Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease†
Potential conflict of interest: R.J.J.: Published a lay book “The Sugar Fix” that discusses the potential role of fructose in obesity and fatty liver and has a patent application on lowering uric acid to reduce fatty liver disease. All other authors have no conflict of interest.
Abstract
The rising incidence of obesity and diabetes coincides with a marked increase in fructose consumption. Fructose consumption is higher in individuals with nonalcoholic fatty liver disease (NAFLD) than in age-matched and body mass index (BMI)-matched controls. Because fructose elicits metabolic perturbations that may be hepatotoxic, we investigated the relationship between fructose consumption and disease severity in NAFLD. We studied 427 adults enrolled in the NASH Clinical Research Network for whom Block food questionnaire data were collected within 3 months of a liver biopsy. Fructose consumption was estimated based on reporting (frequency × amount) of Kool-aid, fruit juices, and nondietary soda intake, expressed as servings per week, and classified into none, minimum to moderate (<7 servings/week), and daily (≥7 servings/week). The association of fructose intake with metabolic and histological features of NAFLD was analyzed using multiple linear and ordinal logistic regression analyses with and without controlling for other confounding factors. Increased fructose consumption was univariately associated with decreased age (P < 0.0001), male sex (P < 0.0001), hypertriglyceridemia (P < 0.04), low high-density lipoprotein (HDL) cholesterol (<0.0001), decreased serum glucose (P < 0.001), increased calorie intake (P < 0.0001), and hyperuricemia (P < 0.0001). After controlling for age, sex, BMI, and total calorie intake, daily fructose consumption was associated with lower steatosis grade and higher fibrosis stage (P < 0.05 for each). In older adults (age ≥ 48 years), daily fructose consumption was associated with increased hepatic inflammation (P < 0.05) and hepatocyte ballooning (P = 0.05). Conclusion: In patients with NAFLD, daily fructose ingestion is associated with reduced hepatic steatosis but increased fibrosis. These results identify a readily modifiable environmental risk factor that may ameliorate disease progression in patients with NAFLD. HEPATOLOGY 2010
The prevalence of obesity in the United States is rising, and with it, the frequency of fatty liver, nonalcoholic steatohepatitis (NASH), “cryptogenic” cirrhosis, hepatocellular carcinoma, and other end-organ complications of the metabolic syndrome.1, 2 The healthcare burden and associated economic implication of the epidemic of obesity, diabetes, and the hepatic complications of the metabolic syndrome are tremendous.3 Unfortunately, no therapy for nonalcoholic fatty liver disease (NAFLD) currently exists. Therefore, a rigorous search for modifiable risk factors or environmental exposures that may increase the risk of developing NASH or its transition to cirrhosis is essential.
The rapid rise in NAFLD supports a role for environmental factors in the pathogenesis of this condition. In this regard, recent studies suggest that overconsumption of high-fructose corn syrup (HFCS), primarily in the form of soft-drink consumption, is linked to weight gain and the rise in obesity, particularly in children and adolescents,4-6 and increases the risk of NAFLD. Table sugar (sucrose) and HFCS are the two major dietary sources of fructose. Intake of dietary fructose, either as a free monosaccharide or bound to glucose in the form of sucrose, has increased 1000% during the past 40 years.5 First introduced into the human diet around 1970, HFCS consumption during the past decade accounts for 10% of caloric food intake.7
Dietary fructose is a major candidate for causing NAFLD. Unlike glucose, fructose ingestion can rapidly cause fatty liver in animals, in association with the development of leptin resistance,8 microvascular disease, and vascular inflammation.9, 10 Recent data suggest that increased fructose consumption increases fat mass, de novo lipogenesis, and inflammation and induces insulin resistance and postprandial hypertriglyceridemia, particularly in overweight individuals.10-15 Furthermore, studies have indicated that the development of NAFLD may be associated with excessive dietary fructose consumption.16, 17 Whether increased fructose consumption correlates merely with the development of NAFLD or promotes the transition from NAFLD to NASH and more advanced stages of liver damage remains unclear. In view of the global increase in fructose consumption and its association with NAFLD, we sought to evaluate the influence of fructose consumption on liver histology in patients with NAFLD.
Patients and Methods
Abbreviations
AMP, adenosine monophosphate; AMPK, adenosine monophosphate kinase; ATP, adenosine triphosphate; BMI, body mass index; CI, confidence interval; HDL, high-density lipoprotein; HFCS, high-fructose corn syrup; HOMA-IR, homeostasis model assessment of insulin resistance; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; OR, odds ratio.
Study Design and Population.
We performed cross-sectional analyses using data from the NASH Clinical Research Network18, 19 of patients diagnosed with NAFLD who were enrolled from September 2004 to March 2007. Patients enrolled in the NAFLD Database Study or in the PIVENS (Pioglitazone vs Vitamin E vs Placebo for Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis) trial who met the following criteria were used for our analysis (n = 427): (1) age 18 years or older; (2) available liver histology data; (3) no significant alcohol consumption (>14 drinks/week in men or >7 drinks/week in women on average within the past 2 years) or other coexisting causes of chronic liver disease; and (4) dietary information available from the Block food questionnaire20 within 3 months of the liver biopsy. The NASH Clinical Research Network studies were approved by the Institutional Review Boards at each participating center.
