Overlapping high-resolution copy number alterations in cancer genomes identified putative cancer genes in hepatocellular carcinoma†
Potential conflict of interest: Nothing to report.
Abstract
Recurrent cancer genome aberrations are indicators of residing crucial cancer genes. Although recent advances in genomic technologies have led to a global view of cancer genome aberrations, the identification of target genes and biomarkers from the aberrant loci remains difficult. To facilitate searches of cancer genes in human hepatocellular carcinoma (HCC), we established a comprehensive protocol to analyze copy number alterations (CNAs) in cancer genomes using high-density single nucleotide polymorphism arrays with unpaired reference genomes. We identified common HCC genes by overlapping the shared aberrant loci in multiple cell lines with functional validation and clinical implications. A total of 653 amplicons and 57 homozygous deletions (HDs) were revealed in 23 cell lines. To search for novel HCC genes, we overlapped aberrant loci to uncover 6 HDs and 126 amplicons shared by at least two cell lines. We selected two novel genes, fibronectin type III domain containing 3B (FNDC3B) at the 3q26.3 overlapped amplicon and solute carrier family 29 member 2 (SLC29A2) at the 11q13.2 overlapped amplicon, to investigate their aberrations in HCC tumorigenesis. Aberrant up-regulation of FNDC3B and SLC29A2 occurred in multiple HCC data sets. Knockdown of these genes in amplified cells decreased cell proliferation, anchorage-independent growth, and tumor formation in xenograft models. Importantly, up-regulation of SLC29A2 in HCC tissues was significantly associated with advanced stages (P = 0.0031), vascular invasion (P = 0.0353), and poor patient survival (P = 0.0325). Overexpression of FNDC3B or SLC29A2 in unamplified HCC cells promoted cell proliferation through activation of the signal transducer and activator of transcription 3 signaling pathway. Conclusion: A standardized genome-wide CNA analysis protocol using data from user-generated or public domains normalized with unpaired reference genomes has been established to facilitate high-throughput detection of cancer genes as significant target genes and biomarkers for cancer diagnosis and therapy. (HEPATOLOGY 2010)
Sequential accumulation of genetic aberrations is a hallmark of cancer genomes and is attributed to the etiology of tumor formation and progression. Genetic aberrations in cancer, including point mutations, amplifications, deletions, and translocations, commonly result in the activation of oncogenes and inactivation of tumor-suppressor genes. For instance, cancer genomes with amplified oncogenes such as epidermal growth factor receptor (EGFR),1 deleted tumor-suppressor genes such as cyclin-dependent kinase inhibitor 2A (CDKN2A),2 and translocated fusion genes such as nucleophosmin/anaplastic lymphoma kinase (NPM/ALK)3 not only demonstrate genetic causes of tumorigenesis but also serve as targets for cancer therapy.
Several systematic approaches have led to a global view of genetic aberrations in cancer. Recent studies using cancer genome sequencing strategies have generated a larger number of infrequently mutated genes with, on average, more than 50 nonsilent mutations in an individual tumor organ, and only a few of these genes are mutated in a high proportion of tumors.4, 5 The low frequency of new commonly mutated genes suggests that the development of new cancer therapeutic targets at a faster pace still remains a big challenge with current sequencing strategies.6 Recent advances in manufacturing high-density oligonucleotide arrays from various commercial sources have revolutionized the detection of genome aberrations through the high-resolution analysis of copy number alterations (CNAs). However, the cloning of putative cancer target genes is still hampered because inevitable DNA contamination of surrounding nonneoplastic cells attenuates the detection of aberrant signals, and there are few systematic approaches to pinpointing altered cancer genes in aberrant regions. It has been suggested that the detection of CNAs in a cancer genome is highly dependent on the purity of the neoplastic DNA.7, 8 More than 10% contamination of nonneoplastic or etiologically heterogeneous cancer cells results in a significant reduction of sensitivity of CNA analysis, especially for the detection of homozygous deletions (HDs).7 Furthermore, with improvements in tumor diagnosis, the availability of primary tumor tissues and their matched normal controls is becoming more limited, and this will eventually be a problem, especially for experiments using genome-wide approaches. Instead, cancer cell lines can provide snapshots of acquired accumulated genomic lesions during tumorigenesis, and they represent an ideal and unlimited source of DNA in the search for novel cancer genes without concerns about contamination from normal cells.
To overcome the limitations of cancer cell lines without matched normal controls, 50 Epstein-Barr virus (EBV)–transformed peripheral blood lymphocytes from healthy individuals were genotyped with Affymetrix GeneChip high-density 500K single nucleotide polymorphism (SNP) arrays to test their use as feasible alternative controls. We established criteria and protocols for CNA analysis and revealed 57 HDs and 653 amplified regions in 23 human cancer cell lines. To pinpoint pivotal genes in human hepatocellular carcinoma (HCC), we overlapped the CNA regions to narrow the common aberrant regions shared by multiple cell lines. Two genes, fibronectin type III domain containing 3B (FNDC3B) at the 3q26.3 amplicon and solute carrier family 29 member 2 (SLC29A2) at the 11q13.1 amplicon, were selected to validate their aberrant roles in tumorigenesis and to correlate them with the clinicopathological features of HCC. Our results suggest that the definition of common HDs and amplicons with high-density SNP arrays is a powerful method for revealing cancer genes involved in tumorigenesis.
