Hepatic iron loading in mice increases cholesterol biosynthesis†
Potential conflict of interest: Nothing to report.
Abstract
Iron and cholesterol are both essential metabolites in mammalian systems, and too much or too little of either can have serious clinical consequences. In addition, both have been associated with steatosis and its progression, contributing, inter alia, to an increase in hepatic oxidative stress. The interaction between iron and cholesterol is unclear, with no consistent evidence emerging with respect to changes in plasma cholesterol on the basis of iron status. We sought to clarify the role of iron in lipid metabolism by studying the effects of iron status on hepatic cholesterol synthesis in mice with differing iron status. Transcripts of seven enzymes in the cholesterol biosynthesis pathway were significantly up-regulated with increasing hepatic iron (R2 between 0.602 and 0.164), including those of the rate-limiting enzyme, 3-hydroxy-3-methylglutarate-coenzyme A reductase (Hmgcr; R2 = 0.362, P < 0.002). Hepatic cholesterol content correlated positively with hepatic iron (R2 = 0.255, P < 0.007). There was no significant relationship between plasma cholesterol and either hepatic cholesterol or iron (R2 = 0.101 and 0.014, respectively). Hepatic iron did not correlate with a number of known regulators of cholesterol synthesis, including sterol-regulatory element binding factor 2 (Srebf2; R2 = 0.015), suggesting that the increases seen in the cholesterol biosynthesis pathway are independent of Srebf2. Transcripts of genes involved in bile acid synthesis, transport, or regulation did not increase with increasing hepatic iron. Conclusion: This study suggests that hepatic iron loading increases liver cholesterol synthesis and provides a new and potentially important additional mechanism by which iron could contribute to the development of fatty liver disease or lipotoxicity. (HEPATOLOGY 2010;)
Iron is an essential trace element and an important structural or functional component of many physiological systems. The liver is the major storage site for iron and is important in the regulation of iron metabolism, being the site of production of the hormone hepcidin, which regulates iron absorption.1 Iron metabolism is tightly regulated; nevertheless, iron deficiency and iron overload can occur and may have serious clinical consequences. The most common disorder associated with iron depletion is iron deficiency anemia, which affects more than 30% of the world's population.2 At the other end of the spectrum, iron overload can occur in subjects with hereditary hemochromatosis, which is caused by mutations in one of several genes, or secondary to iron administration.3 A range of biochemical disturbances may result from dysregulated iron metabolism; these include metabolic disorders affecting glucose and insulin, leading to diabetes,4 and to nonalcoholic fatty liver disease (NAFLD).5
Like iron, cholesterol is essential in normal physiological systems. It is required in cell membranes to maintain cellular integrity and for the formation of bile acids which aid in fat digestion. It is also a precursor of steroid hormones and vitamin D.6, 7 Also like iron, excesses and deficiencies of cholesterol can result in pathophysiological sequelae, including atherosclerosis and NAFLD, skeletal abnormalities, and mental health disorders.8-10
NAFLD is a collective term for chronic liver disorders which can range from fatty deposits in hepatocytes to nonalcoholic steatohepatitis (NASH) and which can progress to cirrhosis and hepatocellular carcinoma.11 It has been proposed that progression of NAFLD from steatosis to steatohepatitis occurs via a number of steps that result from the actions of additional factors upon the steatotic liver, a model known as the “two-hit hypothesis”.12 One of the factors identified as contributing the second hit is the presence of reactive oxygen species that cause oxidative stress.13 Iron is known to catalyze the production of reactive oxygen species which can then initiate cellular damage, including lipid peroxidation,14 and an increase in iron has been shown to increase the oxidation of cholesterol, particularly when the liver is already under conditions of oxidative stress.15 This is supported by a recent study which reported that hepatocyte iron loading was associated with liver fibrosis in patients with NAFLD.16 Thus, excess hepatic iron has been hypothesized to be a cofactor in the progression of steatosis to NASH and, indeed, several studies have reported an association between parameters of iron loading and NASH.17-19
Previous studies investigating the interaction between iron and cholesterol have focused on the plasma and present conflicting information. Administration of a high iron diet to animals has been found to result in an increase in plasma cholesterol in some studies but not in others,20, 21 and intraperitoneal administration of iron has been shown to lower plasma cholesterol.22 In humans homozygous for the Cys282Tyr (C282Y) mutation in HFE, which causes hemochromatosis, plasma low-density lipoprotein (LDL) cholesterol has been found to be reduced.23 In contrast, plasma total and LDL cholesterol were found to be reduced in anemic women.24
Further clarification of the potential role of iron in disordered lipid metabolism is required. To examine this, we studied the effects of iron status on hepatic cholesterol synthesis in mice with iron burdens ranging from deficient to overloaded. We show that increasing iron burden in mice results in an increase in the transcripts of approximately half of the enzymes of the cholesterol biosynthetic pathway, resulting in an increase in hepatic total cholesterol. These results provide a new and potentially important additional mechanism by which iron could contribute to the development of NAFLD or lipotoxicity.
