Hepatocellular adenoma management and phenotypic classification: The Bordeaux experience†
Potential conflict of interest: Nothing to report.
Abstract
We took advantage of the reported genotype/phenotype classification to analyze our surgical series of hepatocellular adenoma (HCA). The series without specific known etiologies included 128 cases (116 women). The number of nodules varies from single, <5, and ≥5 in 78, 38, and 12 cases, respectively. The resection was complete in 95 cases. We identified 46 HNF1α-inactivated HCAs (44 women), 63 inflammatory HCAs (IHCA, 53 women) of which nine were also β-catenin–activated, and seven β-catenin–activated HCAs (all women); six additional cases had no known phenotypic marker and six others could not be phenotypically analyzed. Twenty-three of 128 HCAs showed bleeding. No differences were observed in solitary or multiple tumors in terms of hemorrhagic manifestations between groups. In contrast, differences were observed between the two main groups. Steatosis (tumor), microadenomas (resected specimen), and additional benign nodules were more frequently observed in HNF1α-inactivated HCAs (P < 0.01) than in IHCAs. Body mass index > 25, peliosis (tumor), and steatosis in background liver were more frequent in IHCA (P < 0.01). After complete resection, new HCAs in the centimetric range were more frequently found during follow-up (>1 year) in HNF1α-inactivated HCA. After incomplete resection (HCA left in nonresected liver), the majority of HCA remained stable in the two main groups and even sometimes regressed. Six patients of 128 developed hepatocellular carcinoma (HCC) (all were β-catenin–activated, whether inflammatory or not). Conclusion: There were noticeable clinical differences between HNF1α–inactivated HCA and IHCA; there was no increased risk of bleeding or HCC related to the number of HCAs; β-catenin–activated HCAs are at higher risk of HCC. As a consequence, we believe that management of HCA needs to be adapted to the phenotype of these tumors. (HEPATOLOGY 2009.)
Hepatocellular adenomas (HCAs) are benign monoclonal tumors occurring essentially in young women taking oral contraceptives (OCs). These tumors bleed rather frequently and transform rarely into hepatocellular carcinoma (HCC). Traditionally HCA are transferred to surgical departments for different purposes including (1) emergency, (2) advice for surgery, and (3) identification of nodules recently discovered with no firm radiological diagnosis.
Several molecular features associated with HCA have recently been described (for review, see Rebouissou et al.1). Recurrent mutations were identified in the TCF1 (transcription factor 1) gene encoding the hepatocyte nuclear factor 1α (HNF1 α), in the CTNNB1 (catenin beta-1) gene coding for β-catenin,2 and recently in the IL6ST (interleukin 6 signal transducer) gene, which encodes the signaling coreceptor gp130.3 Genotyping allowed the identification of three subtypes: HNF1α-inactivated (35%-50% of cases), β-catenin–activated (15%-18% of cases), and inflammatory (40%-55% of cases) HCAs which can be identified by immunohistochemistry on paraffin-embedded material4; furthermore, HNF1α and inflammatory adenomas (IHCAs) often display pathological characteristics, and we have already shown that in IHCA, patients were significantly overweight as compared to patients with HNF1α HCA.4 Less than 10% of HCAs do not express any of these phenotypic markers. Apart from the risk of HCC in the β-catenin group,2 we did not report information concerning patient sex, number, size, hemorrhagic risk, and regression of HCA among groups in general and in the two major phenotype subgroups in particular.
The aim of this study was to review the whole Bordeaux series of surgically treated HCAs and analyze clinical data according to the genotype/phenotype classification.
Abbreviations
BMI, body mass index; CRP, C-reactive protein; FNH, focal nodular hyperplasia; HCA, hepatocellular adenoma; HCC, hepatocellular carcinoma; HNF1α, hepatocyte nuclear factor 1α; IHCA, inflammatory hepatocellular adenoma; LFABP, liver fatty acid binding protein; OC, oral contraceptive; SAA, serum amyloid A.
Patients and Methods
Patients.
From January 1984 to October 31, 2008, 135 cases were retrieved from our pathological files (eight before 1990, 36 from 1990 to 1999, and 91 from 2000 to October 31, 2008). Since 2006, immunohistochemistry was performed routinely.4 Thereafter, ancient cases have been retrospectively analyzed. Briefly, taking into account good genotype/phenotype correlations, we classified our cases as follows: (1) nodules lacking liver fatty acid binding protein (LFABP) expression as a presumably HNF1α-mutated subtype; (2) nodules with aberrant nuclear β-catenin expression as presumably β-catenin–mutated; and (3) nodules expressing inflammatory proteins (serum amyloid A [SAA] and/or C-reactive protein [CRP]) as IHCAs. Some of these IHCAs also expressed aberrant nuclear β-catenin; therefore, this group was identified as presumably β-catenin–mutated IHCAs; nodules without any of these above mentioned phenotypic markers were reported as unclassified HCAs. Finally, in six cases, nodules were not suitable for immunohistochemistry analyses because of massive hemorrhage and/or necrosis. In 80% of the cases where genotyping had been performed, the immunohistochemical classification was confirmed.
