Molecular characterization of the vascular features of focal nodular hyperplasia and hepatocellular adenoma: A role for angiopoietin-1†
Potential conflict of interest: Nothing to report.
Abstract
Focal nodular hyperplasia (FNH) and hepatocellular adenoma (HCA) are two hepatic nodular lesions of different etiologies. FNH, a polyclonal lesion, is assumed to be a regenerative reaction following a vascular injury, whereas HCA is a monoclonal, benign neoplastic lesion. In addition to features that are predominantly found in either FNH or HCA (e.g., dystrophic vessels in FNH and single arteries in HCA), FNH and HCA share morphological vascular abnormalities such as dilated sinusoids. We hypothesized that these anomalous vascular features are associated with altered expression of growth factors involved in vascular remodeling. This was based on reports of morphologically abnormal hepatic vasculature and nodular lesions in transgenic models of hepatocytic overexpression of angiopoietin-1 (Ang-1), a member of the angiopoietin family, which is crucially involved in vascular morphogenesis and homeostasis. We investigated gene and protein expression of members of the angiopoietin system and vascular endothelial growth factor A (VEGF-A) and its receptors in 9 FNH samples, 13 HCA samples, and 9 histologically normal livers. In comparison with normal samples, a significant increase in Ang-1 was found in FNH (P < 0.01) and HCA (P < 0.05), whereas no significant changes in Ang-2, receptor tyrosine kinase with immunoglobulin-like and EGF-like domains 2, VEGF-A, or vascular endothelial growth factor receptor 2 (VEGFR-2) were observed. Conclusion: Because of the different etiological contexts of a preceding vascular injury in FNH and a neoplastic growth in HCA, Ang-1 might exert different effects on the vasculature in these lesions. In FNH, it could predominantly stimulate recruitment of myofibroblasts and result in dystrophic vessels, whereas in HCA, it may drive vascular remodeling that produces enlarged vessels and arterial sprouting that generates single arteries. Hepatology 2010
Focal nodular hyperplasia (FNH) and hepatocellular adenoma (HCA) are two hepatic nodular lesions predominantly occurring in otherwise healthy livers in women of reproductive age. FNH is a polyclonal lesion thought to develop as a regenerative parenchymal reaction following a vascular injury.1-3 HCA is a monoclonal, benign neoplastic lesion that rarely transforms into hepatocellular carcinoma (HCC). On the basis of a recent series of mutational analyses, HCAs are now being categorized into subtypes according to the genotypic variants, the phenotypes of which can be visualized at the protein level by immunohistochemistry.4, 5 Although FNH and HCA primarily represent hepatic parenchymal growth, both lesions contain a variety of vascular malformations that are in part morphologically similar. FNH is characterized by dystrophic, thick-walled vessels due to myointimal hyperplasia. These vessels are located in a centrally located star-shaped fibrous scar and its radiating septal extensions. In the parenchyma of both FNH and HCA, dilated vessels and widened sinusoids can be encountered. Additionally, HCA contains haphazardly distributed single arteries, a feature that HCA shares with HCC. Single, with respect to arteries, denotes the absence of an accompanying bile duct and a location outside the context of a portal tract.
The etiological background of these dysmorphic vascular features is as yet undetermined. Paradis et al.6, 7 found highly significant up-regulation of the angiogenic growth factor angiopoietin-1 (Ang-1) in FNH, but this was also seen in HCA and HCC in comparison with normal livers, although it was much less pronounced in comparison with FNH. In our previous study on the molecular identity of vascular remodeling in HCC,8 we also found up-regulation of Ang-1 in HCC of cirrhotic and noncirrhotic livers.
Ang-1 is a member of the angiopoietin system, a family of growth factors that are pivotal in vascular morphogenesis and maintenance of vascular homeostasis.9 This family consists of four ligands, of which Ang-1 and Ang-2 are the best characterized factors: they have similar binding affinities to their specific tyrosine kinase receptor [tyrosine kinase with immunoglobulin-like and EGF-like domains 2 (Tie-2)], to which they bind in a competitive manner. Ang-1 has an antiapoptotic effect on endothelial cells (ECs), stimulates EC sprouting, and increases vascular stability by inducing recruitment of pericytes and stimulation of mesenchymal cells to differentiate into vascular smooth muscle cells (SMCs). As such, Ang-1 has a major role in maintaining vascular quiescence and integrity.9, 10 Ang-1 is predominantly expressed by nonendothelial cells, such as pericytes and myofibroblasts, and in the liver by hepatic stellate cells, cholangiocytes, hepatoblasts, and hepatocytes.8, 11-13
Ang-2 is predominantly produced by ECs and released during EC activation, and it promotes vessel destabilization and increases endothelial responsiveness to other growth factors such as vascular endothelial growth factor A (VEGF-A).