Abbreviations
ADAM15, a disintegrin and metallopeptidase 15; AKT, v-akt murine thymoma viral oncogene homolog 1; AMACR, alpha-methylacyl-coenzyme A racemase; BCAS1, breast carcinoma amplified sequence 1; BRMS1, breast cancer metastasis suppressor 1; CCND1, cyclin D1; CDKN2A, cyclin-dependent kinase inhibitor 2A; CHN2, chimerin 2; CKS1B, CDC28 protein kinase regulatory subunit 1B; CNA, copy number alteration; DAB2, disabled homolog 2, mitogen-responsive phosphoprotein (Drosophila); EBV, Epstein-Barr virus; EGFR, epidermal growth factor receptor; ETV1, ets variant 1; EVI1, ecotropic viral integration site 1; FNDC3B, fibronectin type III domain containing 3B; HCC, hepatocellular carcinoma; HD, homozygous deletion; ICN, inferred copy number; IHC, immunohistochemistry; LRP1B, low-density lipoprotein receptor-related protein 1B; LRP5, low-density lipoprotein receptor-related protein 5; Luc, luciferase; MAGI2, membrane associated guanylate kinase, WW and PDZ domain containing 2; MDS1, myelodysplastic syndrome 1; MTAP, methylthioadenosine phosphorylase; NPC, nasopharyngeal carcinoma; NSCLC, non–small cell lung cancer; OD, optical density; ORAOV1, oral cancer overexpressed 1; PARK2, Parkinson desease (autosomal recessive, juvenile) 2, parkin; PBS, phosphate-buffered saline; qRT-PCR, quantitative reverse-transcriptase polymerase chain reaction; RAC1, ras-related C3 botulinum toxin substrate 1; RIN1, Ras and Rab interactor 1; RNAi, RNA interference; SHC1, Src homology 2 domain containing transforming protein 1; shRNA, short hairpin RNA; SLC29A2, solute carrier family 29 member 2; SNP, single nucleotide polymorphism; STAT3, signal transducer and activator of transcription 3; TERC, telomerase RNA component; TRIO, triple functional domain (PTPRF interacting).
Materials and Methods
Cell Lines and Tissues
Thirteen HCC cell lines (HA22T, HA59T, Hep3B, HepG2, HuH6, HuH7, Mahlavu, PLC/PRF/5, SK-Hep-1, SNU387, SNU398, SNU449, and Tong) and two nasopharyngeal carcinoma (NPC) cell lines (HK1 and CNE1) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 1% nonessential amino acids, and 1% penicillin/streptomycin (Invitrogen). Eight non–small cell lung cancer (NSCLC) cell lines (A549, H23, H358, H928, H1299, H1437, CL1, and CL3) were cultured in Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. All the genomic DNAs were extracted and purified with phenol/chloroform extraction followed by ethanol precipitation. Forty-five archived primary HCCs and their matching adjacent normal liver tissues were obtained from National Taiwan University Hospital, and the institutional review board of National Taiwan University Hospital approved the use of these archived tissues.
Genotyping with a 500K SNP Array and Data Analysis
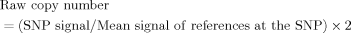
Finally, to ensure the quality of the CNA analysis, we instituted three highly stringent criteria for the definition of HDs and amplicons on cancer genomes: (1) a window size of 10 was used for median smoothing to infer the raw copy number of the SNP; (2) amplicons and HDs were defined by inferred copy numbers (ICNs) greater than 4 and less than 0.4, respectively, in at least 10 consecutive SNPs; and (3) if there were two neighboring amplicons or HDs with a gap less than 10 SNP, they were merged. When common amplicons with ICNs over 4 were observed in two cell lines, the overlapped amplicons in other cell lines with an ICN intensity ≥3 and <4 were also presented.
Immunohistochemical Analysis
Rabbit polyclonal antibodies against human FNDC3B and SLC29A2 were purchased from Sigma and Abcam, respectively. Frozen tissue sections (5 μm) were cut from the recipient blocks and were incubated overnight at −20°C to ensure adherence. The sections were then treated with 3% hydrogen peroxide for 30 minutes to stop the endogenous peroxidase activity and were boiled in a 10 mM citrate buffer (pH 6.0) at 95°C for 15 minutes to unmask the epitopes. After antigen retrieval, the sections were incubated with a 1:50-diluted antibody in phosphate-buffered saline (PBS) for 1 hour, and this was followed by PBS washes. A horseradish peroxidase/fragment antigen binding polymer conjugate (PicTure-Plus kit, Zymed) was then applied to the sections for 30 minutes. After the washing, the sections were incubated with a peroxidase substrate for 2 minutes and were counterstained with hematoxylin.