Methods.
A total of 135 cases constituted the basis of this study. The gold standard for the diagnosis of HCA is the pathological examination of the resected specimen using classical criteria. We used immunohistochemical markers such as LFABP, SAA, CRP, glutamine synthetase, and β-catenin for diagnosis purposes, as well as for the classification of HCA (see below). For differential diagnosis with focal nodular hyperplasia (FNH), we sometimes used other antibodies such as anti-cytokeratin 7 (CK7) and anti-CK19 to highlight the characteristic ductular reaction at the interface between parenchyma and fibrotic bands, and analyzed overall glutamine synthetase staining, which exhibited the characteristic distribution pattern of FNH.5 For the differential diagnosis with HCC, we used classical criteria and, if necessary, glypican 3 and MIB-1, particularly when HCA was associated with HCC.
Clinical, surgical, radiological, and pathological data were reviewed and analyzed. The following items were collected (Table 1): age, sex, pregnancy, drugs, contraception, disease known to be related to HCA, body mass index (BMI), mode of discovery (hemorrhage/shock, abdominal pain, discomfort, mass, abnormal liver function tests, by chance), as well as overt consumption of alcohol (more than 30 g/day for women and 60 g/day for men) and tobacco (≥10 cigarettes/day for women or ≥1 pack/day for men for at least 5 years).
| Characteristic | Value |
|---|---|
| Number (women/men) | 128 (116/12) |
| Age median (extremes) | 41 (21/66) |
| Oral contraception > 2 years (≤ 1 year/≤ 2 years) | 100 (78%) (4/2) |
| BMI <25 (≥ 25 <30/≥ 30) | 86 (67.2%) (21/18) |
| Mode of discovery | |
| Bleeding | 23 (18%) |
| Abdominal pain/disconfort | 32 (25%)/4 |
| Mass | 3 |
| Abnormal liver tests | 23 (18%) |
| By chance | 43 (33.6%) |
| Imaging | |
| Solitary/≥ 2 ≤ 5/>5 | 78 (60.9%)/38 (29.7%)/12 (9.4%) |
| Median size (cm) of the largest HCA (extremes) | 7 (1/18) |
| Surgery | |
| Laparotomy/coelioscopy | 102 (79.7%)/26 |
| Major hepatectomy (RH/LH) | 46 (35.9%) (40/6) |
| LL/segmentectomy/tumorectomy | 11/39/10 |
| Association | 22 |
| Additional discovered HCA (≥ 1cm) (solitary/≥ 2 ≤ 5/>5) | 13 (3/7/3) |
| Complete resection (≥ 1cm) (solitary/≥ 2 ≤ 5/>5) | 95 (74.2%) (77*/15/3) |
| Number of cases HCA ≥ 5cm (solitary/≥ 2 ≤ 5/>5) | 94 (73.4%) (56/28/10) |
| Pathology (HCA) | |
| Total number of HCA (≥1cm) resected | 207 (194/13†) |
| Maximum HCA resected per case | 14 |
| Hematoma (alone/with hemorrhagic necrosis/with peliosis/with both) | 19 (14.8%) (7/6/4/2) |
| Hemorrhagic necrosis (alone/with hematoma/with peliosis/with both) | 22 (17.2%) (9/6/5/2) |
| Peliosis (alone/with hematoma/with hemorrhagic necrosis/with both) | 39 (30.5%) (28/4/5/2) |
| Steatosis | 74 (57.8%) |
| Micro HCA (≤ 1cm) | 32 (25%) |
| HCC | 5 (all women), 1 bordeline lesion‡ (man) |
| IHC (LFABP [−]/SAA [+]/SAA [+] β-Cat (+)/β-Cat [+]/no marker/NA) | 46/54/9/7/6/6 |
| Other types of nodules: total (FNH/hemangioma/both) | |
| Imaging | 27 (21.1%) (18/9/4) |
| Resected | 32 (25%) (21/11/1) |
| Follow-up (≥ 1 year) | |
| Complete resection (≥ 1cm) | 82 (86.3%) |
| No HCA | 71 (86.6%) |
| HCA (< 1cm/≥ 1cm/both) | 4/3/1 |
| Nodules noncharacterized (HCA–hemangioma–FNH) (< 1 cm/≥ 1 cm) | 2/1 |
| Incomplete resection | 27 (81.8%) |
| No HCA | 5 |
| HCA (≥ 1 cm): stable (number/size)/increase/reduction size | 22 (81.5%): 18/1/3 |
| Nodules noncharacterized (HCA–hemangioma–FNH) (< 1 cm/≥ 1 cm) | 0/0 |
| Death/death-related HCC | 4/2 |
- * One patient with part of his solitary HCA left in place was counted as an incomplete resection (Case 1).