The significant role of Ang-1 in hepatic vascular morphogenesis has been demonstrated in several experimental animal studies. Prolonged overexpression of Ang-1 in mouse hepatocytes resulted in abnormal vessel formation, including arterial sprouting, the formation of enlarged arteries, hepatic vein dilation, the loss of portal vein radicles, and arteriovenous shunting. More importantly, Ang-1 overexpression also generated nodular parenchymal changes resembling FNH.14 In a study of transgenic expression of Ang-1 in mouse livers, aberrant dilated vessels resembled the peliotic changes observed in HCA,15 and together, these results emphasize the importance of Ang-1 for hepatic vascular morphology. On the basis of these experimental results, the findings of Paradis et al.,6 and our own previous findings in HCC,8 we hypothesized that altered expression and a distorted balance of angiogenic growth factors could be responsible for the aberrant vascular features in FNH and HCA. Therefore, in the present study, we investigated the expression profiles of the ligands and cognate receptors of the two currently most influential families of angiogenic growth factors, the angiopoietins and VEGF-A. Besides gene and protein expression levels, we established their location in the lesions and in adjacent tissues. Our main finding is that a significant increase in Ang-1 expression exists in FNH and, though less prominently, in HCA without a significant alteration of Ang-2 and VEGF-A expression. As overexpression of Ang-1 in FNH and HCA does not necessarily imply that a similar remodeling process is responsible for the vascular abnormalities in both lesions, we discuss this finding in light of the different etiologies of FNH and HCA. In FNH, the preceding vascular injury evokes vascular or endothelial reparative mechanisms, whereas in HCA, overexpression of Ang-1 stimulates angiogenesis to fulfill the demands of neoplastic growth.
Abbreviations:
α-SMA, alpha-smooth muscle actin; Ang, angiopoietin; Angpt, angiopoietin; EC, endothelial cell; FNH, focal nodular hyperplasia; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GS, glutamine synthetase; HCA, hepatocellular adenoma; HCC, hepatocellular carcinoma; HRP, horseradish peroxidase; HSEC, hepatic sinusoidal endothelial cell; I-HCA, inflammatory-type hepatocellular adenoma; Ig, immunoglobulin; LFABP-1, liver fatty acid binding protein-1; mRNA, messenger RNA; PCR, polymerase chain reaction; RT-PCR, reverse-transcriptase polymerase chain reaction; SAA, serum amyloid A protein; SEC, sinusoidal endothelial cell; SMC, smooth muscle cell; Tie-2, tyrosine kinase with immunoglobulin-like and EGF-like domains 2; VEC, vascular endothelial cell; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Patients and Methods
Patients and Tissue Samples.
All procedures and use of (anonymized) tissue samples were performed according to recent national guidelines. Tissue samples of 9 FNH patients (mean age = 33.1 ± 4.7 years) and 12 HCA patients (mean age = 37.5 ± 10.5 years) who underwent partial liver resection for lesions were included. All patients were females, and all lesions were present in otherwise healthy livers. One patient in the HCA group had 2 separate tumors, so in all there were 9 FNHs and 13 HCAs. We also included nine samples of livers showing normal histological features. These samples were collected from surplus materials of a donor liver, a solitary hemangioma liver, and a traumatic liver rupture. Adjacent, nondiseased liver tissue was also included in the study (n = 5 for FNH and n = 4 for HCA).
Fresh tissue samples were collected from the resection specimens. One part of the samples was snap-frozen in −80°C-cooled isopentane and stored at −80°C for subsequent preparation for quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) analysis and western blot studies. Another part was fixed in buffered formalin (4%) and embedded in paraffin. Paraffin sections were cut to 4 μm and stained with hematoxylin-eosin, periodic acid Schiff after diastase digestion, Masson trichrome, reticulin, and iron stains for the histopathological classification of the lesions. The lesions were histologically and immunohistologically classified according to the latest recommendations for the classification of benign hepatic nodules.4, 5, 16 Paraffin sections were also applied for immunohistological staining with CD34 and alpha-smooth muscle actin (α-SMA) and for immunophenotypical categorization of the lesions.
Quantitative RT-PCR for Messenger RNA (mRNA) Analysis.