Abbreviations:
Abc, adenosine triphosphate-binding cassette; Apo, apolipoprotein; Bhmt2, betaine-homocysteine methyltransferase 2; C/EBPα, CCAAT/enhancer binding protein α; Cyp51, lanosterol-14α demethylase; Cyp27b1, 25-hydroxyvitamin D3-1α-hydroxylase; Cyp7a1, cholesterol 7α-monooxygenase; Ebp, cholestenol-Δ-isomerase; Ggcx, gamma-glutamyl carboxylase; Ggps1, geranylgeranyl diphosphate synthase 1; GSEA, gene set enrichment analysis; Hmgcr, 3-hydroxy-3-methylglutarate-coenzymeA reductase; Hnf4a, hepatocyte nuclear factor 4α; Hsd17b7, 3-keto-steroid reductase; Hsd3b7, hydroxy-Δ5-steroid dehydrogenase; Idi1, isopentenyl-diphosphate-Δ-isomerase; mRNA, messenger RNA; Mvk, mevalonate kinase; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; Nqo1, NAD(P)H dehydrogenase (quinone) 1; Nr1h3, nuclear receptor 1H3; Nsdhl, sterol-4α-carboxylate 3-dehydrogenase; Pmvk, phosphomevalonate kinase; Psap, prosaponin; RT-PCR, real-time polymerase chain reaction; Sc5d, lathosterol oxidase; Srebf2, sterol-regulatory element binding factor 2; Tm7sf2, Δ14-sterol reductase; Tmem97, transmembrane protein 97; Vkorc1, vitamin K epoxide reductase complex (subunit 1); VLDL, very low density lipoprotein; Vrk3, vaccinia-related kinase 3.
Materials and Methods
Animals.
Male AKR mice (Animal Resources Centre, Murdoch, Australia) were fed a diet of normal mouse chow containing 0.01% iron (normal iron diet; Specialty Feeds, Glen Forrest, Australia) ad libitum. A second group of mice were fed a diet supplemented with 2% carbonyl iron (Sigma, Sydney, Australia; iron-loaded) for 3 weeks, and a third group were fed a diet containing no added iron (0.001% iron; iron-deficient) for 7 weeks from 3 weeks of age. Mice were sacrificed at 10 weeks of age following an overnight fast. Organs were perfused with isotonic saline in situ; livers were harvested and snap-frozen in liquid nitrogen. All procedures were approved by the Animal Ethics Committee of the University of Western Australia.
Microarray Experiments.
Total RNA was extracted from the livers of 12 mice (four from each group) using Tri-Reagent (Invitrogen, Sydney, Australia) and treated with deoxyribonuclease I (Ambion, Austin, TX). RNA used for microarray analysis was further purified using an RNeasy kit (Qiagen, Sydney, Australia). RNA purity was confirmed (Agilent 2100 Bioanalyzer) and subjected to microarray analysis using Illumina Sentrix Mouse-6 (version 1.1) Beadarray chips at the Institute for Molecular Bioscience Microarray Facility (Brisbane, Australia).
Real-Time Polymerase Chain Reaction.
Hepatic gene expression was measured in mice independent of those used for the microarray experiments. RNA treated with deoxyribonuclease I was reverse transcribed using Superscript III reverse transcriptase (Invitrogen) and gene expression was measured by real-time polymerase chain reaction (RT-PCR) using a Rotor-Gene (Qiagen, Australia) PCR cycler. Aliquots of complementary DNA from each sample were pooled and used to generate standard curves by serial dilution. Reactions were prepared using FastStart SYBR Green master mix (Roche Applied Science, Sydney, Australia). Transcripts were quantified by the method of Pfaffl25 using the mean of the iron-deficient samples as the calibrator and β-actin as the reference gene. Primer sequences are listed in Supporting Table 1.
Liver Iron and Cholesterol.
Liver nonheme iron was measured by the method of Kaldor26 and plasma iron by the method of Fielding.27 Liver cholesterol was extracted using chloroform:methanol (2:1) as described.28 Following evaporation to dryness under nitrogen, lipids were resuspended in isopropanol. Liver and plasma total cholesterol were measured enzymatically using a Cobas Mini Random Analyzer (Roche Products Pty., Ltd., Basel, Switzerland).
Statistical Procedures.
