Diferentially expressed adenylyl cyclase isoforms mediate secretory functions in cholangiocyte subpopulation†
Potential conflict of interest: Nothing to report.
Abstract
Cyclic adenosine monophosphate (cAMP) is generated by adenylyl cyclases (ACs), a group of enzymes with different tissue specificity and regulation. We hypothesized that AC isoforms are heterogeneously expressed along the biliary tree, are associated with specific secretory stimuli, and are differentially modulated in cholestasis. Small duct and large duct cholangiocytes were isolated from controls and from lipopolysaccharide-treated or α-naphthylisothiocyanate–treated rats. AC isoform expression was assessed via real-time polymerase chain reaction. Secretion and cAMP levels were measured in intrahepatic bile duct units after stimulation with secretin, forskolin, HCO3−/CO2, cholinergic agonists, and β-adrenergic agonists, with or without selected inhibitors or after silencing of AC8 or soluble adenylyl cyclase (sAC) with small interfering RNA. Gene expression of the Ca2+-insensitive isoforms (AC4, AC7) was higher in small duct cholangiocytes, whereas that of the Ca2+-inhibitable (AC5, AC6, AC9), the Ca2+/calmodulin-stimulated AC8, and the soluble sAC was higher in large duct cholangiocytes. Ca2+/calmodulin inhibitors and AC8 gene silencing inhibited choleresis and cAMP production stimulated by secretin and acetylcholine, but not by forskolin. Secretion stimulated by isoproterenol and calcineurin inibitors was cAMP-dependent and γ-aminobutyric acid–inhibitable, consistent with activation of AC9. Cholangiocyte secretion stimulated by isohydric changes in [HCO3−]i was cAMP-dependent and inhibited by sAC inhibitor and sAC gene silencing. Treatment with lipopolysaccharide or α-naphthylisothiocyanate increased expression of AC7 and sAC but decreased expression of the other ACs. Conclusion: These studies demonstrate a previously unrecognized role of ACs in biliary pathophysiology. In fact: (1) AC isoforms are differentially expressed in cholangiocyte subpopulations; (2) AC8, AC9, and sAC mediate cholangiocyte secretion in response to secretin, β-adrenergic agonists, or changes in [HCO3−]i, respectively; and (3) AC gene expression is modulated in experimental cholestasis. (HEPATOLOGY 2009)
The intrahepatic biliary tree is lined by cholangiocytes, specialized epithelial cells that regulate the alkalinity1 and volume of bile.2 Cholangiocyte secretion is modulated by autocrine/paracrine signals and gastrointestinal hormones including secretin.3 Cholangiocytes display morphological and functional heterogeneity.4, 5 Small duct cholangiocytes (SDCs) do not contribute to secretin-regulated ductal secretion4, 5 and are involved in the reparative/regenerative reponse to liver damage.2 Large duct cholangiocytes (LDCs) contribute to secretin-regulated bile secretion4, 5 and express the secretin receptor, the cystic fibrosis transmembrane regulator (CFTR), and the Cl−/HCO3− exchanger (AE2).4-7 In addition to secretin, cholinergic and β- adrenergic agonists, as well as the intracellular HCO3− content, influence bile secretion trough the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) pathway.2, 3
Stimulation of secretin receptor promotes cAMP gradient by adenylyl cyclase (AC) and thereby a PKA-dependent phosphorylation of CFTR.2 This is a complex Cl− channel that facilitates Cl− and HCO3− efflux,8 but also performs important cell regulatory functions, such as modulation of apical Cl−/HCO3− exchange,9 vesicular transport10 and apical adenosine triphosphate release.11 The cAMP/PKA pathway also regulates vesicular trafficking, cell proliferation, and signal transduction from ciliary mechanosensors.12 Cholangiocyte AC activity is altered in inflammatory conditions leading to cholestasis.13 The ability of proinflammatory cytokines and nitric oxide to block secretin-stimulated cholangiocyte secretion by inhibiting AC function suggests that ACs play an important role in cholangiocyte pathophysiology.13, 14
Nine membrane-bound isoforms of AC (AC1 to AC9)15 and one cytosolic isoform, known as soluble adenylyl cyclase (sAC), are known.16 AC activity is relevant for many important functions in the liver, but little information is available concerning the distribution and function of AC isoforms in liver cells and their specific role in the regulation of ductal secretion.17 Recently, AC6, AC4, and AC8 were shown to be expressed in cholangiocyte cilia,12, 18 indicating a preferential coupling between ciliary function and specific AC isoforms. AC isoforms have different expression patterns, a unique regulation and respond to specific stimuli. This allows the integration of multiple and contrasting extracellular signals into a net cellular cAMP content. Therefore, understanding the expression profile of ACs in a given cell type is a relevant piece of information.