Liver Histology.
The primary outcome in this study was liver histology in patients with NAFLD. All liver biopsy specimens were stained with hematoxylin-eosin and Masson's trichrome stains, and reviewed and scored centrally by the Pathology Committee according to the published NASH Clinical Research Network scoring system.21 For the analyses, fibrosis stage 1a, 1b, and 1c were combined and treated as stage 1.
Dietary Information.
Although sugar-sweetened beverages and fruit or fruit juices account for approximately 50% of total fructose consumption,22 we elected to remain conservative in our data acquisition by limiting our dietary assessment of fructose intake to beverage intake only. Dietary information was obtained via a validated dietary questionnaire (Block food questionnaire, version 1998) as self-reported usual eating habits over the prior year. For the calculation of fructose consumption, we first retrieved frequency (per week) and numbers of servings (per day) of fructose-containing beverages. The number of weekly servings of each drink were calculated as a product of frequency per week and number of servings per day and expressed as servings per week. The number of servings were then combined as total servings of fructose-containing drinks per week and used to estimate individual fructose consumption levels. For the analyses, total weekly servings of fructose-containing drinks were classified into three categories: “non-consumers” (0 servings per week), “minimum to moderate consumers” (>0 and <7 servings per week), and “daily consumers” (≥7 servings per week) of fructose. The amount of fructose consumed was the primary predictor in this study. Estimates of total calories, carbohydrates, protein, and fat intake from the food frequency questionnaire were performed as previously published by Block et al.20
Other Study Variables.
Age, sex, ethnicity, race, body mass index (BMI), fasting lipid profiles (triglycerides, high-density lipoprotein [HDL]-cholesterol, and low-density lipoprotein cholesterol), serum uric acid, fasting serum glucose and insulin, as well as data regarding the use of insulin or insulin-sensitizing agents were collected at study enrollment. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as (fasting glucose [g/dL] × fasting insulin [μU/mL])/405.
Statistical Analyses.
Data are reported as mean ± standard deviation or number (proportion) of patients with a condition. The clinical characteristics between the three categories of fructose consumption were compared using analysis of variance with Tukey's post hoc test or chi-squared tests. The associations between fructose consumption and metabolic features were assessed after adjusting for other potential confounders using multiple linear regression models with two dummy variables (“no fructose consumers” as a reference group) and other potential confounders. The associations between fructose consumption and histological features of NAFLD were assessed using ordinal logistic regression models with and without adjusting for other potential confounders. In the models, four binary logistic curves with different cutoffs (stage 0 versus 1-4, 0-1 versus 2-4, 0-2 versus 3-4, 0-3 versus 4) were modeled, and cumulative odds were computed by pooling a set of β estimates. Three multiple ordinal logistic regression models were developed to assess the associations between fructose consumption and each histological feature: (1) only dummy variables of fructose consumption (unadjusted); (2) the variables in (1) plus age, sex, ethnicity, BMI, and total calorie intake (model 1); and (3) the variables in (2) plus triglycerides, HDL-cholesterol, low-density lipoprotein cholesterol, serum uric acid, and HOMA-IR (model 2). Furthermore, to investigate whether the influence of fructose consumption on liver histology in NAFLD differs depending on age, we assessed the associations between fructose consumption and histological features of NAFLD in different age groups. The study population was divided into two age groups by using a median age value (48 years old). Multiple ordinal logistic regression models (models 1 and 2) were then separately developed in each group. For the analyses in the age subgroups, fructose consumption was classified into two groups, “daily consumers” versus others. For analyses, we used JMP statistical software version 7.0 (SAS Institute Inc.) and considered differences statistically significant when the P-value(s) were less than 0.05. Because of the preliminary nature of this subgroup analysis and small sample size, correction for multiple comparisons was not performed.
Results
Clinical Characteristics of the Study Population.
The clinical characteristics associated with different levels of fructose consumption are summarized in Table 1. Median fructose consumption of the study population was one serving per week (first and third quartiles 0 and 7, respectively). When the study population was classified into the following three fructose consumption categories: “no,” “minimum to moderate,” and “daily” fructose consumers, it became apparent that a significant subpopulation (27.9%) consumed the equivalent of at least one fructose-containing beverage per day. The remaining patients consumed either no fructose-containing beverages (84 individuals, 19.7%), or between one and six servings/week (224 individuals, 52.5%). Higher fructose consumption was univariately associated with younger age, male sex, higher BMI, hypertriglyceridemia, lower HDL-cholesterol, hyperuricemia, and higher total calorie intake (as well as calorie intake of all three different nutrients). Fructose consumption was not associated with fasting serum insulin levels or HOMA-IR; however, “minimum to moderate fructose consumers” were associated with lower fasting serum glucose compared with “no fructose consumers.” In the univariate analyses, no difference in histological features was observed among the fructose consumption groups.
