Sites and mechanisms of insulin resistance in nonobese, nondiabetic patients with chronic hepatitis C†
Potential conflict of interest: Nothing to report.
Abstract
Chronic hepatitis C (CHC) has been associated with type 2 diabetes and insulin resistance, but the extent of impairment in insulin action, the target pathways involved, and the role of the virus per se have not been defined. In this study, we performed a euglycemic hyperinsulinemic clamp (1 mU · minute−1 · kg−1) coupled with infusion of tracers ([6,6-2H2]glucose, [2H5]glycerol) and indirect calorimetry in 14 patients with biopsy-proven CHC, who were selected not to have any features of the metabolic syndrome, and in seven healthy controls. We also measured liver expression of inflammatory cytokines/mediators and tested their association with the metabolic parameters. Compared to controls, in patients with CHC: (1) total glucose disposal (TGD) during the clamp was 25% lower (P = 0.003) due to impaired glucose oxidation (P = 0.0002), (2) basal endogenous glucose production (EGP) was 20% higher (P = 0.011) and its suppression during the clamp was markedly reduced (P = 0.007), and (3) glycerol appearance was not different in the basal state or during the clamp, but lipid oxidation was less suppressed by insulin (P = 0.004). Lipid oxidation was higher in patients with CHC who had more steatosis and was directly related to EGP, TGD, and glucose oxidation. The decreased insulin-stimulated suppression of EGP was associated with increased hepatic suppressor of cytokine signaling 3 (SOCS3; P < 0.05) and interleukin-18 (P < 0.05) expression. Conclusion: Hepatitis C infection per se is associated with peripheral and hepatic insulin resistance. Substrate competition by increased lipid oxidation and possibly enhanced hepatic expression of inflammatory cytokines/mediators could be involved in the defective glucose regulation. (HEPATOLOGY 2009.)
Type 2 diabetes (T2DM) is a frequent complication of hepatitis C virus (HCV) infection, second in prevalence only to cryoglobulinemia. The first report by Allison et al.1 of an increased prevalence of T2DM in HCV-positive subjects with cirrhosis has been repeatedly confirmed also at earlier stages of liver disease.2, 3 In a large survey study,3 HCV-positive subjects older than 40 years were three times more likely than HCV-negative controls to have diabetes. In a longitudinal study, the risk of developing diabetes was up to 12 times higher in patients who were HCV-positive than in the general population.4
HCV is associated with insulin resistance (IR) also in nondiabetic patients. In a prospective study including 600 consecutive patients with chronic hepatitis C (CHC),5 IR assessed using the Homeostasis Model Assessment of IR (HOMA-IR) was found in 32% of patients without diabetes and was associated with both the metabolic syndrome (MS) and with high serum HCV RNA levels, suggesting a possible interaction of viral replication with insulin action. The improvement of IR in patients with sustained viral response to antiviral treatment lends further support to this hypothesis.6
Experimental data suggest a direct interference of HCV with the insulin signaling cascade via proteasomal degradation of the insulin receptor substrate-1 (IRS-1) and IRS-27 and their functional impairment through increased levels of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α).8 In a transgenic mouse model, hepatic insulin resistance was induced by the sole expression of the HCV core protein,8 and a defect in IRS-1 tyrosine phosphorylation was observed in liver biopsy specimens from patients with CHC.9 The mechanisms by which HCV per se may induce the development of IR has been explored in in vitro studies, in animal models, and in liver biopsies, but the surrogate markers of IR so far used in human studies do not provide comprehensive information about the in vivo action of insulin. Moreover, in most population-based or cohort studies, the establishment of a definite link between HCV infection and glucose abnormalities is hampered by the presence of additional risk factors such as obesity, advanced age, and liver damage.
IR classically involves multiple sites: the muscle, where it decreases glucose uptake and utilization, the liver, where it is responsible for the overproduction of glucose despite fasting hyperinsulinemia, and the adipose tissue, where lipolysis is not adequately suppressed by insulin, with subsequent release of glycerol and nonesterified fatty acids (NEFAs) into the bloodstream. The gold standard method for the measurement of insulin sensitivity in vivo is the euglycemic hyperinsulinemic clamp, which, coupled with tracer technique, provides a complete picture of the metabolic actions of insulin.10, 11
In this study, we selected a group of patients with CHC who were not obese, and did not have diabetes or cirrhosis to explore the extent, sites, and mechanisms of IR and to assess the relationship with hepatic steatosis, liver damage, and local expression of inflammatory cytokines/mediators.
