, by theoretical infection of adipose tissue, and by interfering with peroxisome proliferator-activated receptor gamma expression,36 a well-known modulator of adipose tissue homeostasis. In addition, we cannot rule out the possibility that the proinflammatory status, as well as the higher availability of fatty substrates due to adipose dysfunction, are able to stimulate HCV RNA replication. Figure 4 displays the putative mechanistic relationship linking HCV, host metabolism, and VAI.
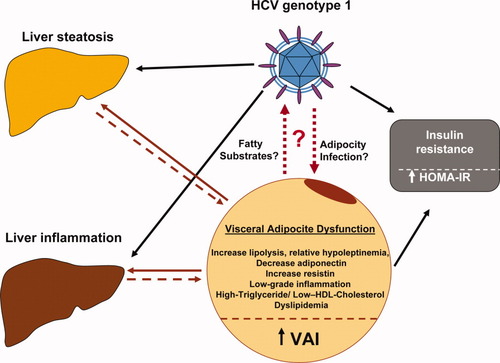
Putative mechanistic relationship linking HCV, host metabolism, and VAI. Adipose tissue dysfunction by way of free fatty acid and proinflammatory cytokine secretion could directly participate in both liver steatosis and inflammation induction. In turn, these features could enhance adipose tissue inflammation and, consequently, its dysfunction. In this complex interplay between the liver and adipose tissue, HCV could have a relevant role. It is possible not only that adipose tissue could offer fatty substrates and a proinflammatory status favoring HCV replication, but also that HCV could interfere with adipocyte function indirectly by increasing the inflammatory status and directly by colonizing adipocytes or immune cells infiltrating adipose tissue.
Finally, we have shown that both IR and VAI score had a nonsignificant trend for predicting failure of SVR achievement after standard antiviral therapy, and that after correction for steatosis, only the latter was significantly associated with a lower likelihood of virological clearance, suggesting an indirect role of both VAI score and IR on SVR achievement by steatosis induction.
The main limitation of this study lies in its cross-sectional nature, making it impossible to dissect the temporal relationship between IR, VAI score, and steatosis, and between VAI score and viral load in G1 CHC patients. A further methodological question is the potentially limited external validity of the results for different populations and settings. Our study included a cohort of European patients, largely overweight, who were enrolled in a tertiary referral center for liver disease, limiting the broad application of the results. Another limitation lies in the interobserver variability of the evaluation of hepatic necroinflammatory activity, which could affect the reproducibility of our results.37 Lack of data on the serum levels and on adipose expression of proinflammatory cytokines and adipocytokines may also have affected our interpretation of the results.
In conclusion, VAI, a new index of both fat function and distribution, appears to be independently associated with steatosis and necroinflammatory activity in G1 CHC patients and has a direct correlation with HCV viral load. These data suggest a direct role of adipose tissue in liver damage and a possible interference of HCV with adipocyte function. Experimental studies are needed to determine the mechanisms responsible for these associations.