Microarray data were initially examined using Illumina BeadStudio version 3.0 (Illumina, San Diego, CA) and were exported to Lumi version 1.1 for Bioconductor 2.4.29, 30 Background correction, variance stabilization, normalization, and quality control were performed as described in the standard methods for the Lumi package. Isotonic regression can provide a better fit test for determining trends across experimental groups compared with traditional linear regression techniques. A modified version of the isotonic regression function from the statistical package R, version 2.9,31 was used to determine whether an increasing or decreasing trend in gene expression level for each probeset was present across the iron-deficient, normal, and iron-loaded groups.32, 33 Gene expression data were compared with hepatic nonheme iron using linear regression analysis on log-transformed data, and pairwise comparisons were performed using the Student unpaired, two-tailed t test. Statistical significance was taken at the nominal 5% level. Other statistical tests and data management were conducted using R.
Gene set enrichment analysis (GSEA) is an analytical method used to identify differences between groups of genes with related biological function.34 We ranked the isotonic regressions by their R2 correlations and used these as input for a preranked GSEA analysis in the GSEA version 2.0 software package.34 Enrichment analysis was performed using the GSEA C2 Molecular Signatures Database, which contains more than 1000 pathways and ontologies curated from online pathway databases and available biomedical literature (www.broadinstitute.org/gsea/msigdb/collection_details. jsp#C2). Input data were collapsed to gene symbols, and the geneset permutation option was used to conduct 1000 gene permutations to determine statistical significance. To minimize false positive findings, we examined resulting genesets with a combination of false discovery rate q value and family-wise error rates below 5%.
Results
Parameters of Iron Metabolism.
Iron status of mice was determined by measurement of liver nonheme iron and the pattern of expression of iron-related genes (Fig. 1). Livers of mice fed a normal diet contained 5.5 ± 0.5 μmol Fe/g wet weight liver, whereas those of mice fed iron-deficient or iron-loaded diets contained 2.4 ± 0.2 and 19 ± 1 μmol Fe/g wet weight liver, respectively (Fig. 1A). Plasma iron concentrations were 30 ± 1 μM in iron-deficient mice, 42 ± 1 μM in normal mice, and 46 ± 1 μM in iron-loaded mice (Fig. 1B). Transcript levels of hepcidin-1 were significantly lower than normal in livers of iron-deficient animals and significantly higher than normal in livers of iron-loaded animals. Transferrin receptor 1 messenger RNA (mRNA) was significantly higher in iron-deficient livers than in normal livers, whereas there were no significant differences in liver transferrin receptor 2 or Hfe mRNA (Fig. 1C-F).
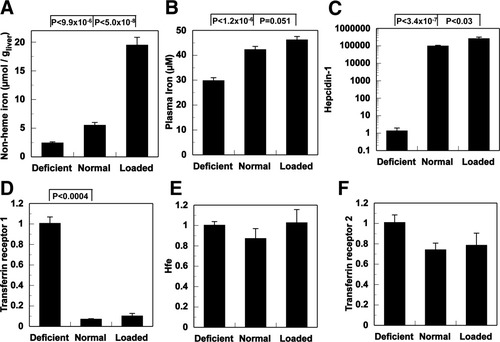
Parameters of iron metabolism. (A) Hepatic and (B) plasma nonheme iron concentrations in mice fed an iron-deficient diet (n = 8), an iron-loaded diet (n = 14), or a normal iron diet (n = 18). Hepatic gene expression of (C) hepcidin-1, (D) transferrin receptor 1, (E) Hfe, and (F) transferrin receptor 2 in iron-deficient (n = 4), normal (n = 14), or iron-loaded (n = 10) mice. Nonheme iron concentrations and gene expression by RT-PCR were determined as described in Materials and Methods. All data are expressed as mean ± SE.
Geneset Enrichment Analysis of Microarray Data.
Gene set enrichment analysis of the ranked isotonic regression values returned the KEGG pathway “HSA00100 Biosynthesis of Steroids” as the most significantly enriched pathway for our data set, with a false discovery rate q value of <10−5 and family-wise error rate P value of 0.001. Of the genes listed in the GSEA geneset, 19 are involved in cholesterol biosynthesis and 16 of these reached GSEA core enrichment status in our data set, suggesting that cholesterol synthesis is significantly increased in response to changes in iron status. The remainder of the genes in this geneset are involved in vitamin metabolism and were not considered further in this study. The mean log2 fold change ratios from the microarray results are presented for the normal and iron-loaded groups relative to the iron-deficient group (Table 1).