RNA Interference (RNAi) Knockdown
Short hairpin RNAs (shRNAs) targeting SLC29A2 and FNDC3B in the RNAi Consortium shRNA library were ordered from the National RNAi Core Facility (Academia Sinica). We selected and mixed the two most effective shRNAs to knock down the expression of SLC29A2 (TRCN0000043658 and TRCN0000043 660) and FNDC3B (TRCN0000082774 and TRCN0 000082776) either by direct transfection to the target cell or by infection with 293T-produced lentivirus. For the lentivirus production, the supernatant of 293T was harvested 24, 48, and 72 hours after transfection with shRNA vectors. Targeted cells were then incubated with lentiviruses for 24 hours with 6 μg/mL polybrene (Sigma-Aldrich). After delivery of the shRNAs to the target cells, the cells were treated with puromycin (Invitrogen) for 3 days of selection.
Cell Proliferation and Colony Formation Assays
For cell proliferation assays, the viability of puromycin-selected cells was determined every 24 hours for 4 days with the reagent alamarBlue (AbD Serotec), and the absorbance at wavelengths of 560 and 590 nm was measured for growth curves. For the colony formation assays in soft agar, 10,000 puromycin-selected cells were mixed in 0.25% top agarose and were plated onto 0.5% bottom agarose in a culture medium in 60-mm dishes. All experiments were conducted in triplicate. The dishes were incubated at 37°C in a 5% CO2 incubator for 3 weeks, and the medium was changed every 3 days. Colonies were photographed by light microscopy and were visualized by staining with 1% crystal violet (Sigma-Aldrich).
Tumorigenesis in Nude Mice

Statistical Analysis
The association of gene expression with the vascular invasion of tumors was calculated with Fisher's exact test. SPSS 17 for Windows XP was applied to the analysis of Kaplan-Meier survival curves. The correlation between SLC29A2 expression and patient survival was calculated with Spearman's rank correlation coefficient (r).
Results
CNA Analysis of Cancer Genomes with Healthy Individual Genomes as References
To perform a comprehensive CNA analysis of cancer genomes without restrictions by available adjacent normal control DNAs, 23 cancer cell lines and 50 EBV-transformed lymphocytes of healthy individuals were genotyped with high-density 500K SNP arrays. As indicated in Fig. 1, we established stringent criteria for defining aberrant regions and especially HDs and amplicons. First, we obtained the raw copy number for each SNP probe by comparing the SNP intensity data with average SNP intensities of 50 healthy individuals. To avoid experimental data variation from an individual SNP, we used a median smoothing method with a window size of 10 continuous SNPs to obtain the ICN of each SNP. We defined amplicons and HDs by ICNs greater than 4 or less than 0.4, respectively, with at least 10 continuous aberrant SNPs. Overall, we identified 57 HDs and 653 amplicons in 23 human cancer cell lines (Supporting Information Fig. 1 and Supporting Information Table 1).
The workflow of genome-wide CNA analysis for amplicons and HDs. There are four major steps for CNA analysis: copy number determination, copy number smoothing, determination of aberrant regions, and refinement of aberrant regions. Representative snapshots of (A) amplicons and (C) HDs and (B) schematic figures of key procedures are shown to demonstrate and illustrate the effects of the data process in each step. In brief, the signal intensities for each SNP in the cancer genome array were compared to the average signal intensities from a reference pool of healthy individuals to obtain the raw copy number of each SNP in the cancer genome. Then, we applied the median smoothing method (window size = 10) to obtain the ICN of each SNP. An amplicon was defined by an ICN ≥ 4 and an HD was defined by an ICN ≦ 0.4 in at least 10 consecutive SNPs. If there were two neighboring amplicons or HDs with a gap less than 10 SNPs, they were merged into one aberration.