Total RNA was isolated with the RNeasy mini kit (Qiagen, Leusden, the Netherlands) with subsequent DNA removal using the RNase-free DNase set (Qiagen); both were used according to the protocol of the manufacturer. RNA was analyzed qualitatively by gel electrophoresis and quantitatively with a Nanodrop ND-100 spectrophotometer (NanoDrop Technologies, Rockland, DE). Reverse transcription and real-time polymerase chain reaction (PCR) were performed as described previously.17 Briefly, 1 μg of total cellular RNA was used for the synthesis of first-strand complementary DNA, and 10 ng of complementary DNA was used for each PCR reaction. Exons overlapping primers and minor groove binder probes used for real-time RT-PCR were purchased as Assay-on-Demand from Applied Biosystems (Nieuwerkerk aan den IJssel, the Netherlands): housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH; assay ID Hs99999905_m1), VEGF (assay ID Hs00173626_m1), vascular endothelial growth factor receptor 1 (VEGFR-1; assay ID Hs00176573_m1), VEGFR-2 (assay ID Hs00176676_m1), Tie-2 (assay ID Hs00176096_ml), angiopoietin-1 (Angpt-1; assay ID Hs00181613_ml), and Angpt-2 (assay ID Hs00169867_ml). TaqMan quantitative PCR was performed with an ABI-Prism 7900HT sequence detector (Applied Biosystems). Amplification was performed under the following cycling conditions: 2 minutes at 50°C, 10 minutes at 95°C, and 40 two-step cycles of 15 s at 95°C and 60 s at 60°C. Triplicate real-time PCR analyses were executed for each sample, and the obtained threshold cycle values (Ct) were averaged. Gene expression was normalized to the expression of the housekeeping gene GAPDH, and this yielded the relative gene expression value. Control samples of distilled water and randomly chosen RNA isolates that were not subjected to reverse transcriptase were consistently found to be negative.
Western Blot Analysis.
Twenty samples of 5-μm-thick tissue slices from each frozen tissue block were lysed in a radioimmunoprecipitation assay buffer [50 mM trishydroxymethylaminomethane hydrochloric acid, pH 7.4, 150 mM sodium chloride, 1% Nonidet P40, 0.25% sodium deoxycholate, 1 mM ethylenediaminetetraacetic acid, 1 mM sodium fluoride, 1 mM sodium orthovanadate, 100 μg/mL phenylmethylsulfonyl fluoride, 1 μg/mL aprotinin (Sigma), 1 μg/mL leupeptin (Roche), and 1 μg/mL pepstatin (Roche)]. Cell debris was removed by centrifugation at 10,000g for 15 minutes, and the protein concentration was measured with a pyrogallol red–molybdate solution. Indicated amounts of lysates were separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (0.45 μm; Bio-Rad Laboratories, Hercules, CA). The membranes were next probed with various primary antibodies (VEGF-A, 1:1000, sc-152, Santa Cruz; Angpt-1, 1:1000, sc-6319, Santa Cruz; Angpt-2, 1:2000, sc-7017, Santa Cruz; and Tie-2, 1:300, sc-324, Santa Cruz), diluted in 5% no-fat milk/0.1% trishydroxymethylaminomethane-buffered saline Tween 20 at 4°C overnight, incubated with peroxidase-labeled secondary antibodies (1:1000), and treated with an enhanced chemiluminescent substrate for the detection of horseradish peroxidase (HRP; Amersham Life Science, London, United Kingdom). Then, the membranes were stripped with a 25 mM glycine/1% sodium dodecyl sulfate (pH 2.0) buffer, and β-actin (mouse anti–β-actin, 1:3000, ab8226, Abcam) was detected as a loading control. Protein expression, observed as electrophoretic bands in X-ray films, was quantified with image analysis software (Quantity One, Bio-Rad) to calculate the volume of bands (intensity × mm2). For each protein, the volume of each sample was divided by the volume of the control (β-actin), and this yielded the relative protein expression value.
Immunohistology.
The antibodies used for immunohistology and their dilutions and sources are listed in Table 1. Staining for VEGF-A, VEGFR-1, VEGFR-2, Ang-1, Ang-2, and Tie-2 was performed on frozen sections according to methods described previously.8
| Antibody | Dilution | Pretreatment | Source/Code |
|---|---|---|---|
| Rabbit anti–VEGF-A (A-20)* | 1/100† | Santa Cruz/sc-152 | |
| Goat anti–Ang-1 (N-18)* | 1/100† | Santa Cruz/sc-6319 | |
| Goat anti–Ang-2 (F-18)* | 1/50† | Santa Cruz/sc-7017 | |
| Rabbit anti–Tie-2 (C-20)* | 1/50† | Santa Cruz/sc-324 | |
| Rabbit anti–VEGFR-1* | 1/100† | Abcam/ab2350 | |
| Rabbit anti–VEGFR-2* | 1/100† | Abcam/ab2349 | |
| Mouse anti-CD34 | Ready to use | Immunotech/QBend10 | |
| Mouse anti–α-SMA | 1/80 | Immunotech/1A4 | |
| Mouse anti-human amyloid A | 1/50 | Microwave | Dako/mc-1 |
| Rabbit polyclonal to LFABP-1 | 1/50 | Microwave | Abcam/ab7807 |
| Mouse anti-GS | 1/400 | Microwave | Biosciences/mab302 |
| HRP-conjugated rabbit anti-mouse Ig | 1/100‡ | Dako/P0260 | |
| HRP-conjugated goat anti-rabbit Ig | 1/100‡ | Dako/P0448 | |
| HRP-conjugated rabbit anti-goat Ig | 1/100‡ | Dako/P0160 |
- * Marked antibodies were applied on frozen sections; unmarked ones were used on paraffin sections.