We have hypothesized that AC isoforms are differentially expressed by cholangiocyte subpopulations, that each AC isoform is functionally coupled to specific mediators able to stimulate bile secretion and that the pattern of expression of ACs is affected by cholestatic injuries. Our results uncover a hitherto unrecognized complexity in the integration of secretory stimuli acting through the cAMP/PKA pathway in the biliary tree both in physiological and pathophysiological conditions.
Abbreviations
AC, adenylyl cyclase; ANIT, α-naphthylisothiocyanate; cAMP, cyclic adenosine monophosphate; CFTR, cystic fibrosis transmembrane regulator; GABA, γ-aminobutyric acid; HEPES, 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid; IBDU, intrahepatic bile duct unit; LDC, large duct cholangiocytes; LPS, lipopolysaccharide; NRC, normal rat cholangiocytes; PCR, polymerase chain reaction; PKA, protein kinase A; PKC, protein kinase C; sAC, soluble adenylyl cyclase; SDC, small duct cholangiocytes; siRNA, small interfering RNA
Materials and Methods
All reagents were obtained from Sigma Chemical Co. unless otherwise indicated. Further details on materials can be found in the Supporting Information. The sAC inhibitor KH7 was a kind gift from Drs. J. Buck and L.R. Levin (Weill Cornell Medical College, New York, NY).19
Animals and Cholestatic Models.
Male Sprague-Dawley rats (150-200 g) were used. Animals received care according to protocols approved by the University of Padua Institutional Veterinary Medicine Service and the Institutional Animal Care and Use Committee of Texas A&M Health Science Center, Texas. Rats were fed a diet containing 0.1% α-naphthylisothiocyanate (ANIT) (70-80 mg/kg) for up to 28 days.20 The endotoxin (lipopolysaccharide [LPS] from Salmonella typhimurium) was given via a single intraperitoneal injection (1 mg/kg in 0.9% sodium chloride)21; control rats received an intraperitoneal saline injection. Rats were anesthetized with pentobarbital sodium (50 mg/kg).
Isolation of Hepatocytes and Small and Large Cholangiocytes and Large Intrahepatic Bile Duct Units.
Hepatocytes were obtained from normal and cholestatic rats as described.22 SDCs (≈8 μm) and LDCs (≈14 μm)5 were purified via counterflow elutriation followed by immunoaffinity separation using a monoclonal antibody.5 Large intrahepatic bile duct units (IBDUs) (mean diameter, 20.8 μm), sealed fragments of isolated bile ducts that retain polarity and secrete into a closed lumen, were prepared and purified as described13 and were plated for 48 hours over a thin layer of Matrigel (Collaborative Research Products, Bedford MA). Cultured normal rat cholangiocytes (NRCs), a polarized rat cholangiocyte cell line, were generated and cultured as described.23, 24
Polymerase Chain Reaction and Quantitative Real-Time Polymerase Chain Reaction Analysis.
Total RNA was isolated from freshly isolated cholangiocytes and NRCs. The following tissues served as controls: kidney for AC1, AC2, AC3, AC4, and AC5; heart for AC6; brain for AC7, AC8, and AC9; and sAC for qualitative polymerase chain reaction (PCR). For details on primers and real-time PCR conditions, see Supporting Table 1 and Supporting Materials and Methods, respectively.