| Gene | GSEA Enriched | Microarray | RT-PCR Versus Nonheme Iron | |||
|---|---|---|---|---|---|---|
| Fold Change Normal/Deficient | Fold Change Loaded/Deficient | Isotonic R2 | R2 | P | ||
| Pmvk | Yes | 1.57 | 2.14 | 1.000 | 0.271 | 0.005 |
| Hmgcr | Yes | 1.37 | 1.79 | 0.999 | 0.326 | 0.002 |
| Nsdhl | Yes | 1.18 | 1.70 | 0.993 | 0.199 | 0.02 |
| Ebp | Yes | 1.21 | 1.39 | 0.988 | 0.602 | 2 × 10–6 |
| Nqo1 | Yes | 1.05 | 1.20 | 0.974 | N/A | N/A |
| Dhcr24 | Yes | 2.00 | 2.27 | 0.965 | 0.088 | 0.12 |
| Lss | Yes | 1.12 | 1.34 | 0.965 | 0.086 | 0.14 |
| Fdps | Yes | 2.56 | 3.38 | 0.958 | 0.136 | 0.06 |
| Sc4mol | Yes | 1.88 | 2.15 | 0.944 | 0.006 | 0.72 |
| Mvd | Yes | 1.14 | 1.26 | 0.927 | 0.033 | 0.37 |
| Sc5d | Yes | 1.04 | 1.05 | 0.894 | 0.520 | 2 × 10–5 |
| Dhcr7 | Yes | 1.83 | 1.96 | 0.888 | 0.090 | 0.13 |
| Hsd17b7 | Yes | 1.04 | 1.02 | (0.871) | (0.241) | 0.008 |
| Ggcx | Yes | 1.14 | 1.28 | 0.855 | N/A | N/A |
| Idi1 | Yes | 1.38 | 1.57 | 0.851 | N/A | N/A |
| Sqle | Yes | 1.29 | 1.80 | 0.828 | 0.027 | 0.40 |
| Cyp51 | Yes | 1.52 | 1.76 | 0.812 | 0.178 | 0.03 |
| Tm7sf2 | Yes | 1.10 | 1.62 | 0.800 | 0.164 | 0.04 |
| Mvk | No | 1.06 | 1.06 | 0.703 | N/A | N/A |
| Vkorc1 | No | 1.08 | 0.92 | (0.638) | N/A | N/A |
| Cyp27b1 | No | 0.99 | 1.00 | 0.632 | N/A | N/A |
| Ggps1 | No | 0.98 | 1.00 | 0.577 | N/A | N/A |
| Fdft1 | No | 0.99 | 0.98 | (0.432) | 0.041 | 0.30 |
- Shown is the GSEA geneset for the KEGG pathway “HSA00100 Biosynthesis of Steroids”, ranked by isotonic regression values. P values for genes shown by RT-PCR to be describing a significant relationship with nonheme iron are shown in bold type. Genes not investigated by RT-PCR are italicized. Values of R2 for which the correlation coefficient was negative are shown in parentheses. Cyp27b1, 25-hydroxyvitamin D3-1α-hydroxylase; Ggcx, gamma-glutamyl carboxylase; Ggps1, geranylgeranyl diphosphate synthase 1; Idi1, isopentenyl-diphosphate-Δ-isomerase; Mvk, mevalonate kinase; N/A, not applicable; Nqo1, NAD(P)H dehydrogenase (quinone) 1; Vkorc1, vitamin K epoxide reductase complex (subunit 1). Other abbreviations are listed in the legend to Figure 2.
Cholesterol Biosynthesis.
RT-PCR was conducted to confirm the changes in gene expression observed in the microarray data along with changes in other genes involved in hepatic cholesterol pathways. The pathways examined are shown schematically in Fig. 2. The transcripts of 3-hydroxy-3-methylglutarate-CoA reductase (Hmgcr), phosphomevalonate kinase (Pmvk), lanosterol-14α demethylase (Cyp51), Δ14-sterol reductase (Tm7sf2), sterol-4α-carboxylate-3-dehydrogenase (Nsdhl), cholestenol-Δ-isomerase (Ebp), and lathosterol oxidase (Sc5d) exhibited significant positive relationships with liver nonheme iron (Fig. 3 and Table 1). Interestingly, 3-keto-steroid reductase (Hsd17b7) exhibited a significant negative relationship with liver iron (Table 1).