Abbreviations
adipose IR, adipose insulin resistance index; BMI, body mass index; CHC, chronic hepatitis C; EGP, endogenous glucose production; FFM, fat-free mass; FM, fat mass; HCV, hepatitis C virus; IR, insulin resistance; IRS, insulin receptor substrate; MS, metabolic syndrome; NAFLD, nonalcoholic fatty liver disease; NEFA, nonesterified fatty acid; Ra, rate of appearance; SOCS3, suppressor of cytokine signaling 3; T2DM, type 2 diabetes mellitus; TGD, total glucose disposal; VCO2, rate of CO2 production; VO2, rate of O2 consumption.
Patients and Methods
Study Subjects.
Fourteen subjects with biopsy-proven CHC (seven infected by genotype 3 and seven by genotypes other than 3 [genotype non-3]) were recruited for this study. CHC was defined by detectable anti-HCV and serum HCV RNA. HCV genotyping was performed with a second-generation reverse hybridization line probe assay (Inno-Lipa HCVII; Innogenetics, Belgium). Patients with concurrent active hepatitis B virus (HBV) (who were positive for hepatitis B surface antigen and core antibody), human immunodeficiency virus infection, autoimmune hepatitis, and/or cholestatic or genetic liver disease were excluded. Alcohol consumption, assessed by interviews extended to family members and general practitioners, was lower than 20 g/day. None of these patients had been previously treated by antiviral therapy.
To assess the contribution of the virus to IR, patients were selected not to have features of MS according to Adult Treatment Panel III classification.12 No patient had evidence of advanced liver disease at the time of biopsy. The control group consisted of seven healthy subjects (with normal fasting glucose levels, lipid profile, and liver function tests) matched by body mass index (BMI) and body composition. Control subjects were recruited from the hospital staff; all subjects had received immunoprophylaxis for HBV and were periodically tested for HCV antibodies. None had seropositivity for anti-HCV. All participants gave their written consent to the study, which was approved by the Institutional Ethics Committee.
Liver Histology.
Liver biopsy specimens were scored according to Ishak.13 The degree of hepatic steatosis was scored as percentage of affected hepatocytes.
Experimental Protocol.
All experiments were performed in the morning after an overnight fast. Teflon catheters were placed into an antecubital vein for isotope infusion, and into a contralateral dorsal hand vein for sampling. Primed-continuous infusions of [6,6-2H2]glucose (0.22 μmol · minute−1 · kg−1; prime 17.6 μmol · kg−1) and [2H5]glycerol (0.1 μmol · minute−1 · kg−1; prime 1.5 μmol · kg−1) were started and continued at a constant rate throughout the study. Blood samples for the determination of tracer enrichments and substrate concentrations were drawn at 10-minute intervals during the last 30 minutes of the basal period (0–120 minutes).
A euglycemic hyperinsulinemic clamp (insulin infusion rate of 1 mU · minute−1 · kg−1; Humulin R; Eli Lilly, Indianapolis, IN) was performed during the 2 hours following the basal period. Plasma glucose concentration was measured every 5 minutes, and a 20% glucose solution was infused at a variable rate to keep plasma glucose constant at the basal level.10 The 20% glucose solution was enriched with [6,6-2H2]glucose, and the constant infusion of [6,6-2H2]glucose was slowly reduced and stopped 20 minutes into the clamp.11 Blood samples were drawn at 20-minute intervals during the first 80 minutes and every 10 minutes during the last 40 minutes of the clamp.
Indirect Calorimetry.
Using an open-circuit canopy system, rates of CO2 production and O2 consumption were determined for 30 minutes during the basal period and during the last 30 minutes of the clamp. Urine was collected at the end of the study, and the amount of protein oxidized was calculated from urinary nitrogen excretion.
Analytical Determinations.
Plasma glucose levels were measured by the glucose oxidase method (Beckman Instruments, Fullerton, CA; interassay coefficient of variation < 4%). Plasma insulin and C-peptide concentrations were assessed by a double-antibody radioimmunoassay (Diagnostic Products Corp., Los Angeles, CA; interassay coefficient of variation < 13%). Serum cholesterol, triglyceride, glycerol, and nonesterified fatty acid (NEFA) levels were measured enzymatically (Alfa Biotech, Rome, Italy) and by NEFA C assay (Wako Chemicals GmbH, Neuss, Germany).
Deuterium (2H) enrichment of plasma glycerol and glucose was determined, as described,14 on a gas chromatography/mass spectrometry system (Hewlett-Packard 5972; Hewlett-Packard, Palo Alto, CA), selectively monitoring ions at a mass-to-charge ratio (m/z) of 200, 201, and 202 for glucose, and 145 and 148 for glycerol. Isotopic enrichments were expressed as tracer/tracee ratios.15
Data Analysis.
Fat-free mass (FFM) was calculated according to Hume's formula16; fat mass (FM) was obtained as the difference between body weight and FFM. Glucose fluxes were expressed per kilogram of FFM (μmol · minute−1 · kgFFM−1).