- † Diluted in 1% bovine serum albumin/phosphate-buffered saline.
- ‡ Diluted in 1% bovine serum albumin/phosphate-buffered saline and 1% human albumin.
The three markers for immunophenotyping HCA and FNH—glutamine synthetase (GS), serum amyloid A protein (SAA), and liver fatty acid binding protein-1 (LFABP-1)—were applied on paraffin sections, as was the staining with anti-CD34 and anti–α-SMA. In short, 4-μm sections were deparaffinized, and microwave pretreatment was applied except for CD34 and α-SMA. After endogenous peroxidase was blocked by H2O2, slides were incubated with the primary antibody. For LFABP-1, GS, and SAA, DAKO EnVision was applied as the amplification system. For CD34 and α-SMA, peroxidase-labeled rabbit anti-mouse immunoglobulin (Ig) was applied as the secondary antibody, and peroxidase-labeled goat anti-rabbit Ig was applied as the tertiary antibody. Diaminobenzidine was applied to visualize the staining reaction, and hematoxylin was used for counterstaining.
Assessment of the Staining Results.
The subclassification of HCA and the confirmation of FNH based on the expression of GS, LFABP-1, and SAA were performed according to profiles recommended by Bioulac-Sage et al.5
The expression of the angiogenic factors on several liver cell constituents [hepatocytes, sinusoidal endothelial cells (SECs), vascular endothelial cells (VECs), bile ducts, and bile ductules] was primarily documented with a binary indication: absence (−) or presence (+). Because of the regular presence of a weaker staining intensity, an intermediate indication of expression (±) was also applied. The most frequently observed pattern for each protein and each cell type in the samples of HCA, FNH, and normal liver was taken as the representative pattern of each group and is summarized in Table 2.
| Protein | Hepatocytes | HSECs | VECs | Bile Ducts | Bile Ductules | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | FNH | HCA | N | FNH | HCA | N | FNH | HCA | N | FNH | HCA | N | FNH | HCA | |
| VEGF-A | − | − | − | + | + | ±* | + | + | + | + | A | A | + | ± | A* |
| VEGFR-1 | − | − | − | ±* | ±* | ±* | ± | ± | ± | + | A | A | + | + | A* |
| VEGFR-2 | − | − | − | ± | + | ± | ± | ± | ± | + | A | A | + | + | A* |
| Ang-1 | + | + | + | − | − | − | − | − | − | + | A | A | ± | ± | A* |
| Ang-2 | − | − | − | + | ± | ± | ± | ± | ± | + | A | A | + | ± | A* |
| Tie-2 | − | − | − | + | + | + | + | ± | ± | − | A | A | − | ± | A* |
- Abbreviations: −, no protein expression; ±, weak protein expression; +, obvious protein expression; A, structures absent; A*, structures usually absent but sometimes present in inflammatory-type hepatocellular adenoma; N, normal liver samples.
- * Protein expression is more obviously present in sinusoidal macrophages.
Statistics.
Quantitative data were expressed as means and standard errors. Logarithmic transformation was performed on data that did not show a normal distribution. A comparison of mean values between groups was performed with the one-way analysis of variance test and Bonferroni post hoc test for multiple comparisons. The paired-sample t test was used for the comparison of mean values between FNH or HCA and adjacent liver tissue.
For all analyses, SPSS 16.0 for Windows statistical software was applied (SPSS, Inc., Chicago, IL). The level of significance was set at 0.05.
Results
The hepatic lesions included in this study were classified according to the latest criteria and immunohistological profiles recommended by Bioulac-Sage et al.4, 5, 16 All nine samples of FNH showed the typical maplike pattern of GS expression. From a total of 13 HCAs, 6 samples showed a diffuse increase in SAA expression compatible with inflammatory-type hepatocellular adenoma (I-HCA), 2 samples demonstrated a lack of LFABP-1, 1 case presented with a diffuse increase in GS, and 4 HCAs did not have any specific immunophenotypic characteristics. The single case in our series showing a diffuse increase in GS showed a monotonous feature containing hepatocytes without cytomorphological atypia arranged in liver cell plates one or two cells thick. Pseudoglandular and trabecular areas were absent. Additional β-catenin staining of multiple samples of the tumor revealed no nuclear β-catenin expression. Now, 6 years after complete surgical resection, the patient is doing well.
CD34 and α-SMA Expression.