| Cytoband | Number of Aberrations | Start (Mb) | End (Mb) | Cell Lines | Known Cancer Genes | ||
|---|---|---|---|---|---|---|---|
| HCC | NSCLC | NPC | |||||
| Amplicons | |||||||
| 1q21.2-22 | 4 | 150.07 | 151.89 | Hep3B | H23, H1437 | SHC1, CKS1B, ADAM15 | |
| 3q26.2-26.31 | 1 | 170.07 | 170.24 | Hep3B | CNE1 | ||
| 1 | 170.28 | 170.99 | Hep3B, PLC/PRF/5 | CNE1 | EVI1, MDS1, TERC | ||
| 2 | 171.21 | 173.50 | Hep3B, PLC/PRF/5, Tong | CNE1 | FNDC3B | ||
| 5p15.33-12 | 77 | 0.40 | 45.14 | HA59T, HA22T | H928, H1437, CL3 | CNE1 | TRIO, AMACR, DAB2 |
| 7p22.2-14.3 | 22 | 4.15 | 32.10 | Hep3B, Huh6 | HK1, CNE1 | RAC1, ETV1, CHN2 | |
| 7p12.1-11.2 | 7 | 52.79 | 55.17 | Hep3B, Tong, Huh6, Huh7 | HK1 | EGFR | |
| 2 | 56.00 | 56.53 | Hep3B, Huh6, Huh7 | HK1 | |||
| 8p11.21 | 1 | 40.44 | 40.62 | Hep3B | H358, H1437 | ||
| 8q24.21 | 1 | 129.21 | 129.29 | H23, H358 | |||
| 11q13.2-13.3 | 1 | 65.85 | 66.44 | SNU387, Huh7, Hep3B | RIN1, BRMS1, SLC29A2 | ||
| 1 | 67.58 | 67.71 | SNU387, Huh7, Hep3B | H23 | |||
| 3 | 67.91 | 69.35 | SNU387, Huh7, Hep3B | H23 | HK1 | LRP5, CCND1, ORAOV1 | |
| 12p12.1 | 2 | 24.36 | 25.54 | Huh6 | H358 | BCAS1, K-ras | |
| 20q13.31 | 1 | 53.94 | 53.96 | HepG2, HA59T, PLC/PRF/5, Hep3B, Huh6, Huh7, Mahlavu | CL3, H23, H928 | CNE1 | |
| HDs | |||||||
| 2q22.1 | 1 | 141.72 | 141.80 | HA22T | CL1 | LRP1B | |
| 7q21.11 | 1 | 77.96 | 78.04 | HepG2, Mahlavu | MAGI2 | ||
| 9p23 | 1 | 9.42 | 9.46 | Mahlavu | H358 | ||
| 1 | 11.90 | 12.00 | PLC/PRF/5 | H1437 | |||
| 9p21.3 | 1 | 21.85 | 21.90 | Sk-Hep-1, SNU387, SNU449 | A549, CL3, H1437 | MTAP, CDKN2A | |
| 1 | 24.27 | 24.84 | SNU387, SNU449 | ||||
- A cell line in italics indicates that its ICN intensity in the amplicon is between 3 and 4. FNDC3B and SLC29A2 as cancer genes are supported by this study.
- Abbreviations: ADAM15, a disintegrin and metallopeptidase 15; AMACR, alpha-methylacyl-coenzyme A racemase; BCAS1, breast carcinoma amplified sequence 1; BRMS1, breast cancer metastasis suppressor 1; CCND1, cyclin D1; CHN2, chimerin 2; CKS1B, CDC28 protein kinase regulatory subunit 1B; DAB2, disabled homolog 2, mitogen-responsive phosphoprotein (Drosophila); ETV1, ets variant 1; EVI1, ecotropic viral integration site 1; LRP1B, low-density lipoprotein receptor-related protein 1B; LRPS, low-density lipoprotein receptor-related protein 5; MAGI2, membrane associated guanylate kinase, WW and PDZ domain containing 2; MDS1, myelodysplastic syndrome 1; ORAOV1, oral cancer overexpressed 1; RAC1, ras-related C3 botulinum toxin substrate 1; RIN1, Ras and Rab interactor 1; SHC1, Src homology 2 domain containing transforming protein 1; TERC, telomerase RNA component; TRIO, triple functional domain (PTPRF interacting).
Validation of HDs and Amplicons by Known Aberrations
We validated our protocols and results on the basis of several known HDs and amplicons, including the HD on exons 3 and 4 of PARK2 in PLC/PRF/5 cells, the HD at 13q12.11 of SK-Hep-1 cells, and the amplicon at 7q22.1 of Tong cells11-13 (Supporting Information Table 2). To detect cancer genes such as EGFR in amplicons and CDKN2A (p16) in HDs, we aligned aberrant loci from multiple cell lines and showed that an EGFR amplicon with an ICN greater than 4 could be detected in Tong, Hep3B, and HK1 cells, and a 9p21.3 HD with an ICN less than 0.4 was detected in six cell lines, with the regions narrowed in A549 and CL3 cells to two tumor-suppressor genes, CDKN2A and methylthioadenosine phosphorylase (MTAP) (Supporting Information Fig. 2A,B). We also validated our protocol for identifying the EGFR amplicon and the MTAP/CDKN2A HD with data from different SNP density arrays and tumor tissues from the Gene Expression Omnibus database of the National Center for Biotechnology Information (Supporting Information Fig. 2C,D). Our results indicate that we have established a protocol for determining the CNAs on cancer genomes with high-density SNP arrays without the need for matched tumor-adjacent normal DNA. Furthermore, our results not only confirm the HDs and amplicons previously reported with low-resolution methods but also refine the boundaries of aberrations to facilitate the cloning of cancer genes.