In the basal state, endogenous glucose production (EGP) was calculated as the glucose rate of appearance (Ra) according to the steady-state equation for stable isotopic tracers.11, 17 During the insulin clamp, EGP was estimated as the difference between glucose Ra calculated using Steele's equation and the exogenous glucose infusion rate.18 The mean rate of EGP during insulin infusion was added to the glucose infusion rate value during the same period to determine total-body glucose disposal (TGD), which provides a measure of peripheral insulin sensitivity. Change in total glucose disposal (Δ TGD) was calculated as the difference between glucose disposal during the clamp and during the basal state.
Because plasma insulin is a strong inhibitory stimulus for EGP,19 an index of hepatic IR was obtained as the product of EGP and plasma insulin concentration.20
Glucose and lipid oxidation rates were obtained from calorimetric measurements.21 Nonoxidative glucose disposal was calculated as the difference between TGD and glucose oxidation. Posthepatic insulin clearance was calculated by dividing the insulin infusion rate during the clamp by the mean peripheral plasma insulin concentration during the last 40 minutes of the clamp.
Glycerol Ra was determined by using the steady-state calculation both in the basal state and during the last 30 minutes of the insulin clamp.14 Studies tracing both endogenous glycerol and endogenous NEFA have established that plasma glycerol Ra reflects hydrolysis of both adipose tissue and very low density lipoprotein triglycerides, in an approximate proportion of 4:1. With this approximation, glycerol Ra was used as an index of whole-body lipolysis.22
Expression of Selected Genes in Liver Biopsies.
A total of 10 liver biopsies were available for nucleic acid analysis. Biopsies were collected in RNAlater (Ambion Inc., Austin, TX) and stored at −80°C until processing. Total RNA extraction was performed using TRIzol reagent (Invitrogen, CA). A commercial total RNA sample from human normal liver was included in the analysis as an expression calibrator (Human Liver Total RNA; Clontech, Mountain View, CA). For each sample, 1 μg of total RNA was treated with deoxyribonuclease I prior to reverse transcription. Primers and probes sets for the selected housekeeping genes (18S ribosomal RNA, RPL13A, TOP1) and the target genes (TNF-α, interleukin-18 [IL-18], suppressor of cytokine signaling 3 [SOCS-3]) were designed and provided as PerfectProbe Gene Detection Kits (Primer Design, Southampton, UK). Quantitative polymerase chain reactions (PCR) were performed using Quantitect probe PCR mastermix (Qiagen, Hilden, Germany) on the iCycler IQ Real-time Detection System (Bio-Rad Laboratories, Hercules, CA). The instrument protocol was 95°C for 15 minutes followed by 50 cycles of 95°C for 15 seconds, 50°C for 30 seconds, and 72°C for 20 seconds. All samples and controls were tested in duplicate, and at least three separate measurements were performed for each target gene. Upon normalization to the geometric mean of the reference genes, the relative expression of each target gene in the test samples compared to the calibrator sample (relative expression ratio [R] or fold change) was calculated using the Pfaffl method.23
Statistical Analysis.
All data were expressed as means ± standard deviation or median and interquartile range if not normally distributed. Data in figures are plotted as means ± standard error of the mean (SEM) unless otherwise specified. Unpaired t-test or Mann-Whitney U test was used to compare groups; proportions were compared by the χ2 test. The mean value of the last four measurements of the basal and clamp period was used in all the analyses. Pearson's correlation and simple regression analysis were used to test for associations between normally distributed variables, while Spearman's rank test was used for non-normal variables. P values less than 0.05 were considered statistically significant. All analyses were performed using Statview for Windows, version 5.0.1 (SAS Institute Inc., Cary, NC)
Results
Clinical and Biochemical Data.
Patients were nonobese (BMI < 30 kg/m2) and had no features of MS according to the Adult Treatment Panel III criteria (Table 1).12 Four of the patients with CHC and two of the control subjects had a familial history of T2DM. All subjects had a sedentary lifestyle. Basal plasma glucose, insulin, triglycerides, and cholesterol levels were within the normal range in all subjects; the HOMA-IR scores were similar (1.05 ± 0.5 versus 0.97 ± 0.40 in patients with CHC and controls, respectively; P = ns) and patients with CHC had a normal glucose tolerance after a 75-g oral glucose load (5.0 ± 0.6 mmol/L at 120 minutes). Patients with genotype 3 CHC had lower levels of total cholesterol than patients with genotype non-3 (2.72 ± 0.51 versus 3.98 ± 0.74 mmol/L; P = 0.003).