In histologically normal livers, CD34 was expressed only by VECs and a small rim of periportal SECs. In FNH, increased CD34 sinusoidal expression was found, mainly around the central scar and scarlike structures within the nodules in a decreasing gradient pattern from the scar deeper in the nodular parenchyma. In HCA, SECs showed an increase in CD34 expression in a variable, nonspecific pattern that was both patchy and diffuse. The expression of α-SMA in histologically normal livers was limited to vascular walls. In FNH, obvious expression of α-SMA was seen in the stromal tissue of the central scar, in the fibrous septa, and in the periseptal sinusoids. There was a gradient pattern similar to that described for CD34 expression. In HCA, a variable increase in sinusoidal α-SMA expression was noted, and the sinusoidal expression ranged from scant to diffuse. The α-SMA staining also emphasized the presence of haphazardly distributed single arteries.
No specific patterns were observed in CD34 and α-SMA expression in the different subtypes of HCA (not shown).
Angiopoietins and Tie-2: Ang-1 Expression Is Increased in FNH and HCA, and Tie-2 Is Increased in FNH.
A highly significant increase in gene expression of Ang-1 was observed in FNH versus normal liver samples (P < 0.01) and HCA (P < 0.05). Also in HCA, Ang-1 expression was increased in comparison with normal livers (P < 0.05; Fig. 1). No significant differences in Ang-1 expression were observed in FNH or HCA in comparison with their nonlesional counterpart. The latter samples showed no significant differences from normal livers.
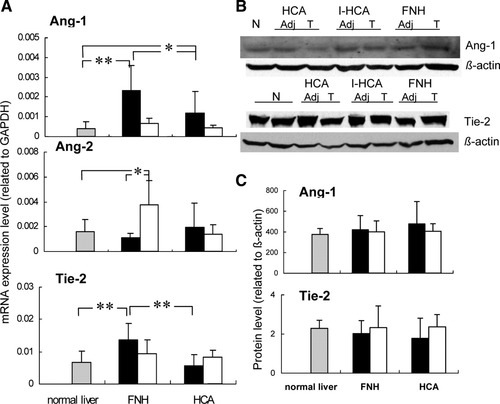
(A) Relative gene expression of Ang-1, Ang-2, and Tie-2 in normal livers (n = 9), FNH (n = 9) and adjacent tissue (n = 5), and HCA (n = 13) and adjacent liver tissue (n = 4) as determined by quantitative RT-PCR. Gene expression values represent relative values adjusted to GAPDH. (B) Western blotting of Ang-1 and Tie-2 (Adj, corresponding adjacent nonlesional liver tissue; T, lesion; N, normal liver). HCA was divided into an I-HCA group (n = 6) and a noninflammatory HCA group comprising the remaining cases (n = 7). (C) Relative protein levels of Ang-1 and Tie-2 in normal livers (n = 9), FNH (n = 9) and adjacent tissue (n = 5), and HCA (n = 13) and adjacent liver tissue (n = 4) as detected by western blotting. Values are given as means; error bars represent the standard error of the mean. *P < 0.05; **P < 0.01. Gray bars represent normal livers, black bars represent lesions, and white bars represent liver tissue adjacent to lesions.
Neither in FNH nor in HCA were significant differences in gene expression seen for Ang-2 in comparison with normal liver samples and each other. A comparison of lesional and nonlesional samples showed increased Ang-2 expression in the adjacent liver tissue of FNH, and it was also increased in comparison with normal samples (both P < 0.05). As Ang-1 and Ang-2 both compete for binding to Tie-2, we also calculated the Ang-1/Ang-2 ratio of gene expression levels in the different tissue samples. The mean values and standard deviations of the Ang-1/Ang-2 ratio were 2.27 ± 1.29 for FNH, 0.96 ± 1.09 for HCA, and 0.32 ± 0.25 for normal liver samples. This resulted in a highly significant increase in the ratio for FNH only in comparison with normal liver samples (P = 0.001) and adenoma (P = 0.03). Tie-2, the tyrosine kinase receptor that binds its ligands Ang-1 and Ang-2, was up-regulated only in FNH and not in HCA (Fig. 1).
At the protein level, the differences in mRNA could not be substantiated for Ang-1 and Tie-2, whereas Ang-2 protein expression was below the detection limit in western blot analysis. Previously, we were able to demonstrate Ang-2 protein expression in renal cell carcinoma protein extracts,8 and this indicated that the experimental protocol used per se is appropriate for the detection of this protein.
In Fig. 2, the cellular localization of Ang-1, Ang-2, and Tie-2 is depicted. In both lesions and normal liver samples, cytoplasmic staining of Ang-1 was observed readily in hepatocytes and less prominently in bile ducts and ductules. Ang-1 was absent in SECs and VECs. Ang-2 was present in SECs and VECs and in bile ducts and ductules, albeit less pronouncedly. In some samples of histologically normal livers and liver tissue adjacent to the lesions, Ang-2 showed a more intense expression in the centrilobular areas. Hepatocytes were negative. Tie-2 expression was strongly positive in SECs and VECs in both types of lesions and in normal livers as well as adjacent liver tissue, whereas no expression was detected in hepatocytes, bile ducts, or ductules. Table 2 summarizes the localization patterns observed in the different tissues.