| Fructose Consumption (Reported Servings) per Week | P-Value | |||
|---|---|---|---|---|
| 0 Servings (N = 84) | >0 and <7 Servings (N = 224) | ≥7 Servings (N = 119) | ||
| Age | 53.9 ± 1.2 | 47.5 ± 0.8* | 41.4 ± 1.0*† | <0.0001 |
| Sex (Male, %) | 22.6 | 38.4 | 56.3 | <0.0001‡ |
| Ethnicity (Hispanic, %) | 10.7 | 14.7 | 16.0 | 0.55‡ |
| Race, (White, %) | 88.1 | 79.9 | 80.7 | 0.24‡ |
| BMI, kg/m2 | 33.7 ± 0.7 | 33.6 ± 0.4 | 35.8 ± 0.6† | 0.008 |
| Triglycerides, mg/dL | 190.9 ± 15.7 | 162.9 ± 9.6 | 203.2 ± 13.2† | 0.03 |
| HDL-cholesterol, mg/dL | 47.8 ± 1.3 | 44.0 ± 0.8* | 39.4 ± 1.1*† | <0.0001 |
| LDL-cholesterol, mg/dL | 120.0 ± 3.8 | 118.9 ± 2.3 | 122.0 ± 3.2 | 0.75 |
| Serum uric acid, mg/dL | 5.9 ± 0.2 | 6.1 ± 0.1 | 6.8 ± 0.1*† | <0.0001 |
| Fasting serum glucose, g/dL | 111.9 ± 3.2 | 97.7 ± 2.0* | 101.6 ± 2.7 | 0.0009 |
| Fasting serum insulin, μU/mL | 26.4 ± 3.2 | 23.7 ± 2.0 | 25.7 ± 2.7 | 0.71 |
| HOMA-IR | 7.5 ± 0.8 | 5.8 ± 0.5 | 6.5 ± 0.7 | 0.19 |
| Total calorie intake, Cal/day | 1315 ± 94 | 1727 ± 58* | 2600 ± 79*† | <0.0001 |
| Carbohydrate, Cal/day | 600 ± 45 | 786 ± 27* | 1310 ± 38*† | <0.0001 |
| Protein, Cal/day | 224 ± 16 | 276 ± 10* | 366 ± 14*† | <0.0001 |
| Fat, Cal/day | 513 ± 43 | 690 ± 26* | 951 ± 36*† | <0.0001 |
| Liver histology | ||||
| Steatosis | 0.27‡ | |||
| Grade 0 | 6.0 | 3.1 | 5.0 | |
| Grade 1 | 26.2 | 37.1 | 37.8 | |
| Grade 2 | 31.0 | 34.8 | 31.9 | |
| Grade 3 | 36.9 | 25.0 | 25.2 | |
| Lobular inflammation | 0.16‡ | |||
| Grade 0 | 0 | 0 | 0 | |
| Grade 1 | 39.3 | 45.5 | 54.6 | |
| Grade 2 | 47.6 | 44.2 | 32.8 | |
| Grade 3 | 13.1 | 10.3 | 12.6 | |
| Ballooning | 0.44‡ | |||
| Grade 0 | 23.8 | 29.0 | 31.1 | |
| Grade 1 | 29.8 | 34.8 | 28.6 | |
| Grade 2 | 46.4 | 36.2 | 40.3 | |
| Fibrosis | 0.23‡ | |||
| Stage 0 | 20.5 | 28.6 | 22.2 | |
| Stage 1 | 26.5 | 32.6 | 33.3 | |
| Stage 2 | 21.7 | 19.2 | 24.8 | |
| Stage 3 | 21.7 | 14.7 | 11.1 | |
| Stage 4 | 9.6 | 4.9 | 8.6 | |
- P-values from chi-squared test or ANOVA (‡chi-squared test).
- * P < 0.05 versus “0 servings per week”;
- † P < 0.05 versus “>0 and <7 servings per week.”
Associations Between Fructose Consumption and Metabolic Parameters.
Because there were significant differences in age, sex, and BMI among the fructose consumption categories, we assessed the associations between fructose consumption and metabolic parameters after adjusting for these factors (Table 2). After adjusting for age, sex, and BMI, daily fructose consumption was significantly associated with lower HDL-cholesterol and higher serum uric acid, compared with no fructose consumption; the estimated differences in mean values of these parameters between “no fructose consumers” and “daily consumers” (in other words, β ± SE) were −5.5 ± 1.8 mg/dL (P = 0.002) for HDL-cholesterol and 0.5 ± 0.2 mg/dL (P = 0.03) for uric acid. Compared with the no fructose consumer group, “minimum to moderate fructose consumers” had lower fasting serum glucoses, triglycerides, and HDL-cholesterol; the estimated differences in means between “no fructose consumers” and “minimum to moderate consumers” were −12.2 ± 3.9 g/dL (P = 0.002) for fasting serum glucose, −37.0 ± 18.8 mg/dL (P = 0.05) for triglycerides, and −2.7 ± 1.5 mg/dL (P = 0.07) for HDL-cholesterol. After adjustment for total calorie intake, the difference in serum uric acid between groups (“no fructose consumers” versus “daily consumers”) was no longer significant. However, the differences in serum glucose and lipids persisted (data are not shown). We repeated the same analyses after excluding subjects who were on insulin or insulin-sensitizing agents (n = 70). With the adjustment for age, sex, and BMI, the association between blood glucose levels and fructose consumption was diminished; however, daily fructose consumption remained associated with lower HDL-cholesterol (P < 0.001) compared with no fructose consumption (Table 3).