- † A total of 13 nodules > 1 cm were discovered on the resected specimen.
- ‡ Borderline lesion between HCC and HCA.
- Abbreviations: β-cat, β-catenin; BMI, body mass index; FNH, focal nodular hyperplasia; HCA, hepatocellular adenoma; HCC, hepatocellular carcinoma; IHC, immunohistochemistry; LFABP, liver fatty acid binding protein; LH, left hepatectomy; LL, left lobe; NA, not available; RH, right hepatectomy; SAA, serum amyloid A.
We recorded the number of HCAs (or potential HCAs) vizualized by imaging and classified them as solitary or multiple (≤5 or >5) hepatocellular nodules without absolute distinction between HCA, FNH, or HCC, as well as the size of the largest HCA. We arbitrarily defined a 1 cm cutoff to identify nodules by imaging. We also recorded the year and type of surgery. On resected specimens, we searched for hematoma (intranodular and extranodular, rupture of Glisson's capsule), hemorrhagic necrosis, peliosis, sinusoidal dilatation/telangiectasia, and steatosis. In the nontumoral liver, when available, we evaluated steatosis (>20%) and the presence of undetected additional nodules (HCA, FNH, hemangioma). Immunohistochemistry was then used to classify newly discovered nodules when necessary. In six cases, we did not have access to the whole liver but to paraffin blocks only (first pathological examination outside our department).
Follow-Up.
Imaging data reports (ultrasound and/or computed tomography scan, magnetic resonance imaging) were reviewed. The surgical cases performed after October 2007 were not analyzed for the follow-up; however, number and size of nodules <1 cm were recorded, because imaging techniques were recent. Only two patients were lost for follow-up and this occurred shortly after surgery. Finally, among the 135 cases, seven cases with rare or specific conditions were analyzed separately. Statistics were performed in the large series of 128 cases.
Statistics.
All P values < 0.05 were considered statistically significant. Pearson χ2 test was used to assess the possible correlation between the HCA subtype (IHCA versus LFABP) and the following criteria: (1) BMI > 25, (2) tumoral peliosis, (3) tumoral steatosis, (4) nontumoral liver steatosis, (5) alcohol exposure, (6) risk of bleeding, (7) association with FNH, and (8) recurrence after complete resection.
Results
Global Analysis (128 Cases)
The cases included 116 women (106 taking OCs of whom 100 had been for more than 2 years) and 12 men (Table 1). None in this group had gross evidence for diseases specifically linked to HCA or adenomatosis such as glycogenosis, etc. The median age was 41 years. Among women, 86 had children, 30 were smokers, and six drank alcohol in excess. Among men, nine drank alcohol in excess and five were heavy smokers. One woman had surgery in two steps and another had two operations. Forty-two women and seven men showed a BMI > 25. The most common mode of discovery was bleeding, abdominal pain, or by chance. Two patients were hospitalized in internal medicine with fever and an inflammatory syndrome with anemia (one case has been previously published6).
Seventy-eight nodules were solitary and 50 were multiple (38 ≤ 5 cm, 12 > 5 cm). The median size of the largest nodule was 7 cm. In 94 cases, the size of the excised nodules exceeded 5 cm. The great majority of patients had a laparotomy, and 46 had a major hepatectomy. Resection was complete in 95 cases. In one case, a solitary large HCA could not be completely excised. A total of 220 nodules were resected in the 128 patients of the cohort. Nodule pathology differed from one case to another. Considering the global analysis, 57.8% of HCAs were steatotic and 30.5% had peliotic areas. Hematoma and hemorrhagic necrosis was observed in each subgroup, globally in 14.8% and 17.2%, respectively. Cytological abnormalities could be found near necrotic areas, but were mainly obvious in the β-catenin–activated subgroup. HCC or borderline lesions between HCC and HCA were also found only in the latter subgroup. There were five HCCs (all in women: in three cases HCC was associated with HCA; in one case there were multiple HCCs on the resected specimen, and in this patient the size of the main nodule decreased after stopping Danazol; in one case HCC was discovered several years after surgery [see below, case 1]) and one borderline lesion in a man. In addition to HCA > 1 cm, microadenomas (<1 cm, ≥1 mm) were found in 32 cases. The volume of nontumoral liver was highly variable from one case to another, and it was totally absent in 21 cases. Additional FNH and hemangioma were resected in 22 and 12 cases, respectively.