| Variable | Controls (n = 7) | Patients with CHC (n = 14) | P Value |
|---|---|---|---|
| Sex (M/F) | 6/1 | 11/3 | 0.69 |
| Age (years) | 28 ± 6 (20–40) | 39 ± 7 (29–57) | 0.002 |
| BMI (kg/m2) | 23.5 ± 2.6 | 23.2 ± 3.1 | 0.84 |
| Waist (cm) | 82 ± 7 | 85 ± 8 | 0.41 |
| Total fat mass (%) | 27 ± 4 | 27 ± 4 | 0.87 |
| Systolic BP (mmHg) | 118 ± 4 | 116 ± 7 | 0.50 |
| Diastolic BP (mmHg) | 70 ± 4 | 72 ± 3 | 0.21 |
| Biochemical data | |||
| Total cholesterol (mmol/L) | 3.6 ± 0.6 | 3.4 ± 1.0 | 0.56 |
| HDL cholesterol (mmol/L) | 1.3 ± 0.2 | 1.2 ± 0.2 | 0.21 |
| Triglycerides (mmol/L) | 0.7 ± 0.1 | 0.7 ± 0.2 | 0.75 |
| AST (U/L) | 21 ± 3 | 48 ± 21 | 0.004 |
| ALT (U/L) | 23 ± 3 | 97 ± 59 | 0.004 |
| Histological data | |||
| Steatosis (%) | ND | 13 ± 11 (0–30) | – |
| Grading (Ishak) | ND | 4 ± 2 (2–7) | – |
- Data are mean ± SD(range). ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; HDL, high density lipoprotein; ND, not determined.
Histology.
Upon analysis of liver biopsy (Table 1), six patients had negligible hepatic fat infiltration (≤5%), while the remaining eight had a variable degree of macrovesicular steatosis (10%–30%). All subjects had necroinflammation (median grading 4/18, range 2/18-7/18). Fibrosis was observed in 13 patients (median 3/6, range 1/6-4/6); none had evidence of cirrhosis. Both genotype groups had similar degrees of hepatic steatosis (15% ± 10% versus 10% ± 12%, in patients infected with genotype 3 and with genotype non-3, respectively; P = 0.44) and liver injury.
Glucose Metabolism.
Fasting levels of glucose and insulin were similar in patients with CHC and controls (Table 2); during the clamp, insulin increased to similar steady-state levels in the two groups.
| Variable | Control Subjects (n = 7) | Patients with CHC (n = 14) | ||
|---|---|---|---|---|
| Basal | Clamp | Basal | Clamp | |
| Glucose Metabolism | ||||
| Glucose (mmol/L) | 5.1 ± 0.3 | 5.0 ± 0.4 | 4.9 ± 0.3 | 5.1 ± 0.3 |
| Insulin (μU/mL)† | 3.3 (3.1) | 87.2 (36.5) | 4.9 (3.3) | 77.7 (42.6) |
| C-peptide (ng/mL) | 1.94 ± 0.79 | 1.17 ± 0.84 | 1.62 ± 0.75 | 1.23 ± 0.68 |
| Total glucose disposal (μmol·minute−1·kgFFM−1) | 10.4 ± 1.6 | 65.5 ± 8.1 | 12.5 ± 1.6* | 48.0 ± 12.1** |
| Glucose oxidation (μmol·minute−1·kgFFM−1) | 10.6 ± 4.4 | 28.8 ± 4.0 | 5.3 ± 2.3** | 15.9 ± 6.1*** |
| Nonoxidative glucose disposal (μmol·minute−1·kgFFM−1) | 0.0 ± 3.1 | 34.9 ± 9.4 | 7.2 ± 2.4 | 32.2 ± 8.7 |
| EGP (μmol·minute−1·kgFFM−1) | 10.4 ± 1.6 | 1.35 ± 1.02 | 12.5 ± 1.6* | 4.75 ± 2.85** |
| Hepatic IR index (μmol·minute−1·kgFFM−1·pM)† | 199 (200) | 402 (874) | 332 (254) | 1804 (2168)* |
| Lipid Metabolism | ||||
| Plasma NEFA (μmol/L) | 626 ± 339 | 96 ± 44 | 542 ± 109 | 111 ± 23 |
| Free glycerol (μmol/L) | 66.7 ± 24.8 | 43.2 ± 26.7 | 45.7 ± 22.3* | 16.9 ± 12.0** |
| β-hydroxy-butyrate (μmol/L) | 39 ± 23 | – | 162 ± 133* | – |
| Glycerol Ra (μmol·minute−1) | 125 ± 28 | 44 ± 10.7 | 159 ± 95 | 51 ± 28 |
| Lipid oxidation (μmol·minute−1·kgFFM−1) | 1.58 ± 0.38 | 0.29 ± 0.38 | 1.73 ± 0.47 | 1.05 ± 0.40** |
- Data are mean ± SD.
- † Data are median (interquartile range).