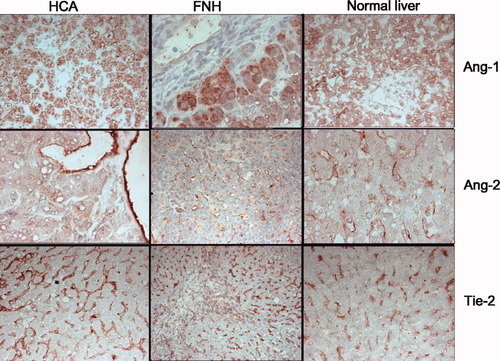
Localization of Ang-1, Ang-2, and Tie-2 expression by immunohistology on frozen sections. In normal livers, FNH, and HCA, Ang-1 was expressed in hepatocytes, whereas Ang-2 and Tie-2 were expressed on VECs and SECs. In HCA, Ang-2 was less conspicuously expressed on SECs versus VECs. Ang-1, Ang-2, and Tie-2 were also expressed on biliary structures in FNH.
VEGF-A and Receptors: No Change in the Expression of VEGF-A and VEGFR-2 in FNH and HCA and Down-Regulation of VEGFR-1 in FNH.
In Fig. 3A,B, the results of the quantitative mRNA and protein expression analyses of the VEGF system are shown. In FNH and HCA, no significant alterations occurred in VEGF-A expression at the gene (Fig. 3A) or protein levels (Fig. 3B) in comparison with normal liver samples. Also, when the HCA group was divided into the I-HCA type (the largest subgroup, n = 6) and the noninflammatory type (n = 7), no significant differences in gene or protein expression levels of VEGF-A could be detected (not shown).
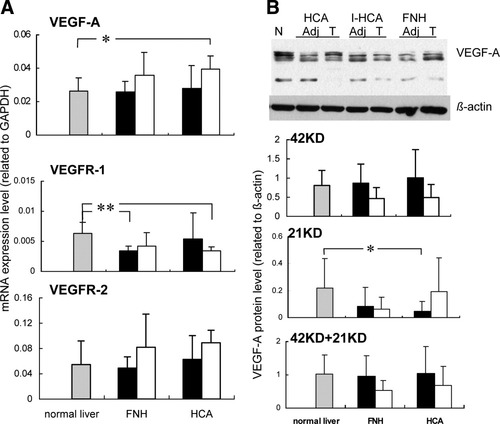
(A) Relative gene expression of VEGF-A, VEGFR-1, and VEGFR-2 in normal livers (n = 9), FNH (n = 9) and adjacent tissue (n = 5), and HCA (n = 13) and adjacent liver tissue (n = 4) as determined by quantitative RT-PCR. Relative gene expression represents values adjusted to GAPDH. Values are given as means; error bars represent the standard error of the mean. *P < 0.05; **P < 0.01. Gray bars represent normal livers, black bars represent lesions, and white bars represent liver tissue adjacent to lesions. (B) The top panel shows western blotting of VEGF-A (Adj, corresponding adjacent nonlesional liver tissue; T, lesion; N, normal liver): HCA was divided into an I-HCA group (n = 6)) and an HCA group comprising the remaining cases (n = 7). The lower three panels show relative VEGF-A protein levels in normal livers (n = 9), FNH (n = 9) and adjacent liver tissue (n = 5), and HCA (n = 13) and adjacent liver tissue (n = 4) as detected by western blotting. Values are given as means; error bars represent the standard error of the mean. *P < 0.05. Gray bars represent normal livers, black bars represent lesions, and white bars represent liver tissue adjacent to lesions.
The VEGFR-1 gene expression level in FNH and in the liver adjacent to HCA was significantly lower than that in normal samples. No other significant differences in VEGFR-1 expression were observed (Fig. 3A).
There were no significant differences in the VEGFR-2 gene expression between normal livers, FNH, and HCA and between lesions and nonlesional counterparts.
The cellular localization of VEGF-A and both receptors was studied by immunohistology (Fig. 4). In normal livers, VEGF-A was expressed by SECs, VECs, bile ducts, and ductules, but hepatocytes were negative. In FNH and HCA, a similar cellular distribution was found, except that FNH and HCA did not contain bile ducts, and only I-HCA contained ductules. VEGF-A expression in SECs of HCA was much less intense than that in FNH and normal livers. In the sinusoidal spaces of HCA, VEGF-A was predominantly seen in macrophages. The adjacent liver of FNH and HCA showed a pattern of VEGF-A expression similar to that seen in normal liver samples.