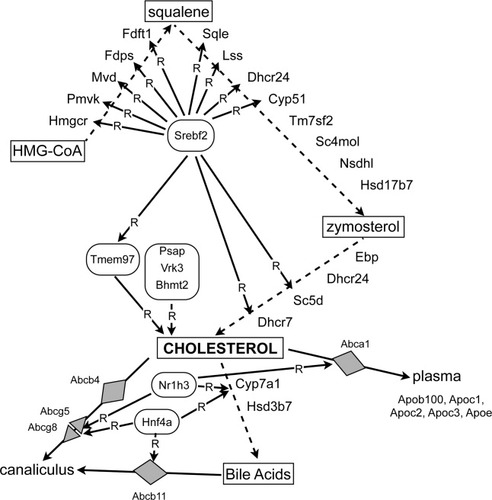
Pathways involved in hepatic cholesterol synthesis. The illustration shows the enzymes and other proteins, whose mRNA expression was measured in the present study, within the context of the major intermediates in the cholesterol biosynthesis pathway. Biochemical intermediates are boxed, transporters are shaded diamonds, and regulators are surrounded by a rounded rectangle. “R” indicates a regulatory interaction. Abc, adenosine triphosphate-binding cassette; Apo, apolipoprotein; Bhmt2, betaine-homocysteine methyltransferase 2; Cyp51, lanosterol-14α demethylase; Cyp7a1, cholesterol-7α monooxygenase; Dhcr24, Δ24-sterol reductase; Dhcr7, 7-dehydrocholesterol reductase; Ebp, cholestenol-Δ isomerase; Fdft1, farnesyl-diphosphate farnesyltransferase; Fdps, dimethylallyltransferase; Hmgcr, 3-hydroxy-3-methylglutarate-coenzymeA reductase; Hnf4a, hepatocyte nuclear factor 4α; Hsd17b7, 3-keto-steroid reductase; Hsd3b7, hydroxy-Δ5-steroid dehydrogenase; Lss, lanosterol synthase; Mvd, diphosphomevalonate decarboxylase; Nr1h3, nuclear receptor 1H3; Nsdhl, sterol-4α-carboxylate-3-dehydrogenase; Pmvk, phosphomevalonate kinase; Psap, prosaponin; Sc4mol, methylsterol monooxygenase; Sc5d, lathosterol oxidase; Sqle, squalene monooxygenase; Srebf2, sterol-regulatory element binding factor 2; Tm7sf2, Δ14-sterol reductase; Tmem97, transmembrane protein 97; Vrk3, vaccinia-related kinase 3.
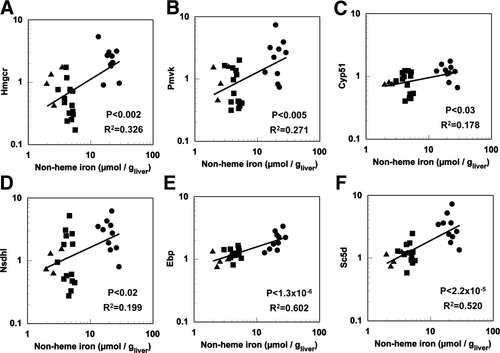
Genes of the cholesterol biosynthesis pathway. Relationship between hepatic nonheme iron and hepatic gene expression of (A) Hmgcr, (B) Pmvk, (C) Cyp51, (D) Nsdhl, (E) Ebp, or (F) Sc5d. Nonheme iron concentrations and gene expression by RT-PCR were determined as described in Materials and Methods (n = 26-28) in iron-deficient (▴), normal (▪), and iron-loaded (•) mice. Abbreviations are listed in the legend to Fig. 2.
Importantly, liver cholesterol also correlated positively and significantly with liver nonheme iron (R2 = 0.255, P < 0.007; Fig. 4A), suggesting that changes in the transcript levels of cholesterol biosynthesis enzymes resulted in an increase in liver cholesterol production. Interestingly, plasma cholesterol levels did not correlate with either iron status or liver cholesterol levels (R2 = 0.014 and 0.101, respectively; Fig. 4B,C). We did not see any inflammatory or fatty changes in liver histology in the mouse models used (RDD, ACGC, RMG, JKO, DT, unpublished observations).
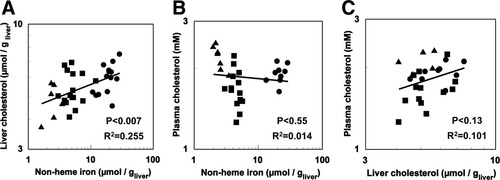
Cholesterol concentrations. Relationship between hepatic nonheme iron and (A) hepatic total cholesterol (n = 34) and (B) plasma total cholesterol (n = 30). (C) Relationship between hepatic total cholesterol and plasma total cholesterol (n = 25). Cholesterol and iron were measured as described in Materials and Methods in iron-deficient (▴), normal (▪), and iron-loaded (•) mice.
Regulation of Cholesterol Biosynthesis.