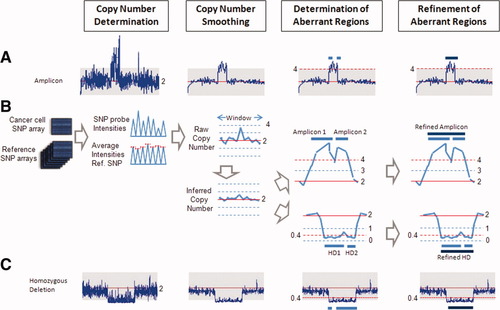
FNDC3B was the only gene at the 3q26.31 overlapped amplicon and was amplified and essential for HCC tumorigenesis. (A) The 3q26.31 overlapped amplicon was 329 kb and was 590 kb and amplified in Hep3B, Tong, and PLC/PRF/5 cells. (B) Concordant expression of FNDC3B was overexpressed in both RNA and protein levels in representative HCC tumor tissues versus matched adjacent normal tissue according to an analysis of qRT-PCR (top panel), IHC (middle panel), and western blotting (bottom panel). Through the introduction of FNDC3B or Luc shRNAs into Hep3B and PLC/PRF/5, validated down-regulation of FNDC3B expression (C) reduced cell proliferation and (D) decreased colony formation in anchorage-independent growth assays (visualized by photography and crystal violet staining). (E) Knockdown of FNDC3B expression by shRNA in Hep3B cells suppressed tumor formation in xenograft tumor models in nude mice (n = 5). **P < 0.005 and *P < 0.05 (significant differences). Abbreviation: OD, optical density.
Detection of Common and Novel Aberration Regions in Multiple Cancer Cells
Because the alignment of aberrant loci could identify frequent alterations and potentially pinpoint commonly embraced cancer genes such as EGFR, CDKN2A, and MTAP in overlapped aberrant loci, we identified 6 HDs and 126 amplicons in 14 cytogenetic loci existing in at least two cancer cell lines (Table 1). Among the six HDs, the 2q22.1, 7q21.11, and 9p21.3 HDs (21.85-21.90 Mb) contained known tumor-suppressor genes. The other three HDs included two HDs at 9p23 (9.42-9.46 and 11.90-12.00 Mb) and one at 9p21.3 (24.27-24.84 Mb) containing neither coding nor noncoding genes. The majority of the 126 amplicons, including 77 amplicons at 5p15.3-12 and 22 amplicons at 7p22.2-14.3, were clustered together because of amplification of the entire 5p in HA59T and H928 and 7p in Hep3B and Huh6 cells (Table 1 and Supporting Information Fig. 1). For the remaining 27 smaller overlapped amplicons, we have legitimate opportunities to pinpoint the amplified target genes after the alignment of amplicons in multiple cell lines. Two novel amplicons with common regions at 3q26.3 in Hep3B and PLC/PRF/5 and at 11q13.2 in Huh7 and SNU387 were selected for further investigation with respect to their roles in HCC tumorigenesis.
FNDC3B at the 3q26.3 Overlapped Amplicon Is Essential for HCC Tumorigenesis
The 3q26.3 overlapped amplicon is a 329-kb region encoding only the gene FNDC3B and exists in three HCC cell lines: Hep3B (ICN = 6.98), PLC/PRF/5 (ICN = 3.62), and Tong (ICN = 3.09; Fig. 2A). The amplification of the FNDC3B gene was confirmed by fluorescent in situ hybridization analysis in Hep3B cells (Supporting Information Fig. 3). We performed quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) on 45 HCC samples at the RNA level and validated the aberrant protein expression of FNDC3B with western blotting or immunohistochemistry (IHC) analysis. Our results indicated that FNDC3B was up-regulated 2-fold in 24.4% of the HCC tumors (11/45) at the RNA level with a high concordance of altered protein expression in tumor tissues (Fig. 2B). To further explore the roles of FNDC3B in HCC, we knocked down FNDC3B expression by shRNAs with validation by western blot analysis (Supporting Information Fig. 4) in amplified HCC cells and performed various in vitro and in vivo tumorigenesis assays. Our results indicated that FNDC3B down-regulated by shRNAs, in comparison with parental and control luciferase (Luc) shRNA–transfected Hep3B and PLC/PRF/5, decreased cell proliferation and colony formation in anchorage-independent growth (Fig. 2C,D). In addition, FNDC3B knocked down by shRNA in Hep3B cells also shrank the tumor volumes in a xenograft nude mouse model (Fig. 2E). Our results indicate that up-regulation of FNDC3B is required for tumor cell proliferation and tumor growth in a subset of HCC tumors.