| Fructose Consumption (Reported Servings per Week) | |||||
|---|---|---|---|---|---|
| 0 Servings | >0 and <7 Servings | ≥7 Servings | |||
| β ± SE | P Value | β ± SE | P Value | ||
| Triglycerides, mg/dL | – | −37.0 ± 18.8 | 0.05 | −1.4 ± 22.0 | 0.95 |
| HDL-cholesterol, mg/dL | – | −2.7 ± 1.5 | 0.07 | −5.5 ± 1.8 | 0.002 |
| LDL-cholesterol, mg/dL | – | −0.2 ± 4.6 | 0.97 | 4.6 ± 5.5 | 0.40 |
| Serum uric acid, mg/dL | – | 0.1 ± 0.2 | 0.60 | 0.5 ± 0.2 | 0.03 |
| Fasting serum glucose, g/dL | – | −12.2 ± 3.9 | 0.002 | −6.7 ± 4.6 | 0.15 |
| Fasting serum insulin, μU/mL | – | −2.5 ± 3.9 | 0.52 | −1.2 ± 4.6 | 0.79 |
| HOMA-IR | – | −1.5 ± 1.0 | 0.13 | −0.8 ± 1.2 | 0.51 |
- Multiple lineal regression models were used to compute adjusted mean differences (versus 0 servings). Adjusted means are presented as β-coefficient ± SE (P-value) in the table.
| Fructose Consumption (Reported Servings per Week) | |||||
|---|---|---|---|---|---|
| 0 Servings | >0 and <7 Servings | ≥7 Servings | |||
| β ± SE | P Value | β ± SE | P Value | ||
| Triglycerides, mg/dL | – | −20.6 ± 19.5 | 0.29 | 9.4 ± 22.7 | 0.68 |
| HDL-cholesterol, mg/dL | – | −3.6 ± 1.7 | 0.04 | −6.5 ± 2.0 | 0.001 |
| LDL-cholesterol, mg/dL | – | −1.4 ± 5.3 | 0.79 | 1.5 ± 6.2 | 0.81 |
| Serum uric acid, mg/dL | – | −0.06 ± 0.21 | 0.77 | 0.45 ± 0.24 | 0.06 |
| Fasting serum glucose, g/dL | – | −1.9 ± 2.8 | 0.50 | −0.8 ± 3.2 | 0.81 |
| Fasting serum insulin, μU/mL | – | 1.9 ± 4.4 | 0.67 | 3.7 ± 5.1 | 0.47 |
| HOMA-IR | – | 0.38 ± 1.01 | 0.71 | 0.96 ± 1.18 | 0.42 |
- Multiple lineal regression models were used to compute adjusted mean differences (versus 0 servings). Adjusted means are presented as β-coefficient ± SE (P value) in the table.
Associations Between Fructose Consumption and Histological Severity of NAFLD.
To investigate relationships between fructose consumption and histological features of NAFLD, we first assessed the associations in the entire study population with and without adjustment for age, sex, Hispanic ethnicity, BMI, total calorie intake, and metabolic parameters. The cumulative odds ratios with 95% confidence intervals (CI) of the fructose consumption categories for steatosis, lobular inflammation, ballooning, and fibrosis are summarized in Table 4. Higher fructose consumption was less likely to be associated with higher histological grades of steatosis; cumulative odds ratios with 95% CI of “minimum to moderate consumers” and “daily consumers” versus “no fructose consumers” were 0.7 [0.4, 1.1] (P = 0.10) and 0.4 [0.2, 0.9] (P = 0.02), respectively (in the full models/model 2). Conversely, daily fructose consumption was more likely associated with higher histological stages of fibrosis; cumulative odds ratios with 95% CI of “daily consumption” versus “no fructose consumption” were 2.6 [1.4, 5.0] (P = 0.004) (in the full models/model 2).