Assessment of Ductular Secretion by Video-Optical Planimetry in IBDUs.
Expansion of IBDU lumen over time was quantified using video-optical planimetry as a measure of ductal secretion rate.13, 25 After a 10-minute baseline period, IBDUs were exposed to secretin (50 nM), forskolin (10 μM), acethylcholine (10 μM), or isoproterenol (10 μM) in the presence or absence of specific inhibitors at the concentrations indicated in the Results and the figures. In experiments studying the effect of isohydric increases in intracellular [HCO3−], cells were equilibrated in HCO3−-free 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES)-buffered media or in KRB-buffer-25 (25 mM HCO3/5% CO2) and exposed for 30 minutes to KRB-buffer-25 or KRB-buffer-50 (50 mM HCO3/10% CO2).7,26,27 Serial images of IBDUs were acquired using a JVC TKC 1380 video camera (Galileo Siscam, Firenze, Italy) every 5 minutes for 30 minutes. Luminal areas were determined from the recorded images, using an ARKON image processor from Nikon (Galileo Siscam). Data are expressed as percent variation over the 30-minute recording compared with baseline.13, 25
RNA Interference Silencing.
Silencer Cy3-labeled custom small interfering RNA (siRNA) for AC8 was purchased from Ambion (Austin, TX) according to a previously published sequence (5′-GGUUUGUCGUCCUAGAAAUTT-3′).26 Scramble negative control was also purchased from Ambion. NRCs were plated on a 10-cm plate at approximately 60% confluence and allowed grow for 24 hours. NRCs were transfected according the manufacturer's protocol with the TransIT-TKO tranfection reagent (Mirus, Madison, WI). The final concentration of siRNA used was 30 nM. Twenty-four hours after transfection, cells were harvested and stimulated with secretin (100 nM) for the analysis of intracellular cAMP levels or processed for the isolation of total RNA. In IBDUs, AC8 siRNA and scramble RNA were added immediately after isolation for 24 hours at a concentration of 30 nM. To silence sAC in IBDUs, we used siRNA against four different sequences of sAC and Alexa fluor 488 negative control siRNA (Qiagen, Valencia, CA)19 using the same procedure described above. Forty-eight hours after transfection, IBDUs were used to measure fluid secretion via videoptical planimetry.
Intracellular Cyclic Adenosine Monophosphate Assay.
cAMP was measured in cell extracts by RIA (see Supporting Materials and Methods for details).
Statistical Analysis.
Results are shown as the mean ± standard deviation. Statistical comparisons were made using the Student t test or analysis of variance with Tukey's post test where appropriate. Graph-Pad software (Biosoft, Cambridge UK) was used, and P < 0.05 was considered significant.
Results
Gene Expression Pattern of AC Isoforms in Normal Cholangiocytes and Hepatocytes.
Expression of messenger RNA for AC isoforms was evaluated in freshly isolated hepatocytes, SDCs, LDCs, and NRCs via qualitative real-time PCR using β-actin as internal control (see Supporting Table 1 for the list of primers). In hepatocytes, expression of all AC isoforms was observed except for AC2 and AC8. AC isoforms 1-3 were not expressed in cholangiocytes that, on the contrary, expressed AC8 (Supporting Fig. 1). Real-time PCR revealed quantitative differences in AC gene expression between SDCs and LDCs. Interestingly, transcripts of the so-called Ca2+-insensitive AC4 and AC7 were, on average, 2.4- and 6.3-fold more abundant (P < 0.05) in SDCs than in LDCs, respectively. On the other hand, transcripts of the Ca2+-inhibitable isoforms (AC5, AC6, AC9) of the Ca2+/calmodulin-activated AC8 and of sAC were more abundant (2.4-, 4.5-, 2.3-, 6.8-, and 5.9-fold, respectively) in LDCs (Supporting Table 2). AC gene expression in NRCs was quantitatively similar to that of large cholangiocytes.