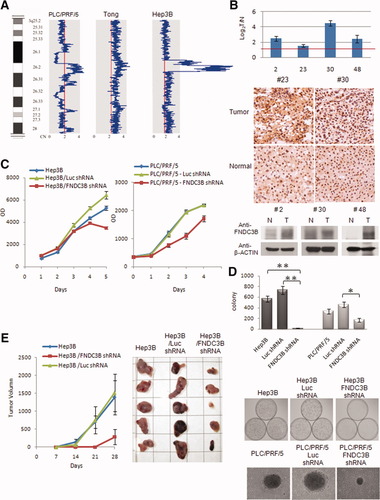
SLC29A2 at the 11q13.2 common amplicon was highly amplified and was essential for HCC tumorigenesis. (A) The 11q13.2 overlapped amplicon was 590 kb and was amplified in Huh7, SNU387, and Hep3B cells. The shadow line across the amplicons indicates the overlapped amplicon, and the red arrow indicates the location of SLC29A2. (B) Concordant expression of SLC29A2 was overexpressed in both RNA and protein levels in representative HCC tumor tissues versus matched adjacent normal tissue according to an analysis of qRT-PCR (top panel), IHC (middle panel), and western blotting (bottom panel). Through the introduction of SLC29A2 or Luc shRNAs into Huh7 and PLC/PRF/5, validated down-regulation of SLC29A2 expression (C) resulted in reduced cell proliferation and (D) decreased colony formation in anchorage-independent growth assays (visualized by photography and crystal violet staining). (E) Knockdown of SLC29A2 expression by shRNA in Huh7 cells suppressed tumor formation in xenograft tumor models in nude mice (n = 5). **P < 0.005 and *P < 0.05 (significant differences). Abbreviation: OD, optical density.
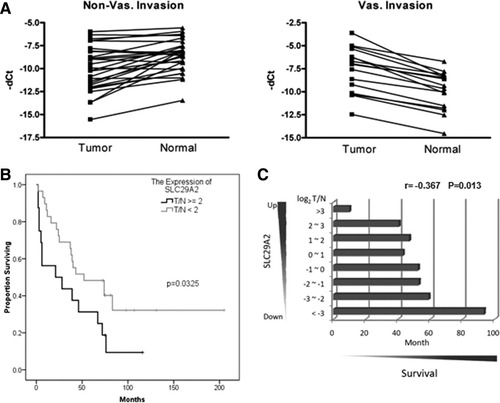
Up-regulation of SLC29A2 was significantly associated with vascular invasion and the survival of patients with HCC. (A) qRT-PCR analysis of SLC29A2 expression in 45 HCC tissues with or without vascular invasion. (B) Kaplan-Meier survival curves for HCC patients showing a statistical association with the tumor expression of SLC29A2 (T/N ≥ 2, P <0.0325). (C) Dosage effects of the aberrant expression of SLC29A2 in HCC tissues versus the survival time of patients.
Integrated Analysis Reveals SLC29A2 as an HCC Cancer Gene at the 11q13.2 Amplicon
Unlike the single gene amplicon at 3q26.3, a 590-kb overlapped amplicon at 11q13.2 was amplified in SNU387 (ICN = 5.34), Huh7 (ICN = 4.28), and Hep3B (ICN = 3.54) and encoded 25 known and predicted genes ( Fig. 3A). We selected SLC29A2 as a candidate HCC cancer gene because it resided at the highest amplification signals in multiple HCC cells. The amplified SLC29A2 gene was confirmed by fluorescent in situ hybridization analysis in Hep3B cells (Supporting Information Fig. 3). In addition, an insilico search of our integrated and open-access HCC database, OncoDB.HCC, also suggested that SLC29A2 was amplified and overexpressed in HCC tumor tissues.14SLC29A2, also known as equilibrative nucleotide transporter protein 2, is essential for the nucleotide synthesis of salvage pathways in cells but is unknown in tumorigenesis. qRT-PCR results indicated more than 2-fold up-regulation of SLC29A2 in 35.6% of HCC tumors (16/45) in comparison with adjacent normal tissues. The up-regulation of SLC29A2 was further confirmed at the protein level by IHC and western blot analysis of HCC tumor pairs (Fig. 3B). To investigate whether up-regulated SLC29A2 is involved in tumorigenesis, knockdown of SLC29A2 with shRNAs, confirmed by western analysis (Supporting Information Fig. 4), was performed in SLC29A2–up-regulated HCC cells with various in vitro and in vivo tumorigenesis assays. Our results showed that down-regulation of altered SLC29A2 in Huh7 and PLC/PRF/5 significantly reduced cell proliferation and colony-forming capability (Fig. 3C,D). The tumor volume of the Huh7-injected xenograft model was suppressed when amplified SLC29A2 was knocked down by shRNA (Fig. 3E). We have concluded that up-regulation of SLC29A2 plays a pivotal role in the growth and formation of a subset of HCC tumors.
Up-Regulation of SLC29A2 Is Associated with Advanced Stages, Vascular Invasion, and Poor Survival of HCC Patients
Our results failed to show a significant correlation of up-regulated FNDC3B with clinicopathological features of 45 HCC samples (Supporting Information Table 3). In contrast, 35.6% of HCC patients (16/45) with up-regulated SLC29A2 tended to have advanced stages (P = 0.0031), vascular invasion ( Fig. 4A; P = 0.0353), metastasis (P = 0.0499), and poor survival (Fig. 4B; P = 0.0325). Interestingly, a dosage effect was observed, with higher SLC29A2 up-regulation correlating with shorter patient survival in our 45 HCC samples (Fig. 4C; r = −0.367, P = 0.013). Our results indicate that up-regulation of SLC29A2 could potentially be an important predictive biomarker for vascular invasion and poor outcome for patients with HCC.