The samples displayed the following characteristics upon immunohistochemical analysis: LFABP-negative (n = 46), SAA/CRP-positive (n = 59), SAA/CRP-positive and β-catenin–positive (n = 9), β-catenin–positive (n = 7), and without specific marker (n = 6). In six cases, the hemorrhage or necrosis was too extensive to rely on the results. Eighty-two cases with complete resection were followed for more than 1 year (median = 82 months; range = 12-245 months). In 71 cases, there were no new nodules. HCAs or nonidentified nodules were observed in eight and three cases, respectively. Among the 27 cases with incomplete resection (nodules left in surrounding liver) during follow-up (median = 49 months; range = 12-163 months), nodules were no longer visible in five cases and were still present in 22 cases. In the great majority of cases, the size remained stable. Fourteen female patients gave birth after surgery (11 after complete resection of their HCA). Two patients are presently pregnant. A woman (case 1) with incomplete resection of one single large HCA died of HCC 12 years after surgery of her HCA. The retrospective analysis of this case showed it was corresponding to a β-catenin–activated IHCA. In our series, four patients died, two of HCC (see below) and the two other deaths being unrelated to HCC.
Analysis According to Subgroups
LFABP-Negative (HNF1α-Inactivated) HCA (Table 2; Supporting Table 1).
There were two men out of 46 cases. These two cases were peculiar: one had a constitutional mutation and a family history of multiple HCA (this case has been previously published7); the other patient had multiple black adenomas and died of lung cancer (case reported8). Bleeding was independent of the number of nodules. Homogeneous steatosis was a common finding, and in 54% of the cases, micronodules were discovered in the resected specimen in addition to the known HCA. In 24% of cases, there were additional type of nodules which were mainly FNH. Median follow-up was 85 months (range = 12-245). One year after complete resection (median = 96 months, range = 24-245), nodules were still detected in nine of 25 cases (36%) during follow-up (median = 1.8 cm; range = 0.7-2.8 cm); some nodules <1 cm were or were not characterized as HCA. After incomplete resection (median = 49 months; range = 12-163 months), the median size of HCAs not removed was 2.7 cm (range = 1-7.5 cm). All persisted with little variation in size. There was no HCC detected.
| Characteristic | LFABP(−) HCA | SAA/CRP(+) HCA* | β-Catenin HCA | HCA | |
|---|---|---|---|---|---|
| Without Specific Marker | Not Suitable for Immunohistochemistry | ||||
| Number of cases (women) | 46 (44) | 63 (53) | 7 (7) | 6 (6) | 6 (6) |
| Median age (extremes) | 40 (23/65) | 41 (25/59) | 35 (21/66) | 28 (24/48) | 47 (35/52) |
| BMI ≥ 25 | 7 | 27† | 1 | 1 | 3 |
| Steatosis (nontumoral liver) | 5 | 24† | 2 | 3 | 2 |
| Bleeding (tumor, liver, peritoneum) | 4 | 10 | 1 | 2 | 6 |
| HCC/death related to HCC/death unrelated | 0/0/2 | 2/2/0 | 3/0/0 | 0/0/0 | 0/0/0 |
| Number of nodules (unique/>5) | 22/5 | 42/6 | 4/1 | 5/0 | 5/0 |
| Median size (cm) of the largest HCA (extremes) | 5.5 (1/14) | 7 (2.8/18) | 8 (3.8/10) | 5.5 (2/8) | 8.5 (2.2/15) |
| Resected HCA (≥5 cm/<5 cm) | 26/20 | 50/13 | 5/2 | 5/1 | 4/2 |
| Complete resection | 31 | 47 | 6 | 6 | 6 |
| Outcome (>1 year) after complete resection | 25 | 41 | 6 | 6 | 5 |
| No HCA | 16 | 40 | 5 | 6 | 5 |
| New HCA/HCC/nodules noncharacterized | 7/0/2 | 0/0/1 | 0/1/0 | 0 | 0 |
| Outcome (>1 year) after incomplete resection | 13 | 13 | 1 | 0 | 1 |
| No HCA | 0 | 4 | 0 | 0 | 1 |
| HCA/HCC/nodules noncharacterized | 13/0/0 | 7/2/0 | 0/0/1 | 0 | 0 |
- * There were 54 cases of SAA/CRP(+) (47 women) and 9 cases of SAA/CRP(+) β-catenin (+) (6 women).