| Unadjusted | Adjusted (Model 1) | Adjusted (Model 2) | ||||
|---|---|---|---|---|---|---|
| OR[95%CI] | P Value | OR[95%CI] | P Value | OR[95%CI] | P Value | |
| Steatosis | ||||||
| Fructose consumption | ||||||
| 0 serving | – | – | – | – | – | – |
| 0-7 servings | 0.7 [0.4, 1.1] | 0.09 | 0.6 [0.4, 0.9] | 0.02 | 0.7 [0.4, 1.1] | 0.10 |
| ≥7 servings | 0.6 [0.4, 1.0] | 0.06 | 0.4 [0.2, 0.8] | 0.007 | 0.4 [0.2, 0.9] | 0.02 |
| Lobular inflammation | ||||||
| Fructose consumption | ||||||
| 0 serving | – | – | – | – | – | – |
| 0-7 servings | 0.8 [0.5, 1.3] | 0.30 | 0.9 [0.5, 1.4] | 0.55 | 0.8 [0.5, 1.4] | 0.53 |
| ≥7 servings | 0.6 [0.4, 1.0] | 0.06 | 0.9 [0.5, 1.8] | 0.86 | 1.1 [0.6, 2.3] | 0.70 |
| Ballooning | ||||||
| Fructose consumption | ||||||
| 0 serving | – | – | – | – | – | – |
| 0-7 servings | 0.7 [0.4, 1.1] | 0.13 | 0.9 [0.5, 1.4] | 0.62 | 0.9 [0.5, 1.5] | 0.73 |
| ≥7 servings | 0.7 [0.4, 1.2] | 0.25 | 1.3 [0.7, 2.4] | 0.44 | 1.4 [0.7, 2.7] | 0.32 |
| Fibrosis | ||||||
| Fructose consumption | ||||||
| 0 serving | – | – | – | – | – | – |
| 0-7 servings | 0.6 [0.4, 0.9] | 0.01 | 0.8 [0.5, 1.3] | 0.44 | 0.9 [0.6, 1.5] | 0.78 |
| ≥7 servings | 0.7 [0.4, 1.2] | 0.19 | 1.7 [1.0, 3.2] | 0.07 | 2.6 [1.4, 5.0] | 0.004 |
- Fructose consumption is expressed as reported servings per week. Cumulative odds ratio (OR) and P-values were derived from ordinal logistic regression models (Model 1: adjusted for age, sex, BMI, Hispanic ethnicity, and total calorie intake; Model 2: adjusted for age, sex, BMI, Hispanic ethnicity, total calorie intake, triglycerides, HDL-cholesterol, LDL-cholesterol, uric acid, and HOMA-IR).
Associations Between Fructose Consumption and Histological Severity of NAFLD in Different Age Groups.
Age or aging-related mitochondrial dysfunction is associated with a decline in the intrinsic metabolic activity of the liver and fibrosis progression.23, 24 Therefore, we further evaluated the association between fructose consumption and histological severity of NAFLD in different age groups to see whether the influence of fructose consumption on liver histology in NAFLD differs depending on age. The cumulative odds ratios with 95% CI of “daily consumption” for steatosis, lobular inflammation, ballooning, and fibrosis are summarized in Table 5. Among older subjects, “daily consumers” were less likely to have higher grades of steatosis (cumulative OR [95% CI] = 0.2 [0.1, 0.5], P = 0.0008 in model 2) and were more likely to have higher grades of lobular inflammation (cumulative OR [95% CI] = 2.5 [1.0, 6.2], P < 0.05 in model 2) and ballooning (cumulative OR [95% CI] = 2.5 [1.0, 6.0], P = 0.05 in model 2). Compared with nonconsumers of fructose beverages, both older and younger “daily fructose consumers” were more likely to have higher stages of liver fibrosis; cumulative OR and 95% CI in model 2 were 3.2 [1.7, 6.1], P = 0.0003 for the younger group and 3.2 [1.4,7.4], P = 0.006 for the older group.
| Younger Group (<48 years old) | Older group (≥48 years old) | |||||||
|---|---|---|---|---|---|---|---|---|
| Adjusted (Model 1) | Adjusted (Model 2) | Adjusted (Model 1) | Adjusted (Model 2) | |||||
| OR [95%CI] | P Value | OR [95%CI] | P Value | OR [95%CI] | P Value | OR [95%CI] | P Value | |
| Steatosis | ||||||||
| Fructose consumption | ||||||||
| <7 servings | – | – | – | – | – | – | – | – |
| ≥7 servings | 1.1 [0.6, 2.0] | 0.72 | 1.0 [0.6, 1.9] | 0.95 | 0.3 [0.1, 0.6] | 0.0009 | 0.2 [0.1, 0.5] | 0.0008 |
| Lobular inflammation | ||||||||
| Fructose consumption | ||||||||
| <7 servings | – | – | – | – | – | – | – | – |
| ≥7 servings | 0.7 [0.4, 1.3] | 0.24 | 0.9 [0.5, 1.8] | 0.83 | 2.1 [1.0, 4.8] | 0.07 | 2.5 [1.0, 6.2] | 0.05 |
| Ballooning | ||||||||
| Fructose consumption | ||||||||
| <7 servings | – | – | – | – | – | – | – | – |
| ≥7 servings | 1.3 [0.7, 2.3] | 0.40 | 1.5 [0.8, 2.8] | 0.19 | 2.1 [0.9, 4.5] | 0.07 | 2.5 [1.0, 6.0] | 0.05 |
| Fibrosis | ||||||||
| Fructose consumption | ||||||||
| <7 servings | – | – | – | – | – | – | – | – |
| ≥7 servings | 2.5 [1.4, 4.4] | 0.003 | 3.2 [1.7, 6.1] | 0.0003 | 2.1 [0.1, 4.3] | 0.05 | 3.2 [1.4, 7.4] | 0.006 |
- Fructose consumption is expressed as reported servings per week. Cumulative odds ratio (OR) and P-values were derived from ordinal logistic regression models (Model 1: adjusted for age, sex, BMI, Hispanic ethnicity, and total calorie intake; Model 2: adjusted for age, sex, BMI, Hispanic ethnicity, total calorie intake, triglycerides, HDL-cholesterol, LDL-cholesterol, uric acid, and HOMA-IR.