- EGP, endogenous glucose production; FFM, fat-free mass; IR, insulin resistance; NEFA, nonesterified fatty acid; Ra, rate of appearance.
- * P < 0.05;
- ** P < 0.01;
- *** P < 0.001 versus controls.
Total glucose disposal (TGD), a measure of peripheral insulin sensitivity, was 25% lower in patients with CHC compared to controls irrespective of genotype (Table 2 and Fig. 1A), but the variability was considerable in the CHC group, ranging from the lower end of normal to severely impaired (range 64.6–30.4 μmol minute−1 · kg FFM).
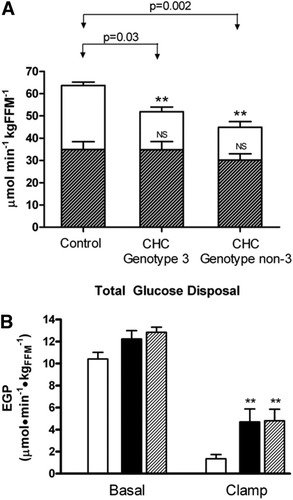
Glucose metabolism. (A) Total glucose disposal. The proportional contribution of oxidative (open bars) and nonoxidative (shaded bars) glucose pathways to total glucose disposal (represented by the entire bar) in the control and CHC groups during the insulin clamp. Bars represent respective mean (± SEM). **P < 0.01 compared to control group for oxidative glucose disposal. (B) Endogenous glucose production (EGP) in the controls (open bars), CHC genotype 3 (black bars), and CHC genotype non-3 (shaded bars) groups in the basal state and during the insulin clamp. Data are represented as mean (± SEM). **P < 0.01 compared to controls during the clamp.
The impairment in TGD in patients with CHC was almost entirely accounted for by a defect in glucose oxidation (Table 2; P = 0.0002) and was already evident even in those patients with a TGD comparable to controls, in whom it was compensated by an increased nonoxidative glucose disposal, i.e., glycogen synthesis (data not shown). This pattern was similar between patients with CHC who were infected with genotype 3 and those with genotype non-3 (Fig. 1A).
Basal EGP was 20% higher in patients with CHC than in controls (Table 2; P = 0.011). During the clamp, the ability of insulin to suppress EGP, i.e., hepatic insulin sensitivity, was markedly reduced in patients with CHC in both genotype groups (Table 2 and Fig. 1B; P = 0.0069), resulting in a 3.5-fold higher EGP than controls. The hepatic IR index was three-fold increased during the clamp in patients with CHC (Table 2; P < 0.05).
Posthepatic insulin clearance was similar between subjects with CHC and control subjects (13.5 ± 5.2 versus 12.5 ± 4.0 mL · minute−1 · kg−1, P = 0.66) and plasma C-peptide levels were comparable in the basal state and under clamp conditions (basal: 1.2 ± 0.8 versus 1.9 ± 0.8; clamp: 1.2 ± 0.7 versus 1.6 ± 0.7 ng/mL in subjects with CHC versus controls; P = n.s. for both), suggesting normal insulin degradation and insulin-mediated suppression of endogenous insulin release, respectively.
Lipid Metabolism.
In the basal state, circulating NEFA concentrations were comparable in the two groups (Table 2), but plasma-free glycerol was reduced by 30% (P = 0.005) and β-hydroxy-butyrate was increased four-fold (P = 0.04) in patients with CHC. During the clamp, NEFA and glycerol concentrations were inhibited by insulin to the same extent in all patients (Table 2) and similarly in both genotypes (Fig. 2A). Fasting glycerol Ra rates (an index of lipolysis) were marginally increased in patients with CHC, but were appropriately suppressed during the clamp. Basal rates of whole-body lipid oxidation were not different between the two groups, but the ability of insulin to suppress lipid oxidation was significantly reduced in patients with CHC compared to controls (Table 2; P = 0.004) irrespective of genotype (Fig. 2A).
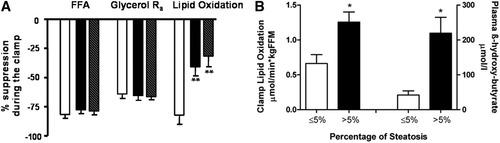
Lipid metabolism. (A) Percent suppression of free fatty acids (FFA), rate of appearance of glycerol (Glycerol Ra), and lipid oxidation during the insulin clamp in the control group (open bar), CHC genotype 3 (black bar), and CHC genotype non-3 (shaded bar) groups. Data are represented as mean (±SEM). **P < 0.001 when compared to controls. (B) The increase in lipid oxidation during the insulin clamp (P = 0.02) and intrahepatic lipid oxidation (P = 0.01) in patients with steatosis (black bar, n = 8) compared to those without steatosis (open bar, n = 6). Data are represented as mean (±SEM).