Overexpressed FNDC3B or SLC29A2 in Huh6 Promotes Cell Proliferation Through Activation of the Signal Transducer and Activator of Transcription 3 (STAT3) Signaling Pathway
To investigate the role of amplification of FNDC3B and SLC29A2 in HCC tumorigenesis, we overexpressed FNDC3B or SLC29A2 in unamplified HCC cells (Huh6) and examined the potential activation of the downstream signaling pathway. Our results demonstrated that overexpressed FNDC3B and SLC29A2 increased cell proliferation (Supporting Information Fig. 5A) and were validated by up-regulation of Ki-67 protein expression (Supporting Information Fig. 5B). To further dissect the activation of downstream signaling pathways involved in augmenting cell proliferation, we examined the expression and activation of v-akt murine thymoma viral oncogene homolog 1 (AKT) and STAT3. Our results showed that activation of the STAT3 pathway through increased Tyr705 phosphorylation was detected in FNDC3B- and SLC29A2-overexpressed Huh6 cells, but phosphorylation of Ser427 of AKT was not (Supporting Information Fig. 5C). The relatively activated STAT3 signaling pathway was also detected in FNDC3B- and SLC29A2-amplified Hep3B but not in unamplified Huh6 (Supporting Information Fig. 5D). Together, our results suggest that amplification of FNDC3B or SLC29A2 could lead to activation of the downstream STAT3 signaling pathway, confer selective growing advantages, and promote tumorigenesis of HCC.
Discussion
In this study, we developed a comprehensive approach to analyzing genome-wide CNAs of cancer genomes with high-density SNP arrays and to searching for cancer genes by identification of overlapped amplicons and HDs in multiple cancer cell lines. First, we established the CNA analysis protocol and criteria without the need for precious genomic DNAs isolated from tumor-adjacent normal tissues. Our results suggest that a reference pool of high-density SNP arrays requires at least 10 reference samples from healthy individuals to minimize signal variations between the SNP arrays (Supporting Information Fig. 6). The number of reference samples required for CNA analysis was based on the minimal number of reference samples required to obtain the minimal variation of standard deviations of SNP intensities and to stabilize the number of aberrant SNPs from tested cancer genome arrays. Because our main purpose was searching for target genes in cancer genomes with the exclusion of false-positive signals, we established highly stringent criteria for defining amplicons (at least 10 continuous SNPs with an ICN ≥ 4) and HDs (at least 10 continuous SNPs with an ICN ≦ 0.4). The defined threshold for amplicons (ICN ≥ 4) was intended to avoid the inclusion of common trisomic regions, and the defined threshold for HDs (ICN ≦ 0.4) was aimed at the direct exclusion of loss-of-heterozygosity regions in cancer genomes. A total of 653 amplicons and 57 HDs were identified, and this allowed us to align CNAs to further narrow the target genes from common aberrant regions in multiple cancer cell lines.
Furthermore, our highly stringent criteria should not influence the accuracy of determining the total number of amplicons because amplification could be legitimately covered by high-density SNP probes in SNP arrays. On the contrary, it might potentially lead to the miscalling of smaller HD regions in less than 10 continuous SNPs with an ICN ≦ 0.4. The underestimated HDs could be detected if we lower the number of continuous SNPs during data processing, but this raises the risk of inclusion of false-positive results, which then will require additional experimental validations. Nevertheless, using our highly stringent criteria and protocol, we have already discovered numerous known and novel amplicons and HDs, and they provide at least three advantages: (1) the detection of more precise aberrant boundaries and targeted genes in comparison with previous reports, (2) the reduction of the cost of genotyping references and precious adjacent normal samples, and (3) the allowance of high-throughput and insilico CNA analyses of cancer genomes downloaded from public domains.