Secretin-Stimulated and Acethylcholine-Stimulated Ductal Secretion and cAMP Production in Normal IBDUs Are Ca2+/Calmodulin-Dependent.
Secretin and acethylcholine stimulate cAMP production and fluid secretion in large cholangiocytes.2-4 The effects of cholinergic agonists on cAMP production in cholangiocytes is [Ca2+]i-dependent. AC8 is the only Ca2+−activated AC expressed by cholangiocytes. Therefore, to study if secretin-stimulated fluid secretion was functionally linked to AC8 in LDCs, we evaluated the effects of inhibition of calmodulin, a Ca2+-binding protein that is required to stimulate AC8.27 As shown in Fig. 1A and Supporting Table 3, exposure of IBDUs to secretin (50 nM), forskolin (10 μM), or acetylcholine (10 μM) stimulated fluid secretion, and the effect was inhibited by the PKA inhibitor H89 (10 μM). In IBDUs stimulated with secretin or acetylcholine, inhibition of calmodulin with ophiobolin-A (1 μM)28 or W7 (1 μM),29 resulted in a significant decrease in secretion. Conversely, inhibition of calmodulin in IBDUs treated with 10 μM forskolin (stimulating all ACs except AC9 and sAC)30, 31 did not affect cholangiocyte secretion (Fig. 1A). Calmodulin may interfere with secretory events other than AC stimulation; therefore, we measured the effects of ophiobolin A and W7 on basal and secretin-stimulated cAMP formation in IBDUs. Ophiobolin A and W7 had no effect on basal cAMP levels, but they decreased secretin-stimulated cAMP production significantly (Fig. 1B). These data indicate that a calmodulin-dependent AC modulates secretin signaling.
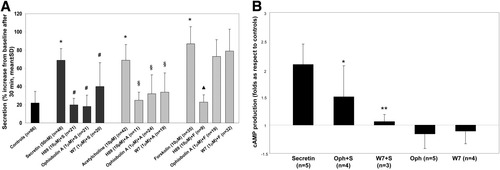
Calmodulin inhibitors diminish secretin- and acetylcholine- but not forskolin-stimulated fluid secretion and intracellular cAMP levels in normal rat IBDUs. (A) Secretion was measured as luminal expansion over time. A, acetylcholine; F, forskolin; S, secretin. H89 is an inhibitor of PKA. *P < 0.001 versus controls. #P < 0.001 versus secretin. §P < 0.001 versus acetylcholine. ▴P < 0.001 versus forskolin. (B) Controls or cells pretreated with ophiobolin-A (1 μM) or W7 (1 μM) were stimulated with secretin (50 nM) for 30 minutes. Ophiobolin-A and W7 inhibited secretin-stimulated increase in cAMP, but not basal levels of cAMP. Columns represent the mean ± standard deviation of indicated replicas normalized to controls. *P < 0.05 versus secretin 50 nM. **P < 0.01 versus secretin 50 nM.
Effect of Secretin on Intracellular cAMP Levels and on Fluid Secretion in AC8-Silenced NRCs and IBDUs.
We used an siRNA approach to test the involvement of AC8 in secretin-stimulated cAMP production and fluid secretion, using NRCs and IBDUs, respectively. NRCs are a polarized normal cholangiocyte cell line whose AC expression resembles that of large cholangiocytes. Using real-time PCR, we established that transfection with AC8 siRNA results in a greater than 90% reduction in the fold expression of the messenger RNA for AC8. Scramble siRNA did not alter basal levels of AC8 gene expression (Fig. 2A). Secretin did not increase cAMP levels in silenced NRCs, whereas secretin increased cAMP levels in NRCs exposed to scramble siRNA (Fig. 2B). Forskolin instead increased cAMP levels in both silenced and nonsilenced NRCs (Fig. 2B). We measured fluid secretion via video-optic planimetry in IBDUs following silencing of AC8. Secretin-stimulated fluid secretion was reduced in silenced IBDUs (68 ± 29% versus 24 ± 16%), whereas the forskolin-stimulated secretion (110 ± 56%) was not (97 ± 46% in forskolin-treated silenced IBDUs) (Fig. 2C). These data provide strong evidence for the role of AC8 in the regulation of secretin-stimulated ductal secretion.