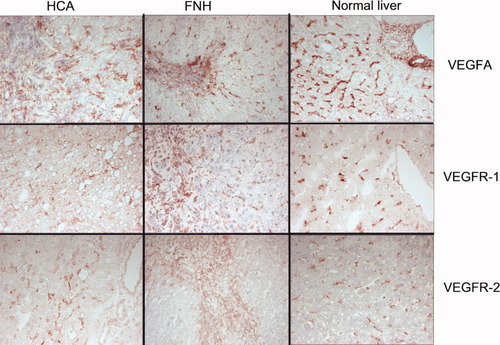
Localization of VEGF-A, VEGFR-1, and VEGFR-2 expression by immunohistology on frozen sections. In HCA, FNH, and normal livers, VEGF-A and its receptors are expressed on hepatic SECs and sinusoidal macrophages, whereas in FNH, there is also expression in stromal cells in the central scar and on ductular structures. In this particular case of FNH, VEGF-R2 expression in the sinusoidal compartment adjacent to the central scar area was much less conspicuous than that in the central scar.
VEGFR-1 and VEGFR-2 showed similar cellular localization in normal livers, FNH, HCA, and their nonlesional adjacent counterparts. Both receptors were absent in hepatocytes but were expressed by bile ducts and ductules when present (e.g., ductules in FNH and I-HCA). The most obvious expression of VEGFR-1 and VEGFR-2 was present in sinusoidal macrophages, SECs, and VECs. Stromal cells and macrophages in fibrous scars and septa of FNH also expressed both receptors (Fig. 4). The patterns observed in the different tissues are summarized in Table 2.
Discussion
FNH and HCA are two nodular hepatic lesions of different etiological backgrounds. HCA is a benign, neoplastic lesion of several mutational backgrounds, whereas FNH is thought to represent a hyperplastic reaction following a vascular injury.3, 5 FNH and HCA contain various morphological vascular abnormalities, the pathogenesis of which is not clear. Some vascular features are shared by the two lesions, whereas some are lesionally restricted. Studies in transgenic mice have shown that overexpression of the angiogenic growth factor Ang-1 results in hepatic vascular anomalies and generates hepatocellular nodules similar to FNH.14, 15 We hypothesized that the various abnormal vascular features prominent in human FNH and HCA are related to increased vascular remodeling with a central role for the angiopoietin system. To test this hypothesis, we investigated the gene and protein expression pattern of growth factors belonging to the angiopoietin system: Ang-1, Ang-2, and their cognate receptor Tie-2. VEGF-A and its receptors VEGFR-1 and VEGFR-2 were included in the analysis as these factors are known to act in concert with the angiopoietins.18 We observed a significant increase of Ang-1 in FNH and to a lesser extent in HCA in comparison with histologically normal livers, with a concurrent increase in the Ang-1/Ang-2 ratio. In FNH, this increase existed next to a significant increase in Tie-2 expression. In contrast, changes in VEGF-A and VEGFR expression were not prominent in either type of lesion.
Our results support the concept, schematically depicted in Fig. 5, that in FNH and HCA, the Ang-1/Tie-2 system may have a regulatory role in the development of the characteristic vascular features of these lesions without signs of robust involvement of the VEGF system. Studies addressing the molecular background of vascular remodeling in FNH and HCA are rare. Paradis et al.6 investigated 209 genes in FNH and were the first to report that Ang-1 gene expression was enhanced in FNH, whereas Ang-2 was decreased, but without a concurrent increase in Tie-2 as we observed. The same group also studied telangiectatic FNH and postulated that this FNH subtype resembles HCA more than it resembles FNH on the basis of the expression patterns of Ang-1 and Ang-2.7 In a similar pursuit to classify telangiectatic FNH, Bioulac-Sage et al.19 confirmed these results. In these two studies, the telangiectatic FNHs were monoclonal lesions, and this supports the concept that they represent an HCA subtype. Currently, lesions that were previously designated as telangiectatic FNH are categorized as the inflammatory subtype of HCA on the basis of their immunophenotype.5 All cases of FNH and HCA that were included in our present study were categorized according to their immunophenotypes. Although our study was focused on the possible role of the angiopoietins in the development of the vascular lesions of FNH and HCA and not on the classification of the lesions, our findings of increased Ang-1 in FNH and HCA are in line with the aforementioned studies.
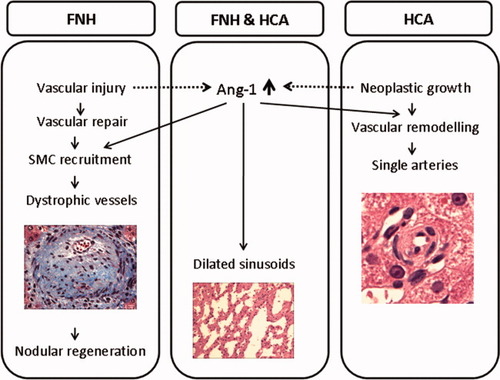
Schematic representation of a possible sequence of Ang-1–related effects during the morphogenesis of the vascular changes in FNH and HCA.