To investigate the possible mechanisms of regulation involved in increased production of hepatic cholesterol, we measured the expression of sterol-regulatory element binding factor 2 (Srebf2), a regulator of many genes in the cholesterol biosynthesis pathway.35 In addition, we measured the expression of four other genes recently identified as modifying cellular cholesterol levels: transmembrane protein 97 (Tmem97), betaine-homocysteine methyltransferase 2 (Bhmt2), vaccinia-related kinase 3 (Vrk3), and prosaponin (Psap).36 As shown in Fig. 5 and Table 2, neither Srebf2 nor Tmem97, a target of Srebf2, exhibited significant relationships with liver nonheme iron. The same was true of Psap, knockdown of which has been shown to increase cellular cholesterol, as well as Vrk3 and Bhmt2, knockdown of which have been shown to decrease cellular cholesterol.36
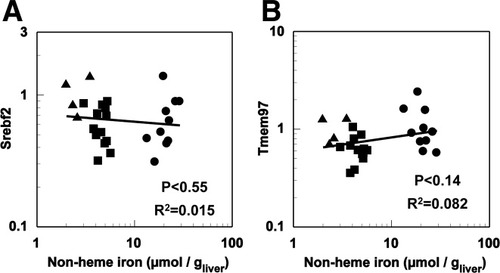
Potential regulators of cholesterol biosynthesis. Relationship between hepatic nonheme iron and hepatic gene expression of (A) Srebf2 and (B) Tmem97. Nonheme iron concentrations and gene expression by RT-PCR were determined as described in Materials and Methods (n = 28) in iron-deficient (▴), normal (▪), and iron-loaded (•) mice. Abbreviations are listed in the legend to Fig. 2.
| Gene | R2 | P Value |
|---|---|---|
| Regulation of cholesterol biosynthesis | ||
| Srebf2 | (0.0154) | 0.53 |
| Tmem97 | 0.0819 | 0.14 |
| Bhmt2 | (0.1283) | 0.07 |
| Psap | (0.0756) | 0.16 |
| Vrk3 | 0.0331 | 0.35 |
| Bile acid synthetic enzymes, regulators and transporters | ||
| Cyp7a1 | (0.0946) | 0.11 |
| Hsd3b7 | (0.5937) | 2 × 10–6 |
| Hnf4a | 0.0470 | 0.27 |
| Nr1h3 | 0.0058 | 0.70 |
| Abcb11 | 0.0021 | 0.82 |
| Cholesterol transport genes | ||
| Abca1 | 0.2023 | 0.02 |
| Abcb4 | (0.2240) | 0.02 |
| Abcg5 | 0.2093 | 0.02 |
| Abcg8 | (0.0068) | 0.68 |
| Apob100 | (0.0280) | 0.39 |
| Apoc1 | 0.0318 | 0.36 |
| Apoc2 | (0.0002) | 0.94 |
| Apoc3 | 0.2430 | 0.009 |
| Apoe | 0.0518 | 0.25 |
- Values of R2 for which the correlation coefficient was negative are shown in parentheses. P values for genes shown by RT-PCR to be describing a significant relationship with nonheme iron are shown in bold type (n = 27-28). Abbreviations are listed in the legend to Figure 2.
Fate of Cholesterol.
Bile acid synthesis represents the major metabolic route for hepatic cholesterol.6, 7 To determine whether the bile acid synthesis pathway was up-regulated in response to increasing iron burden, we measured the gene expression of the rate-limiting enzyme in bile acid synthesis, cholesterol-7α-monooxygenase (Cyp7a1), as well as that of hydroxy-Δ5-steroid dehydrogenase (Hsd3b7), another early enzyme in the bile acid synthesis pathway. In addition, we measured hepatocyte nuclear factor 4α (Hnf4a) and nuclear receptor 1H3 (liver X receptor α; Nr1h3), two regulators of bile acid synthesis and adenosine triphosphate-binding cassette B11 (bile salt export protein; Abcb11), a bile acid transporter.37-39 No significant correlations were observed between liver nonheme iron and Cyp7a1, Hnf4a, Nr1h3, or Abcb11 transcripts, whereas Hsd3b7 mRNA exhibited a significant negative relationship (Fig. 6 and Table 2).
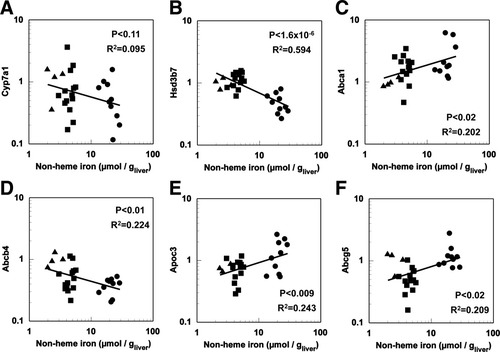
Genes involved in bile acid synthesis and cholesterol export. Relationship between hepatic nonheme iron and hepatic gene expression of (A) Cyp7a1, (B) Hsd3b7, (C) Abca1, (D) Abcb4, (E) Apoc3 and (F) Abcg5. Nonheme iron concentrations and gene expression by RT-PCR were determined as described in Materials and Methods (n = 27-28) in iron-deficient (▴), normal (▪), and iron-loaded (•) mice. Abbreviations are listed in the legend to Fig. 2.