Discussion
Recent data suggest that intake of more simple carbohydrates and less saturated fat is higher in patients with NAFLD compared with the general population, suggesting that dietary imbalances play a role in the development and progression of NAFLD.25 The ideal diet for NAFLD should reduce fat mass and inflammation in the adipose tissue, restore insulin sensitivity, and provide low amounts of substrates for de novo lipogenesis26; however, scientific evidence to recommend specific diets is currently lacking. Although prior studies suggest an association between increased fructose consumption with NAFLD, no study has implicated a dietary risk factor in NAFLD progression. Defining modifiable risk factors for liver disease progression in NAFLD would have significant public health implications for the development of strategies that may decrease risk for liver fibrosis and associated health-related complications. Evidence that childhood obesity and pediatric NALFD are becoming epidemic, particularly in young boys who tend to consume soft drinks,27, 28 suggests that there is a significant opportunity to improve risk factors for progressive liver damage at early stages of life.
In this study, we investigated the impact of increased fructose consumption on the metabolic syndrome and histological features of NAFLD. In patients with established NAFLD, increased consumption of fructose was associated with younger age, male sex, increased BMI, increased serum triglycerides, lower HDL cholesterol, and higher uric acid levels. To our surprise, increased fructose consumption appeared to improve systemic insulin sensitivity (in other words, lowered fasting serum glucose, slight decrease in serum insulin. and HOMA-IR). Although this observation was diminished when excluding all subjects requiring insulin or insulin-sensitizing agents, this finding is particularly notable because it was observed despite evidence that daily fructose ingestion was accompanied by a significant increase in daily consumption of total calories, carbohydrates, proteins, and fats, as well as increased BMI. Furthermore, based on our extended analysis, such associations still appeared to exist among subjects who were on insulin or insulin-sensitizing agents (data not shown). The limited sample size in the subgroup and the cross-sectional nature of this analysis limits the ability to draw any conclusions regarding causality or the impact of increased fructose consumption on the natural history of NAFLD. Further studies are required to delineate potential differential influences of fructose consumption on insulin sensitivity. Also, from the time of diagnosis of NAFLD to the time of study participation, patients may have spontaneously initiated lifestyle modification (such as decreased sugar consumption, dietary modification, or increased exercise), which led to improved insulin sensitivity. Although we attempted to decrease the window between liver biopsy and study participation to only 3 months, even a modest dietary change or weight loss could improve insulin sensitivity. Although a dose–response relationship between fructose and low HDL cholesterol was observed, the apparent lack of a dose–response relationship between fructose intake and insulin resistance may potentially be explained by other confounders (such as use of insulin-sensitizing agents or lipid-lowering agents), which may alter peripheral or hepatic insulin sensitivity and decrease hepatic steatosis.
Despite our inability to link increased fructose consumption to worsened insulin resistance, daily fructose consumption was associated with metabolic abnormalities that typically accompany insulin resistance, including lower HDL-cholesterol and higher serum uric acid, even after adjusting for age, sex, and BMI. In this regard, our findings reproduce other reports that have linked such metabolic derangements with increased consumption of fructose.4, 5, 29-34 Moreover, after controlling for factors that have been shown to influence NAFLD (such as age, sex, BMI, Hispanic ethnicity, and total calorie intake), we found that increased fructose consumption was associated with decreased hepatic steatosis and increased fibrosis. When lipid parameters (triglycerides, HDL and low-density lipoprotein cholesterol), uric acid, and HOMA-IR were incorporated into the analytical model, the association of increased fructose intake with decreased steatosis and increased fibrosis persisted. In addition, older subjects (age ≥ 48 years) with NAFLD who consumed increased amounts of fructose (≥ 7 servings/week) had increased lobular inflammation and ballooned hepatocytes. Other studies have also identified older age as an independent predictor of NAFLD severity.35 Together with those data, our results raise the possibility that habitual ingestion of fructose exacerbates liver injury and promotes fibrosis progression in NAFLD. However, the research tools used to collect dietary fructose consumption do not allow us to ascertain whether some other dietary constituent for which fructose is simply a “marker” accounts for our findings.