- † P < 0.005.
Inflammatory HCA (SAA/CRP-Positive), Inflammatory (SAA/CRP-Positive) and β-Catenin–Activated HCA (Table 2; Supporting Tables 2a,2b, Respectively).
There were 54 SAA/CRP-positive cases and nine additional cases which were SAA/CRP-positive and β-catenin–activated. Men were present in both groups (seven and three, respectively). There were no major distinguishable differences between the two groups except that HCC (two women cases) and one borderline lesion between HCC and HCA (one man) were found in the SAA/CRP-positive and β-catenin–activated group. Interestingly enough, HCAs of this group occurring in men were always solitary. Cases in the IHCA subgroup with or without additional β-catenin activation were characterized by a BMI > 25 in 45%, peliosis in more than 50%, and by steatosis in the nontumoral liver in 40% of the cases. In 17% of the cases, there were additional types of nodules mainly represented by FNH. One year after complete resection (median = 55 months; range = 13-201 months), nodules were absent in 40 of 41 cases during follow-up. In one case, a centimeter-sized nodule was not precisely characterized. After incomplete resection (median = 52 months; range = 13-201 months), no nodules were seen in four of 13 cases. The majority of those which remained were stable in size and number. The median size of HCA not removed was 2.3 cm (range = 1-9 cm). In one case not taken into account, a 10-cm nodule could not be removed during surgery.
Comparison between LFABP-negative HCA and IHCA with or without β-catenin activation showed differences in many aspects. Patients with IHCA were more obese: BMI > 25: 27/63 = 43% versus 7/46 = 15%; P < 0.01); nodules were more peliotic (33/63 = 52% versus 4/46 = 9%; P < 0.01) and less steatotic (27/63 = 43% versus 38/46 = 82%; P < 0.01). When present, the type of steatosis was quite different: more macrovesicular and heterogeneously distributed in the IHCA subgroup. The nontumoral liver was also more frequently steatotic (24/63 = 38% versus 5/46 = 11%; P < 0.01), and patients were more frequently exposed to alcohol (14/63 = 22% versus 0/46; P < 0.01). In contrast, there was no significant difference in the two subgroups for the bleeding rate (10/63 = 16% versus 4/46 = 9%; P = 0.2) and absence of HCA 1 year after complete resection (40/41 = 98% versus 16/25 = 64%; P < 0.01). After incomplete resection, HCAs were still present in the 13 LFABP-negative cases versus seven of 13 IHCA cases.
β-Catenin HCA (Table 2; Supporting Table 3a).
The seven cases with β-catenin–activated HCA were women. Four cases were discovered by chance. HCAs were solitary in five of the seven cases. There were three cases with HCC (present on the resected specimen). In two cases (one with HCC), women had received Danazol in addition to OCs.
Unclassified HCA (That Is, with no Specific Phenotypic Markers) (Table 2; Supporting Table 3b).
The six cases were all women, of which two were addressed for hemorrhage. HCAs were solitary in five of the six cases. There was no recurrence.
HCA Not Suitable for Immunohistochemistry Analysis (Table 2; Supporting Table 4).
The six cases were all women addressed for hemorrhage; all cases but one were solitary. There was no recurrence.
Rare and Specific Etiologies
There were five men (one child) out of seven cases (Table 3). Among the etiologies known to be linked to HCA, there were three glycogenosis (two underwent transplantation; one did not undergo transplantation with HCC), one had familial adenomatous polyposis coli, one child was treated for aplastic anemia with Danazol, and one who had a portacaval shunt in infancy had hepatoportal sclerosis. Finally, one case (a woman) with Cushing syndrome was classified in this group; she had a β-catenin–activated HCA and died of HCC 5 years after surgery.