Cholesterol may also be exported to the plasma or into the canaliculus. Abca1 is an exporter which transports cholesterol to the plasma, whereas Abcb4, Abcg5, and Abcg8 are involved in cholesterol export to the canaliculus.40 The apolipoproteins (Apo) B100, C1, C2, C3, and E are all constituents of very low-density lipoproteins (VLDLs), one of the forms in which cholesterol is exported from hepatocytes and the major lipoprotein exported from the liver.41 Transcripts of Abca1 and Abcb4 correlated significantly with liver nonheme iron; however, the former correlation was positive and the latter negative. Of the apolipoprotein transcripts, only Apoc3 exhibited a significant correlation with liver nonheme iron. Liver nonheme iron exhibited a significant positive relationship with Abcg5 whereas that with Abcg8 was not significant (Fig. 6C-F and Table 2).
Discussion
Iron is an essential trace element which plays a role in many physiological systems. Perhaps not surprisingly, it has also been linked to changes in many metabolic processes, including disorders of lipid metabolism.5, 42 Here, we present data indicating a link between hepatic iron status and the production of cholesterol by the liver.
Hepatic iron stores were two-fold lower than normal in iron-deficient mice and eight-fold higher than normal in iron-loaded mice. This was reflected in the transcript levels of the iron hormone hepcidin-1, which were up-regulated in the presence of increasing iron. Conversely, transferrin receptor 1 transcript, which contains several iron-responsive elements in its 3′ untranslated region,43 was substantially up-regulated in iron deficiency. Hfe and transferrin receptor 2, neither of which are regulated by iron at the transcriptional level,44, 45 exhibited no regulation by hepatic iron levels.
Cholesterol is an important molecule in homeostasis. It is a component of lipid membranes and can be further metabolized either within the liver or extrahepatically. Like iron, excess cholesterol can be toxic, being deposited in arteries to form atherosclerotic plaques,8 or in the liver, where it may contribute to NALFD. The present study suggests a role for iron in cholesterol synthesis: increasing hepatic iron was positively associated with increasing hepatic cholesterol, and significant positive correlations of liver iron with transcript levels of enzymes involved in cholesterol biosynthesis were seen. Seven enzyme transcripts exhibited significant positive relationships with hepatic iron levels, including Hmgcr, which codes for the rate-limiting enzyme. Nine did not exhibit significant associations with hepatic iron and one, Hsd17b7, exhibited a significant negative correlation. It is unclear why transcript levels of Hsd17b7 decreased with increasing iron; however, the decrease did not appear to affect cholesterol production, because this increased with increasing hepatic iron.
Bile acid synthesis represents the major metabolic route for hepatic cholesterol.6, 7 The current results suggest that cholesterol produced in response to increased liver iron is not directed to bile acid synthesis. Cytochrome P450 7a1 (Cyp7a1) mRNA, which encodes the rate-limiting enzyme in bile acid synthesis,46 did not significantly correlate with liver iron and Hsd3b7 mRNA, which encodes another enzyme involved early in bile acid synthesis, declined in response to increasing iron. Additionally, transcript levels of the bile acid transporter Abcb11 and two regulators of bile acid synthesis, Hnf4a and Nr1h3, did not exhibit significant correlations with liver iron.
Cholesterol may also be exported to other organs for further processing, for example, for the manufacture of steroid hormones.7 Abca1 and Apoc3 mRNA exhibited significant positive correlations with liver iron. Abca1 is a transporter which exports cholesterol to ApoA147 and, recently, overexpression of Apoc3 has been shown to enhance VLDL secretion from a hepatoma cell line.48 Although these results may suggest that cholesterol produced in response to iron loading might be exported to other organs, the observation that plasma cholesterol levels showed no relationship with either liver iron or cholesterol raises the possibility that much of the cholesterol produced by the liver under these conditions remains there. This may also explain the lack of agreement in other studies which have examined iron status and plasma levels of cholesterol.
Cholesterol may also be exported directly into the canaliculus. Abcg5 is a half-transporter which dimerizes with Abcg8 to export cholesterol and plant sterols into the canaliculus,49 whereas Abcb4 is a transporter which exports cholesterol and phosphatidylcholine into the canaliculus.50 Investigation of these transporters revealed that Abcg5 mRNA correlated positively with liver iron, whereas Abcb4 mRNA correlated negatively. Superficially, this suggests up-regulation of cholesterol export into the bile, particularly given that the substrate preference for Abcb4 is phosphatidylcholine rather than cholesterol.51 However, Abcb4 knockout mice overexpressing Abcg5 and Abcg8 have only very low levels of cholesterol in the bile,52 and the presence of bile salt micelles is required to accept cholesterol.53 Thus, in the present study, despite the increase in Abcg5 transcript with increasing iron, the down-regulation of bile acid synthetic enzymes and Abcb4 mRNA under the same conditions suggests that transport of cholesterol to the bile does not increase to accommodate the increase in cholesterol production.