The concept that excessive consumption of fructose might promote progression of NAFLD is biologically plausible given experimental evidence that high-fructose corn syrup-55 (HFCS-55) increases endoplasmic reticulum stress, promotes activation of the stress-related kinase, Jun N-terminal kinase, induces mitochondrial dysfunction, and increases apoptotic activity36-40 in liver cells. Furthermore, a link between dietary fructose intake, gut-derived endotoxemia, toll-like receptor 4, and NAFLD has been suggested by the results of human and animal studies.17, 41 Mice fed water enriched with 30% fructose develop hepatic triglyceride accumulation, altered markers of insulin resistance, portal endotoxemia, and increased hepatic lipid peroxidation, MyD88, and tumor necrosis factor alpha levels. Such data suggest that fructose-induced NAFLD or NASH associated with intestinal bacterial overgrowth and increased intestinal permeability, subsequently leading to an endotoxin-dependent activation of hepatic Kupffer cells.41 As discussed subsequently, habitual fructose consumption also may lead to an unfavorable energy balance in the liver, which enhances the susceptibility of hepatocytes to injury.42
The lipogenic and proinflammatory effects of fructose appear to be attributable to its unique metabolism, which involves a period of transient adenosine triphosphate (ATP) depletion because of its rapid phosphorylation within the cell and from its unique ability among sugars to raise intracellular and serum uric acid. In experimental animals, lowering uric acid concentrations ameliorated features of the metabolic syndrome induced by fructose, including weight gain, hypertriglyceridemia, hyperinsulinemia and insulin resistance, and hypertension.34 These findings were surprising, because most authorities had considered uric acid to be either biologically inert or an important antioxidant in the plasma.43 However, uric acid was found to have numerous deleterious biological functions. Uric acid stimulates both vascular smooth muscle cell proliferation and the release of chemotactic and inflammatory substances, induces monocyte chemotaxis, inhibits endothelial cell proliferation and migration, and causes oxidative stress in adipocytes, which results in the impaired secretion of adiponectin.1, 44-48 Fructose-related reductions in hepatic ATP also may help to explain why we observed a relationship between chronic ingestion of fructose, hyperuricemia, and NAFLD severity in our patients. However, after adjusting for total calorie intake and other metabolic features, the association between increased fructose consumption and liver injury persisted, suggesting that an alternative mechanism other than hyperuricemia may be involved.
During hepatic fructose metabolism, two molecules of ATP are consumed per each fructose molecule that is metabolized. The resultant adenosine diphosphate is then further degraded to adenosine monophosphate (AMP). The fate of this AMP, in turn, is dictated by the relative activities of two competing enzymes, AMP kinase (AMPK) and xanthine dehydrogenase. When AMPK is more active than xanthine dehydrogenase, AMP is “recycled” to restore hepatocyte ATP content. Conversely, when xanthine dehydrogenase is more active than AMPK, AMP is converted to uric acid, delaying recovery of hepatic ATP stores (Fig. 1). Intravenous administration of fructose to healthy subjects increases blood levels of uric acid, the urinary excretion of urate and xanthine, and acutely reduces hepatic ATP.49, 50 Furthermore, obese patients with NASH were less efficient than healthy controls at recovering from fructose-induced depletion of hepatic ATP stores.51 Exercise, metformin, thiazolidinediones, and adiponectin,12, 52-54 all of which have been shown to improve NASH, activate AMPK. Together, these data support the concept that hepatic AMPK activity is relatively inhibited in NASH, rendering hepatocytes more vulnerable to ATP depletion when ATP is consumed during fructose metabolism. Hence, the presence of hyperuricemia may be a surrogate measure of chronic hepatic ATP depletion in habitual fructose consumers.55 In addition, hyperuricemia has long been recognized as a marker of advanced liver disease.49, 56 More recently, multivariate analysis demonstrated that hyperuricemia is also an independent risk factor for NASH.57 Thus, studies in animals and humans suggest a mechanism by which habitual fructose consumption promotes progression of liver damage by exacerbating underlying abnormalities in hepatic energy homeostasis. Impaired hepatic energy homeostasis (that is, ATP depletion) may also explain the observed associations of increased fructose consumption with decreased steatosis and increased hepatic inflammation; inability to supply ATP for triglyceride synthesis may fail to transform toxic free fatty acids to a safer form of lipids (that is, triglycerides), constrain accumulated free fatty acids in the liver, and thus exacerbate lipotoxicity.
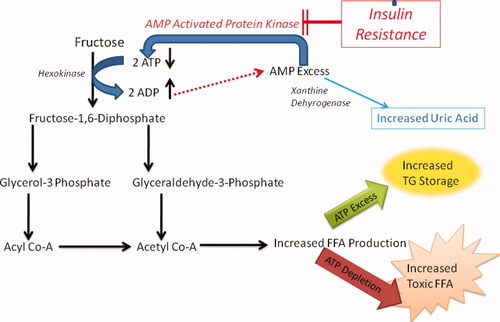
Fructose-associated ATP depletion. For each fructose molecule that is metabolized, two molecules of ATP are consumed. The resultant adenosine diphosphate is then further degraded to AMP. The fate of this AMP is dictated by the relative activities of two competing enzymes, AMP kinase (AMPK) and xanthine dehydrogenase. When AMPK is more active than xanthine dehydrogenase, AMP is “recycled” to restore hepatocyte ATP content. Conversely, when xanthine dehydrogenase is more active than AMPK, AMP is converted to uric acid, delaying recovery of hepatic ATP stores. Insulin resistance, which decreases AMPK activity, further augments the effect of fructose metabolism, resulting in hepatic ATP depletion.
Although further research is necessary to confirm these results and evaluate this hypothesis directly, data from the current cross-sectional analysis are exciting because they not only lend credence to this concept but they suggest both a novel biomarker (serum uric acid) and a modifiable risk factor (dietary fructose) for liver fibrosis in patients with NAFLD. Given the latter, well-designed prospective controlled dietary intervention studies are necessary to evaluate whether a low-fructose diet improves the metabolic disturbances associated with NAFLD and alters the natural history of NAFLD in those at risk of disease progression.