| Cases | Age/Sex | Year (Type of Surgery) | Size of the Largest HCA (cm) (Imaging) | Diseases | Pathology IHC | Outcome (2008) |
|---|---|---|---|---|---|---|
| 2 | 43 F | 1986 (RH) | 11.5 | Cushing syndrome 1984 (bilateral) surrenalectomy 1990 HCC reccurence | 1983 biopsy: HCA 1986: HCA and HCC (β-cat+) | (1991)* |
| 11 | 18 M | 1990 (OLT) | 3.5 | Glycogenosis type 1 | adenomatosis (SAA + β-cat+) | Alive |
| 13 | 27 F | 1991 (OLT) | 6 | Glycogenosis type 1 | Adenomatosis (no marker) | (Postoperatively)* |
| 15 | 33 M | 1992 (OLT) | 12 | HPS-Portacaval shunt | 1 HCA (SAA+) | Alive |
| 18 | 27 M | 1993 (V, VI) | 9 | FAP | 1 HCA (SAA + β-cat+) | Alive, no recurrence |
| 20 | 14 M | 1993 (LL) | 5 | Refractory anemia Danazol treatment | 3 HCA (β-cat+) | Alive, no recurrence |
| 73 | 37 M | 2003 (V, VI, VII) | 8 | Glycogenosis type 1 | Adenomatosis (1 HCC - several HCA β-cat+) | Alive, adenomatosis |
- * death.
- Abbreviations: β-cat, β-catenin; FAP, familial adenomatosis polyposis; HCA, hepatocellular adenoma; HCC, hepatocellular carcinoma; HSP, hepatoportal sclerosis; LL, left lobectomy; OLT, orthotopic liver transplantation; RH, right hepatectomy; SAA, serum amyloid A.
Discussion
Since the first publication linking HCA to OCs9 in 1973, and the first article in 1985 individualizing adenomatosis10 as a different entity from HCA and still considered as such,11, 12 the same questions can still be raised. In spite of the fact that a reasonable algorithm has been proposed regarding surgery,13 there is a lack of consensus regarding the best strategies concerning the size of nodules to be removed to prevent complications13-15 and the best way to follow up patients with HCA (uncompletely resected and not resected). The most important findings in recent years was the recognition that telangiectatic FNH was not atypical FNH but a group of HCAs today classified as IHCA16 with specific clinical/biological features such as a high BMI and raised gamma glutamyl transpeptidase and CRP,4 and that mutations could be identified in different HCA subgroups.1-3 The analysis of our series of 135 cases in light of the phenotypic classification clearly revealed that HCA is a heterogeneous entity. To avoid any background noise in data analysis, it appears relevant from a clinical perspective to separately consider HCA that is linked to specific etiologies, such as glycogenosis,17 vascular disorders,18 therapeutics for aplastic anemia,19 or some drugs (other than OCs), all conditions known to induce HCA.
Immunohistochemical Classification/Molecular Analysis
Immunohistochemical classification is based on the correlation between genotype and phenotype. The correspondence between pathological description and immunophenotyping classification was highly concordant most of the time, particularly for the very homogeneous LFABP-negative subgroup with marked steatosis and lobulated contours, as well as for the SAA/CRP-positive HCA subgroup which exhibited inflammatory infiltrate, telangiectasia, thickened arteries, and more or less obvious ductular reaction, as previously described.4 However, if the LFABP-negative phenotype is excellent for correlation with HNF1α inactivation, it does not identify HNF1α constitutional (MODY3) versus somatic mutations. We have to also highlight that some characteristic HNF1α-inactivated HCAs may be difficult to identify by immunohistochemistry when the expression of LFABP in the background liver is low, resulting in a low LFABP ratio in the tumor versus surrounding liver. Another difficulty can occur for some typical IHCAs when the expression of SAA or CRP is faint inside the tumor with little contrast with the surrounding liver or when the nontumoral liver may express SAA or CRP after massive bleeding or portal embolization (unpublished data).
Global Analysis (128 Cases)
There is a strong relationship between the occurrence of HCA and use of OCs. The best arguments are the time concordance between the beginning of OC marketing and the first publications about HCA, and the almost absence of reported cases in Japan where the use of OC pills is limited to medical indications and not for contraception. The number of HCAs has not decreased as predicted with the use of third-generation OCs that contain less estrogen. The increasing use of imaging techniques probably explains in part the lack of expected decline. This study does not bring new data regarding age, predominance of females, the role of OCs, or the rate of complications of either hemorrhage or HCC.
The Phenotypic Classification (Major Subgroups)
This study confirms that LFABP-negative HCA and IHCA are the two main subgroups which differ on clinical and pathological grounds.4 Schematically, LFABP-negative HCAs are more steatotic, and IHCAs are more peliotic and occur more often in overweight patients with liver steatosis. It also confirms that β-catenin–activated HCAs are associated with the risk of HCC.2 The individualization of the two main subgroups, which represents more than 80% of the cases, helps to provide better interpretation of the results than the global analysis.