Both iron and cholesterol metabolism are under complex regulatory control. Hence, we investigated some of the potential regulators that may explain the observed up-regulation of cholesterol biosynthesis. Srebf2 preferentially activates many of the genes in the cholesterol biosynthesis pathway.35 In the present study, four of these genes—Hmgcr, Pmvk, Cyp51 and Sc5d—were significantly up-regulated in response to increasing liver iron levels; however, the mechanism leading to this up-regulation appears to be independent of Srebf2 expression, which did not change in response to iron status. Srebf2 is regulated both transcriptionally and posttranscriptionally35 and, although we cannot rule out a posttranscriptional response of Srebf2 to iron, we believe this to be unlikely given that the majority of known targets of Srebf2 measured in the present study were not up-regulated. Similarly, expression of several genes measured in the present study—Dhcr7, Fdps, Abcg5, and Apob—is known to be regulated by CCAAT/enhancer binding protein α (C/EBPα), which is induced by iron loading.54-56 However, of these genes, only Abcg5 increased with increasing hepatic iron concentration, suggesting that C/EBPα is also unlikely to be involved in the observed up-regulation of cholesterol synthesis. Finally, expression of Tmem97, a gene newly identified as being associated with cholesterol regulation and a target of Srebf2,36 was unchanged in the face of increasing iron burden. The same authors reported a number of other genes as potential regulators of cholesterol metabolism, including Bhmt2 and Vrk3, knockdown of which reduced intracellular cholesterol, and Psap, knockdown of which increased intracellular cholesterol.36 In the present study, neither Bhmt2, Vrk3, nor Psap correlated significantly with liver iron, suggesting that regulation of cholesterol metabolism by iron is independent of these three genes.
In a previous study, Brunet et al.57 observed a pronounced plasma hypercholesterolemia in iron-loaded rats compared with wild-type controls. Hepatic cholesterol concentration did not change and the activity of Hmgcr decreased. These parameters correlated significantly with hepatic malondialdehyde, a marker of oxidative stress, and led the authors to suggest that the changes were due to oxidative damage of the membranes in which the enzymes of cholesterol metabolism reside. It is interesting that in the present study, hepatic total cholesterol increased with increasing iron burden, suggesting that enzyme activity was not disrupted. The difference between the two studies is likely to be explained by the different feeding regimes employed: 12 weeks on a 3% carbonyl iron diet in the study by Brunet et al. compared with 3 weeks on a 2% carbonyl iron diet in the present study. The longer exposure to a higher iron diet is likely to have generated higher levels of oxidative stress than in the present study.
The current study may have important clinical implications for a role of iron in contributing to the pathogenesis of NAFLD. Alterations in cholesterol metabolism have been reported to be associated with iron parameters in many disease states, including iron overload,58 iron deficiency,24 peripheral artery disease,59 and NAFLD.60-62 In the present study, Apoc3 was seen to increase with increasing hepatic iron, and overexpression of Apoc3 has been reported to result in hepatic steatosis.63 Furthermore, a recent, large, multicenter study of patients with NAFLD reported that deposition of iron in hepatocytes was associated with increased risk of moderate to severe liver fibrosis16 and increasing hepatic iron has been shown to be associated with increased lipid peroxidation.14 It has been proposed that the development of NASH occurs in two stages: (1) the deposition of fat, resulting in steatosis and (2) the intervention of another factor which causes steatohepatitis.12 It has been hypothesized that the second stage involves oxidative stress, which can be caused by iron-generated reactive oxygen species. These reactive oxygen species can initiate lipid peroxidation which can lead to cellular damage.5 Increased production of cholesterol by the liver with increasing liver iron burden is, in itself, consistent with a contribution of iron loading to steatosis. The presence of iron may additionally contribute the reactive oxygen species, which can allow further progression of the disease.
In summary, the findings reported in this study suggest that hepatic iron loading increases the synthesis and deposition of cholesterol in the liver. The mechanism appears to be independent of Srebf2. The observations are consistent with a role for iron in the development of NAFLD, with iron contributing, first, to increased cholesterol production and, second, to increased oxidative stress leading to lipid peroxidation.
Acknowledgements
We are grateful to Mary Anne Townsend and the staff at PathWest Laboratory Medicine WA, Fremantle Hospital, for performing the total cholesterol measurements.