Correlations.
In this lean, nondiabetic CHC patient group, no correlations were observed between the degree of peripheral (muscle) IR or hepatic IR and BMI, percentage of body fat, viral load, amount of fatty infiltration of the liver, or histological grading and staging of disease severity. In the pooled CHC and controls dataset, the increase in total glucose disposal (Δ TGD) during the clamp was directly related to the increase in glucose oxidation (r = 0.76, P = 0.0001; Fig. 3A) and inversely related to the suppression of lipid oxidation (r = −0.72, P = 0.0005; Fig. 3B), and EGP was positively related to lipid oxidation (P < 0.0001) in both the control (P = 0.0002) and CHC (P = 0.0009) groups (Fig. 3C).
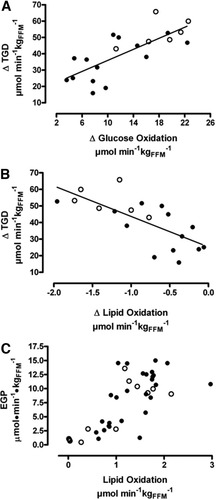
Correlations between the delta total glucose disposal (ΔTGD = change between clamp and basal conditions) with (A) delta glucose oxidation (r = 0.76, P = 0.0001) and (B) delta lipid oxidation (r = −0.72, P = 0.0005) in the whole population (controls, open circles; CHC, black circles.). During both basal and clamp conditions, endogenous glucose production (EGP) was significantly associated with lipid oxidation (C) in controls (P = 0.0002) and patients with CHC (P = 0.0009).
When patients were split by amount of steatosis (irrespective of genotype), those with increased liver fat (>5% hepatocytes affected) had significantly enhanced intrahepatic lipid oxidation, reflected by higher plasma β-hydroxy-butyrate levels (219 ± 129 versus 42 ± 27 μmol/L, P = 0.01) as well as increased whole-body lipid oxidation during the clamp (1.26 ± 0.41 versus 0.66 ± 0.31 μmol · minute−1 · kgFFM−1, P = 0.02) compared to those without steatosis (Fig. 2B). Insulin-stimulated whole-body lipid oxidation was associated with plasma β-hydroxy-butyrate (rs = 0.71, P = 0.009) and tended to be associated with more severe steatosis (P = 0.059).
Liver Expression of Cytokines.
Patients had an increased hepatic expression of TNF-α (median, 5.7-fold increase; range two-fold to 10-fold), IL-18 (median, 5.7-fold increase; range three-fold to 11-fold) and SOCS3 (median, 0.84-fold increase; range 0.5-fold to 1.2-fold). The three cytokines were positively correlated with each other. Notably, in patients with CHC, a decreased insulin-stimulated suppression of EGP was associated with increased hepatic IL-18 (r = 0.63, P < 0.05; Fig. 4A) and SOCS3 expression (r = 0.68, P < 0.05; Fig. 4B), whereas the hepatic expression of TNF-α showed only a positive trend (P = 0.09).
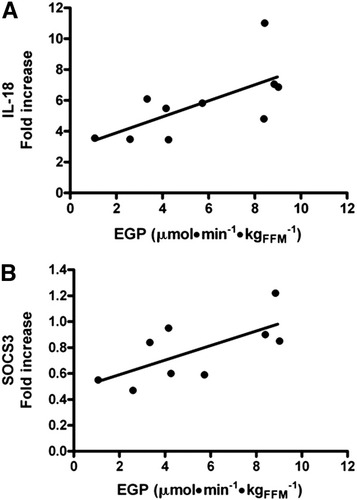
Correlation between endogenous glucose production and inflammatory mediators. The fold increase of hepatic expression of (A) IL-18 and (B) SOCS3 is directly proportional to the amount of endogenous glucose production (EGP) during clamp conditions in patients with CHC (r = 0.63, P < 0.05 and r = 0.68, P < 0.05 respectively).
Discussion
In order to dissect out the contribution of the HCV virus to alterations in insulin action in its target tissues (muscle, liver, and adipose tissue), we studied a highly selected group of patients with CHC with no features of the MS and no histological evidence of cirrhosis, because each of these conditions is itself associated with IR.
The overall results indicate that HCV infection per se is associated with IR in the target pathways of endogenous glucose production (hepatic IR) and total body glucose disposal (peripheral or muscle IR), mainly through the impairment of one of its components (glucose oxidation). Also, the ability of insulin to suppress lipid oxidation is reduced, and increased lipid oxidation is related to impaired glucose disposal.
Hepatic Insulin Resistance.