Comparison between the groups revealed the following interesting data:
Number of HCAs.
LFABP-negative HCA and IHCA are the only two subgroups with a wide range of nodules per case, from solitary to multiple (<5 and >5, respectively). (1) There is no additional risk of bleeding or HCC transformation, whether the nodule is solitary or multiple; (2) the number of detectable HCAs is less than the true number of HCAs because microadenomas are not detected by conventional imaging. Many authors have used the term “adenomatosis” with great freedom regarding this number, some even using the term when there are little more than three nodules.20 The term adenomatosis—if kept and which should mean many HCAs—is not a specific type of HCA. From a clinical point of view, size, genotype, and underlying disease are far more important than number.
HCA in Men.
We observed that men distributed almost exclusively in the IHCA subgroup with or without activation of the β-catenin pathway. These tumors occurring in men were always solitary with no microadenomas in the surrounding liver. The associated factors included alcohol use, tobacco use, elevated BMI, and are those encountered in HCC on noncirrhotic liver.21 We found only two cases in the HNF1α-inactivated subgroup (see above); both were rare cases.
Bleeding Risk.
Our results appear to indicate that there is no difference in the risk of bleeding for IHCA and HNF1α-inactivated HCA (10/63 = 16% versus 4/46 = 9%; P = 0.27). However, the frequency of peliosis (33/63 = 52% versus 4/46 = 4%; P < 0.01) is significantly greater in IHCA than in HNF1α-inactivated HCA. We observed (in imaging and resected HCAs) small hematomas in IHCAs < 5 cm in three cases; however, we do not know if those IHCAs display a higher risk of hemorrhage. Additional data are needed to evaluate if the hemorrhagic risk is the same or different among the two groups.
IHCA and HCC Risk.
There was no death related to HCC in the LFABP group versus two in the SAA/CRP group. The risk of HCC is linked to the mutation of β-catenin. In patients with IHCA, we suspect that β-catenin mutation is a secondary event that occurs at a later time. In men, however, the risk seems high enough that destruction of even small nodules (< 5 cm) appears reasonable.
Growth of Unresected HCA After Stopping OCs.
There are few case reports showing HCA regression after interruption of OCs,22 and there are, to our knowledge, no reports of HCA in old women. Together, these two observations can be reconciled if we accept that regression is a very slow but constant process, particularly after menopause. In this study, we confirmed that nonresected HCAs (usually of small size) remain stable for the great majority of cases, and tend to disappear in a small percentage of cases and in general do not grow. The observation that IHCAs may disappear more rapidly is interesting and needs to be confirmed. Follow-up of patients with LFABP-negative HCAs as well as IHCAs with complete resection can reasonably be stopped a few years after surgery; for those with incomplete resection with no significant change in HCA size during the first years, the follow-up can also probably be performed at longer intervals.
Association of HCA with FNH and Hemangioma.
This study confirmed that HCAs are significantly associated with FNH (P < 0.01), as reported earlier.23 The association with hemangioma is significant (P < 0.01) if we consider a mean hemangioma prevalence of 7% in the general population. These results may, however, be taken with caution in the absence of a control group. The mechanism responsible for this association remains unknown.
Phenotypic Classification (Minor Subgroups)
The other phenotypic subgroups were small. It is, however, important to outline that β-catenin activation (present also in the IHCA subgroup) represents a major potential risk of HCC.2 Unfortunately, we do not have the tools to solve this issue without a liver biopsy yet. The issues that remain unclear concern unclassified HCAs without known phenotypic markers. Thus far, this group appears independent. One patient in six had an elevated BMI. Two patients with a BMI near 25 had liver steatosis. Finally, the unexplored hemorrhagic/necrotic HCA could belong to any of the above groups. It is likely that some cases of this necrotic subgroup belong to the IHCA group; indeed, three cases had a raised BMI, but liver steatosis was observed in one case only.
HCA Within a Specific Context
In this small group of our whole series, we encountered the well-described glycogenosis context,17 children with aplastic anemia exposed to Danazol,19 but also rare entities such as familial adenomatous polyposis coli24 and vascular disorders.18 We did not observe cases in body builders. From this very short series of seven particular cases, we noted again that men were often affected and that β-catenin mutation was a frequent observation associated with a high risk of HCC.
During the past 10 years, great progress has been made concerning HCA.1-4, 6, 7 The organization of clinical data according to phenotypic classification presented in this large series from a single center, if not likely to change our management strategy, still offers new paradigms about the disease. This has led our multidisciplinary team to adopt a novel management decision tree (Fig. 1). The main driving ideas are the role of imaging techniques (magnetic resonance imaging, contrast agent ultrasound)25, 26 combined with clinical and biological data,4 the size and number of nodules, along with biopsy and immunohistochemistry (biopsy and resection).