Acknowledgements
Members of the Nonalcoholic Steatohepatitis Clinical Research Network:
Clinical Centers
Baylor College of Medicine, Houston, TX: Stephanie Abrams, M.D.; Diana Arceo, M.D., M.S.; Denise Espinosa; Leanel Angeli Fairly, R.N.
-
MetroHealth Medical Center, Cleveland, OH: Carol Hawkins, R.N.; Yao-Chang Liu, M.D.; Margaret Stager, M.D.
-
Cleveland Clinic Foundation, Cleveland, OH: Arthur McCullough, M.D.; Srinivasan Dasarathy, M.D.; Ruth Sargent, L.P.N.
Seattle Children's Hospital & Research Institute, WA: Melissa Coffey; Karen Murray, M.D.; Melissa Young
Children's National Medical Center, Washington, D.C.: Parvathi Mohan, M.D.; Kavita Nair
Duke University Medical Center, Durham, NC: Manal F. Abdelmalek, M.D., M.P.H.; Anna Mae Diehl, M.D.; Marcia Gottfried, M.D. (2004-2008); Cynthia Guy, M.D.; Paul Killenberg, M.D. (2004-2008); Samantha Kwan; Yi-Ping Pan; Dawn Piercy, F.N.P.; Melissa Smith
-
Riley Hospital for Children, Indianapolis, IN: Elizabeth Byam; Ann Klipsch, R.N.; Jean Molleston, M.D.; Girish Subbarao, M.D.
Johns Hopkins Hospital, Baltimore, MD: Kimberly Pfeifer; Ann Scheimann, M.D.; Michael Torbenson, M.D.
St Louis University, St Louis, MO: Sarah Barlow, M.D. (2002-2007); Jose Derdoy, M.D. (2007-); Joyce Hoffmann; Debra King, R.N.; Andrea Morris; Joan Siegner, R.N.; Susan Stewart, R.N.; Brent A. Tetri, M.D.; Judy Thompson, R.N.
University of California San Diego, San Diego, CA: Cynthia Behling, M.D., Ph.D.; Lisa Clark, Ph.D., M.P.H.; Janis Durelle; Tarek Hassanein, M.D.; Joel E. Lavine, M.D., Ph.D.; Susana Mendoza; Jeffrey B. Schwimmer, M.D.; Claude Sirlin, M.D.; Tanya Stein, M.D.; Zobeida Palomares
University of California San Francisco, San Francisco, CA: Bradley Aouizerat, Ph.D.; Kiran Bambha, M.D.; Nathan M. Bass, M.D., Ph.D.; Linda D. Ferrell, M.D.; Danuta Filipowski, M.D.; Raphael Merriman, M.D. (2002-2007); Mark Pabst; Monique Rosenthal; Philip Rosenthal, M.D.; Tessa Steel (2006-2008)
University of Washington Medical Center, Seattle, WA: Matthew Yeh, M.D., Ph.D.
Virginia Commonwealth University, Richmond, VA: Sherry Boyett, R.N.; Melissa J. Contos, M.D.; Michael Fuchs, M.D.; Amy Jones; Velimir A. C. Luketic, M.D.; Bimalijit Sandhu, M.D.; Arun J. Sanyal, M.D.; Carol Sargeant, R.N., M.P.H.; Kimberly Selph; Melanie White, R.N.
Virginia Mason Medical Center, Seattle, WA (original grant with University of Washington): Kris V. Kowdley, M.D.; Jody Mooney, M.S.; James Nelson, Ph.D.; Sarah Ackermann; Cheryl Saunders, M.P.H.; Vy Trinh; Chia Wang, M.D.
Washington University, St. Louis, MO: Elizabeth M. Brunt, M.D.
Resource Centers
National Cancer Institute, Bethesda, MD: David Kleiner, M.D., Ph.D.
National Institute of Child Health and Human Development, Bethesda, MD: Gilman D. Grave, M.D.; Terry T. K. Huang, Ph.D., M.P.H.
National Institute of Diabetes, Digestive and Kidney Diseases, Bethesda, MD: Edward Doo, M.D.; James Everhart, M.D., M.P.H.; Jay Hoofnagle, M.D.; Patricia R. Robuck, Ph.D., M.P.H. (Project Scientist); Leonard Seeff, M.D.
Johns Hopkins University, Bloomberg School of Public Health (Data Coordinating Center), Baltimore, MD:
Patricia Belt, B.S.; Frederick L. Brancati, M.D., M.H.S.; Jeanne M. Clark, M.D., M.P.H.; Ryan Colvin, M.P.H.; Michele Donithan, M.H.S.; Mika Green, M.A.; Rosemary Hollick (2003-2005); Milana Isaacson; Wana Kim; Alison Lydecker, M.P.H. (2006-2008); Pamela Mann, M.P.H.; Laura Miriel; Alice Sternberg, Sc.M.; James Tonascia, Ph.D.; Aynur Ünalp-Arida, M.D., Ph.D.; Mark Van Natta, M.H.S.; Laura Wilson, Sc.M.; Katherine Yates, Sc.M.