Insulin exerts a key regulatory action on hepatic glucose production by down-regulating gluconeogenesis. Recent studies have suggested a specific interaction of the HCV core protein with the signaling pathway of insulin in the hepatocyte. A disturbance in tyrosine phosphorylation of insulin receptor IRS-1 has initially been described by Aytug et al.9 in liver biopsy specimens from patients with CHC, resulting in defective phosphoinositide 3-kinase and Akt phosphorylation. In a mouse model transgenic for the HCV core protein,8 insulin failed to inhibit EGP but stimulated muscle glucose uptake normally. Interestingly, in this model, hepatic IR was present before the occurrence of hepatic steatosis, although overt diabetes developed after the onset of steatosis induced by a high-calorie diet.
The results of our study confirm the presence of hepatic IR in all patients examined, irrespective of genotype. The defect is barely evident in the basal state, but highly significant under hyperinsulinemic conditions. Impaired EGP suppression was associated with increased intrahepatic and peripheral lipid oxidation and enhanced hepatic expression of IL-18 and SOCS3.
In normoglycemic individuals, increased gluconeogenesis is the basis for increased EGP and hepatic IR, i.e., rates of glucose release that are inappropriately elevated for the prevailing insulin levels. In the liver, high rates of lipid oxidation provide the energy and biochemical signals to stimulate the gluconeogenic pathway.24 Increased intrahepatic as well as peripheral lipid oxidation are prevalent in subjects with steatosis, possibly as an attempt to remove excess fat in the liver. Although we could not find a direct association between liver fat content and hepatic IR, the significant increase in lipid oxidation observed only in subjects with steatosis lends support to this hypothesis (Fig. 5).
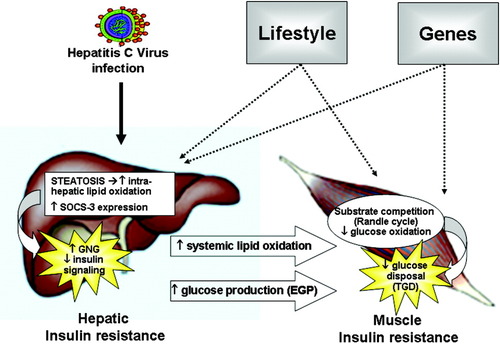
Speculative mechanisms of alterations in insulin sensitivity and glucose metabolism induced by the hepatitis C virus (HCV) in the liver and in the muscle. Hepatic IR in patients with CHC is associated with steatosis and enhanced intrahepatic expression of SOCS3. Steatosis promotes intrahepatic lipid oxidation, which in turn provides the energy and biochemical signals to stimulate gluconeogenesis (GNG) and to increase endogenous glucose production (EGP). SOCS3 can interfere with insulin signaling. Hepatic steatosis is also associated with increased systemic lipid oxidation. In the peripheral tissues (muscle), increased fat oxidation may impede total glucose disposal (TGD) by decreasing glucose oxidation through substrate competition (Randle cycle). The net result is peripheral insulin resistance. The phenotypic expression of metabolic derangements in any individual patient will be determined by the interaction between HCV infection, environment and genetic predisposition (see text for further explanations).
A crucial role of inflammatory cytokines in the pathogenesis of the HCV-associated IR state has been suggested, with most studies involving the role of TNF-α and the suppressor of cytokine signaling 3 (SOCS 3). TNF-α impairs insulin signaling by inhibiting the function of IRS-1 through serine phosphorylation25; but increased circulating and intrahepatic levels of TNF-α occur in chronic viral liver diseases irrespective of etiology. More specific to HCV is the association of hepatic IR and liver SOCS3 expression. In human hepatoma cells, HCV core protein up-regulates SOCS3 and causes proteasomal degradation of both IRS-1 and IRS-2 through ubiquitination.7 Recent reports have related increased plasma levels of IL-18 to obesity, IR, and hypoadiponectinemia.26 IL-18 can suppress adiponectin expression in adipocytes27 and stimulate SOCS3 in the adipose tissue of obese mice.28 With the limitation of the small number of samples available, our results suggest a possible regulation of glucose metabolism in the liver by SOCS3 and IL-18. (Fig. 5)
Peripheral Insulin Resistance.
The ability of insulin to stimulate peripheral glucose uptake, if generally compromised, was very variable in our patients with CHC and was unrelated to viral genotype. The defect was mostly accounted for by decreased glucose oxidation, which in turn was tightly related with the increased (or less suppressed) lipid oxidation. Classically, in peripheral tissues, increased fat oxidation may impede glucose disposal through substrate competition (Randle cycle).29 Although the current results do not speak to cause-effect relationships, the pattern of correlations between lipid oxidation and glucose production/glucose disposal, as measured by independent techniques (calorimetry, tracer infusions, and clamp, respectively), suggest that defective suppression of lipid oxidation plays an important role in the development of both hepatic and peripheral IR of patients with CHC (Fig. 5).