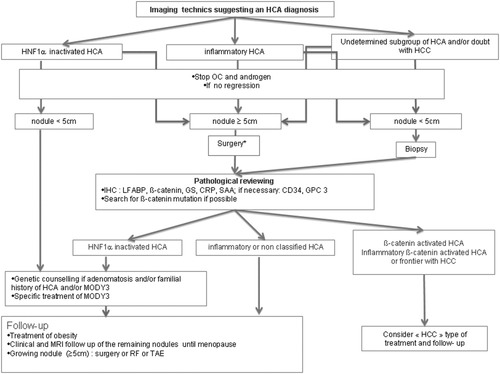
Hepatocellular adenoma (HCA) management decision tree (BORCELINO). This decision tree is based on the results of the radiological investigation25, 26 and/or the pathological phenotype (biopsy, surgical specimen).4 *“Surgery” indicates surgical resection or consideration of biopsy + radio frequency or transcatheter hepatic arterial embolization if surgery is hazardous. BORCELINO, Bordeaux Centre Liver Nodules; CRP, C-reactive protein; GPC3, glypican 3; GS, glutamine synthetase; HCC, hepatocellular carcinoma; IHC, immunohistochemistry; LFABP, liver fatty acid binding protein; MRI, magnetic resonance imaging; OC, oral contraceptives; SAA, serum amyloid A.
- 1
Adenomatosis is not a specific entity as thought before.2 LFABP-negative HCA and SAA/CRP+IHCA, which represents 80% of HCAs, can appear singly or as multiples. Nodules in great number (>10) are most often found in LFABP-negative HCA. It is the size and not the number of nodules which are important to consider for possible complications. Fortunately, cases with multiple large nodules are infrequent. The diagnosis of LFABP-negative adenomatosis should evoke the possibility of MODY3.7
- 2
HCA is a multifactorial disease. Although age, female sex, and use of OCs are the major factors, HCA can still occur in women not taking OCs and in men. HCAs in men are usually SAA/CRP-positive and occur singly, and arise from factors also found in development of HCC. This justifies a more aggressive approach to treatment.
- 3
The risk of HCC is linked to β-catenin mutation. Liver biopsy remains the sole option to detect β-catenin mutation. Reading β-catenin staining on a liver biopsy is difficult, because the staining can be extremely heterogeneous. We rely mainly on glutamine synthetase staining. A strong diffuse staining represents a convincing argument favoring β-catenin mutation. A heterogeneous glutamine synthetase staining is puzzling. If there is no size argument for resection, we recommend a strict follow-up and a rapid destruction if the size of the HCA increased after stopping OCs. The possibility of a biopsy should be discussed for newly discovered nodules in the 3-4 cm range growing in women who have stopped OCs, particularly in those women in the SAA/CRP category. Data are needed to estimate the success rate of the biopsy and of the surrogate markers to classify subgroups. We also need to better understand the link between the β-catenin mutation and the occurrence of HCC. Other factors such as tobacco or alcohol use could play a role.
- 4
The stability or even the regression of small LFABP-negative and SAA/CRP-positive nodules after incomplete resection is a strong argument to modify our surveillance strategy. It is likely that all nodules will tend to disappear with time. However, we still ignore the speed of regression, which may depend on numerous factors (cessation of OCs, menopause, etc.) and if true differences do exist between those two groups.
- 5
The association of HCA and FNH observed particularly in the LFABP-negative and SAA/CRP-positive groups can be a source of difficulties, especially if the size of either type of nodules differ strikingly (i.e., large adenomas and small FNH or vice versa) because the temptation is great to identify all the tumors under the same label.
We definitely need additional data from different centers before modifying our global management strategy, particularly concerning the risk (bleeding and HCC) independent of the size of the nodule. The present data indicate that HCA is a complex entity; as a consequence, we believe that management of HCA needs to be adapted to the phenotypic classification.
Acknowledgements
We thank J. Carles, B. Masson, D. Collet, N. Keroui, J. Drouillard, R. Lecesne, H. Trouette, P.H. Bernard, V. de Lédinghen, and P. Couzigou for contributing to the collection of the clinical, pathological, and radiological data. This work was supported by the Réseau: Génétique des tumeurs hépatocytaires développées sur foie sain (Inserm U674, Paris, France and Université Paris Descartes, Paris, France).