The most characteristic vascular features of FNH are the thick-walled vessels with myointimal hyperplasia located in the central scar and in the radiating septa, and they exist next to the periseptal sinusoidal enhanced α-SMA and CD34 expression, which is indicative of sinusoidal capillarization and vascular remodeling.20, 21 The increased expression of Ang-1 and Tie-2 without a concurrent increase in Ang-2 expression creates a condition that can facilitate Ang-1/Tie-2 signaling. Among other things, this can lead to recruitment of SMCs and promotion of differentiation of mesenchymal cells into vascular SMCs.22, 23 Gain-of-function studies have shown that prolonged expression or overexpression of Ang-1 results in various vascular abnormalities, including larger, more numerous, and highly branched vessels in the skin, vascular enlargement in hepatic microvascular remodeling, and cardiac allograft vasculopathy, which are all dysmorphic vascular changes that resemble the vascular features found in FNH and HCA.14, 15, 24, 25
In cardiac graft vasculopathy, inflammation and arterial injury initiate subsequent myointimal proliferation. Transgenic overexpression of both Ang-1 and Ang-2 decrease inflammation, whereas induced Ang-1 expression (not Ang-2) stimulates activation of vascular SMCs, which results in myointimal growth and development of cardiac vasculopathy.25 It is conceivable that in FNH, overexpression of Ang-1 and a relative lack of Ang-2 lead to a similar course of action. Within the context of the assumed primary vascular injury, the dominant Ang-1 overexpression in FNH, which is emphasized by the significantly enhanced Ang-1/Ang-2 ratio, might stimulate excessive recruitment of vascular SMCs and elicit myointimal hyperplasia. As a result, the dystrophic vessels characteristic of FNH can form. The subsequent compromised vascular supply may underlie local hemodynamic changes leading to regenerative parenchymal hyperplasia; this finding is similar to the FNH-like nodules in mouse livers under the influence of overexpression of Ang-1.14
Also, the occurrence of other vascular abnormalities found in HCA and FNH is supportive of the concept that they are related to excessive Ang-1 activity. In the studies of transgenic expression of Ang-1 in hepatocytes, a spectrum of hepatic vascular changes were documented, and they consisted of arterial sprouting, loss of portal triads, peliotic changes, and vessel dilatation.14, 15 On gross examination of HCA specimens, subcapsular vascular dilatation is frequently observed, and this feature matches the subcapsular dilated vessels in the livers of the Ang-1 hepatic transgenic mice. The parallel findings also extend to the dilated sinusoids and peliotic changes, which are regular features of HCA and FNH. Arterial sprouts, described in Ang-1 transgenic mice, might well represent the equivalent of the single arteries that are regularly found in HCA. The large dystrophic vessels with robust myointimal thickening that characterize FNH are, however, not found in HCA. These differences indicate a preponderance of the myofibroblast recruitment effect of Ang-1 in FNH, whereas in HCA, up-regulation of Ang-1 seems to be predominantly stimulating vascular remodeling. These variable effects of Ang-1 could be due to the difference in the magnitude of Ang-1 enhancement, although the etiological context in which Ang-1 exerts its effects is probably similar if not more important.
The etiological context of FNH is a liver already affected by vascular injury that possibly has stimulated reparative mechanisms to restore vascular integrity and triggered recruitment of vascular SMCs. Of note is the fact that the nodular lesions resembling human FNH in the hepatic Ang-1 transgenic mice were also preceded by an obliterative vascular lesion.14 It remains to be elucidated whether the early stage of vascular injury itself provokes Ang-1 overexpression in FNH.
HCA is a primary neoplastic lesion and lacks a primary vascular anomaly such as that in FNH. As such, in HCA, the vascular integrity is not jeopardized, and recruitment of myofibroblasts to stabilize vessels is less stimulated; this is compatible with the less prominent increase in Ang-1 observed in HCA. The emphasis of Ang-1 overexpression in HCA seems to lie in the induction of vascular remodeling. Whether these vascular changes represent angiogenesis effective at providing neoplastic growth with its increasing vascular demands is yet unclear. The single arteries observed in HCA are also regularly found in HCC, and in our previous study, we also found increased Ang-1 in human HCC.8 However, studies on the expression of Ang-1 in tumor models have reported variable results in different types of tumors, including the induction of tumor angiogenesis in astrocytomas, glioblastomas, and cervical cancer but a decrease in angiogenesis in colorectal cancer and liver metastases of colorectal cancer (reviewed by Shim et al.26).
In conclusion, we have demonstrated increased expression of the angiogenic growth factor Ang-1 in FNH and HCA with maintenance of the expression of its receptor Tie-2, which potentially underlies the development of the dysmorphic vascular features in these two lesions. Further studies analyzing the spatiotemporal changes occurring while lesions are forming will be needed to investigate the causal or consequential nature of these angiogenic factors in the morphogenesis of the vascular lesions.