In a previous study in nondiabetic subjects with nonalcoholic fatty liver disease (NAFLD) using the same methodology, total glucose disposal was reduced by 45%, whereas the dose-response curve for insulin-mediated hepatic suppression was only slightly different from the control group.14 With the understanding that a direct comparison is inappropriate, it appears that in the two models (NAFLD and CHC), target tissues are differently affected by IR, with more prominent hepatic resistance in CHC and dominant peripheral resistance in NAFLD. One potential explanation is that the subjects with NAFLD in the previous study were heavier (by ∼8 kg) than the current patients with CHC. The larger fat mass in NAFLD would add a quota of peripheral IR but cannot provide an explanation to the increased degree of hepatic IR observed in patients with CHC. In our lean subjects with CHC, the primary insult is to the liver and the resulting IR may spread to the peripheral tissues and be superimposed on whatever degree of IR pre-existed in any given individual. Coherent with this interpretation is the wide range of TGD that was measured in the present group of patients. A potential confounder in the comparison of the two studies is the different duration of the clamp period. In the NAFLD study, the longer, two-stage clamp may have been more accurate at measuring glucose rate of disappearance, while the 2-hour clamp in the present study might be too short to adequately label the glucose pool, although glucose infusion rates reached a plateau during the last 40 minutes of the clamp (data not shown).
Lipid Metabolism.
The pattern of lipid metabolism in our patients with CHC showed the same general relations as described in NAFLD, namely, the positive association of increased lipid oxidation with decreased glucose oxidation on the one hand and increased EGP on the other. However, the lipid abnormalities were less marked than in NAFLD, because plasma NEFA levels and glycerol Ra were closer to control values. This quantitative difference may be ascribed to the high dose of insulin infused or again to less adiposity; nevertheless, some qualitative difference could be identified. In NAFLD, the main source of hepatic fat accumulation is increased whole-body lipolysis, whereas in patients with CHC it may be related to host factors or to a “steatogenic” effect of the virus. However, only half our patients had significant liver fat accumulation, and hypertriglyceridemia was conspicuously absent despite the IR. This metabolic picture is reminiscent of the one observed in liver-specific insulin receptor knockout mice.30 In these mice, insulin cannot suppress gluconeogenesis, and thus the liver continues to secrete glucose. Insulin also cannot activate SREBP-1c (sterol regulatory element binding protein 1c), which enhances transcription of genes required for triglyceride biosynthesis, and circulating very low density lipoproteins are low. The net result is hyperinsulinemia and hyperglycemia without hypertriglyceridemia. In patients with CHC, the low levels of triglycerides are partially due to entrapment of lipids in the liver mediated by the HCV core protein.31 As a consequence, lipid oxidation is more selectively increased in the liver as compared with peripheral tissues. In support of this contention is the observed correlation between amount of liver fat and raised β-hydroxy-butyrate levels.
Although the increased liver fat is an evident explanation for the increased intrahepatic lipid oxidation, we can only speculate the reason(s) for the correlation between liver fat and systemic lipid oxidation and for its impact on peripheral insulin sensitivity, because muscle biopsies were not performed. Ectopic liver fat per se may activate some signaling aimed at increasing local as well as whole-body lipid oxidation. Leptin and adiponectin are classical mediators of metabolic information, but the sympathetic nervous system plays a major role in regulating energy expenditure and lipolysis.32 A recent study showed that neuronal pathways, consisting of the afferent vagus from the liver and efferent sympathetic nerves to adipose tissues, are involved in the regulation of energy expenditure, insulin sensitivity, glucose metabolism, and fat distribution between the liver and the periphery.33 In this setting, increased liver fat content would act as a signal of “energy overload” and activate whole-body lipid oxidation. We may thus speculate that the variable degree of peripheral IR in patients with CHC is induced by hepatic steatosis. This possibility needs to be further investigated in ad hoc studies.
In summary, CHC differs somewhat in sites/degree of IR from other well-characterized insulin-resistant states, suggesting that the underlying mechanisms may, at least in part, differ, although the duration of the clamp study and the insulin dose used might have potentially amplified the differences in glucose and glycerol kinetics. In any case, other factors are frequently superimposed to the metabolic disturbances caused by the virus per se, such as the degree of liver inflammation and fibrosis and the classical risk factors for T2DM, all of which contribute to IR. This suggests that HCV infection has the potential to “trigger” the phenotypic expression of metabolic derangements on a genetically determined, environmentally induced, susceptible soil (Fig. 5). Further genetic and mechanistic studies are required to qualify this hypothesis.
Acknowledgements
The authors are indebted to Barbara Uberti, Demetrio Ciociaro, Maura Pettiti, and Emma Buzzigoli for their technical support.