FNDC3B, covering a large area (360 kb), is the only gene annotated in the common 3q26.3 amplicon. Although its biological functions remain largely unclear, FNDC3B with nine fibronectin domain III repeats has been rationally speculated to play a role in the regulation of cell interaction and adhesion in development and cancers. An alternative name, factor for adipocyte differentiation 104, indicates its potential role as a positive regulator of adipogenesis.15, 16 FNDC3B is also called nonstructural protein 5A–binding protein 37 because of its interaction with hepatitis C virus nonstructural protein 5A, which is essential for viral RNA replication, and it may play a role in subverting host intracellular signaling pathways.17 Interestingly, we reanalyzed two sets of gene expression data in the public domain and found that FNDC3B was up-regulated 2-fold in 40.3% of HCC samples (52/129) with unknown hepatitis virus infections and in 13.2% of hepatitis C virus–positive HCC samples (12/91).18 As for hepatitis B virus–positive HCC, two independent HCC data sets from Taiwan and Hong Kong illustrated 2-fold up-regulation of FNDC3B in 24.4% of cases (11/45) and in 25% of cases (14/56), respectively, versus tumor-adjacent normal tissue. On the other hand, a recent study showed hepatitis B viral X protein–mediated nuclear factor kappa B transcription and up-regulated miR-143 expression in an HCC subset with hepatitis B virus positivity and tumor metastasis. Up-regulated miR-143 could target FNDC3B, suppress its expression, and correlate with the enhancement of tumor metastasis.19 These results demonstrate that the modulation of FNDC3B expression in different stages or virus-related HCCs might play distinct but pivotal roles in tumorigenesis. In addition to HCC, a recent integrated microarray study searching for common up-regulated and down-regulated genes in 20 different cancer types indicated that FNDC3B is one of the common up-regulated genes in nearly all cancerous tissues, regardless of their tumor origins.20 Because the expression of FNDC3B was observed in both nucleus (case 30) and cytoplasmic (case 23) compartments by IHC analysis, we further examined endogenous subcellular localization of FNDC3B in HCC cells. Western blot analysis illustrated that two FNDC3B isoforms with estimated molecular weights of 140 and 110 kDa were detected. The 140-kDa FNDC3B isoform was detected mainly in the nucleus compartment, and the 110-kDa isoform was found in both the nucleus and cytoplasmic compartments (Supporting Information Fig. 7). Although isoform-specific alterations of FNDC3B in HCC remain to be determined, we speculate that both isoforms are up-regulated by amplification and potentially play a role in the tumorigenesis of HCC and other cancers.
The significant association of up-regulated SLC29A2 with the vascular invasion of HCC tumors prompted us to study its role in tumorigenesis and as a prognostic biomarker for HCC. HCC is known to be a hypervascular tumor, and vascular invasion, independent of tumor size, is the most consistent predictor of tumor recurrence and poor outcomes for HCC patients.21 Interestingly, a recent study using expression profiling also revealed aberrant up-regulation of SLC29A2 (one of five genes) to be an optimized survival predictor for mantle cell lymphoma.22 SLC29A2 is a member of the equilibrative nucleoside transporter family, which facilitates the transport and uptake of a broad range of purine and pyrimidine nucleosides and their analogues for salvage pathways of nucleotide synthesis and for anticancer and antiviral therapy.23, 24 There are four SLC29 isoforms in humans, including SLC29A1, SLC29A2, SLC29A3, and SLC29A4, but only SLC29A2 was amplified in our 23 cancer cell lines. Instead of abundant expression of SLC29A1 in normal human livers,25 SLC29A2 was aberrantly up-regulated in a subset of HCC samples. A better understanding of the molecular mechanism by which aberrant SLC29A2 expression leads to HCC progression and affects its prognosis requires further studies. We speculate that aberrant up-regulation of SLC29A2 may alter cellular nucleotide synthesis and nucleotide pool balance and thus cause cancer genome instability and subsequently provide growth advantages to tumors.26 Because SLC29A2 is a known adenosine transporter and adenosine released from hypoxic tissue stimulates the release of vascular endothelial growth factor, which then binds to its receptor on endothelial cells to stimulate endothelial proliferation, migration, and tumor angiogenesis,27 we theorize that aberrant expression of SLC29A2 in HCCs may facilitate HCC tumor angiogenesis and result in poor patient outcome.
Our discovery that up-regulated FNDC3B or SLC29A2 in HCC cells increases Tyr705 phosphorylation of STAT3 to activate the STAT3 signaling pathway is a novel finding. STAT3 activation is commonly observed in HCC progression and is known to be triggered by cytokines such as interleukin-6 through Janus kinases, by activated tyrosine kinase receptors such as EGFR, or by nonreceptor tyrosine kinases such as SRC.28 Future experiments designed to identify pathways mediated by the amplification of two genes and increasing the phosphorylation of STAT3 could lead to the development of new HCC therapeutics.
In conclusion, we have systematically developed a highly applicable protocol and criteria with commercially accessible high-density SNP arrays and freely available analysis software to search for target genes in cancer genomes via the scanning of common amplicons and HDs in multiple cancer cells. Our current protocol for CNA analysis, featuring the use of healthy individuals as normal reference controls (either self-prepared or downloaded), allows researchers to rapidly analyze on a large scale cancer genomes from public domains, regardless of the platform formats. Using stringent criteria and a validation process, our approach may facilitate the discovery of common and novel cancer genes and thus result in a better understanding of the mechanisms of tumorigenesis and in diagnostic and predictive biomarkers and therapies for cancer.
Acknowledgements
The authors thank Dr. Pei-Jer Chen and Dr. Ding-Shinn Chen (School of Medicine, National Taiwan University) for their advice on this work. The authors also acknowledge the core facilities of the National Research Program for Genomic Medicine of the National Science Council of Taiwan, including the National Genotyping Center for its help with SNP genotyping and the National RNAi Core Facility for its provision of shRNAs.