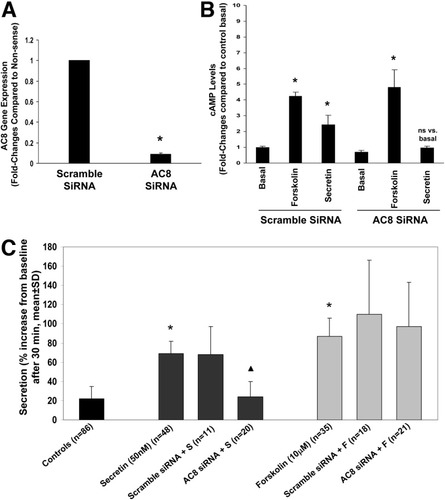
(A) Evaluation of AC8 message expression in NRCs following AC8 silencing. AC8 siRNA results in a >90% reduction in the fold expression in AC8 messenger RNA (mean ± standard error of the mean of three experiments). *P < 0.05 versus scramble SiRNA. (B) Effect of forskolin and secretin on cAMP levels in AC8-silenced NRCs. Contrary to NRCs exposed to scramble siRNA, secretin did not increase cAMP levels in AC8-silenced cells. Forskolin increased cAMP levels of both silenced and nonsilenced NRCs (mean ± SEM of seven experiments). *P < 0.05 versus the corresponding basal value. (C) Effects of AC8 silencing on secretin-stimulated and forskolin-stimulated secretion in IBDUs. A significant reduction in secretin but not forskolin luminal secretion in AC8-silenced IBDUs was found. *P < 0.001 versus controls. ▴P < 0.001 versus secretin + scramble siRNA.
β-Adrenergic–Induced Cholangiocyte Secretion Is Mediated by AC9.
IBDUs exposed to the calcineurin inhibitors FK506 or cyclosporin-A, in the absence of secretin, showed a significant secretory response that was inhibited by treatment with H89 (Fig. 3 and Supporting Table 3). Calcineurin inhibits the activity of AC9, an AC isoform expressed in large cholangiocytes. Consistent with the activation of AC9 by β2-adrenergic receptor agonist isoproterenol, reported in airways epithelial cells,32 we tested the effects of the isoproterenol (10 μM) stimulated secretion in IBDUs. This effect was inhibited by γ-aminobutyric acid (GABA) (10 μM) (Fig. 3 and Supporting Table 3). GABA is known to promote calcineurin-dependent dephoshorylation of AC9,33 indicating that AC9 in LDCs is tonically inhibited by calcineurin-dependent processes, but that it can be activated by β2 agonists and mediates substantial bile secretion.
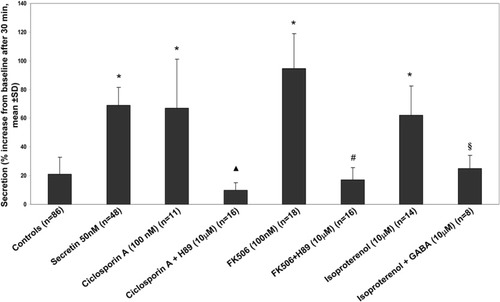
Evidence for β-adrenergic–activated calcineurin-inhibitable AC9. Secretion was measured as luminal expansion in IBDUs as in Figs. 1 and 2A. H89-dependent stimulation of secretion by calcineurin inhibitors cyclosporin A or FK50 (P < 0.001) is consistent with the presence of AC9. Secretion was also stimulated by administration of isoproterenol, a β-adrenergic agonist known to stimulate AC9 in airways32 (P < 0.001). Coadministration of GABA inhibited isoproterenol-stimulated secretion. *P < 0.001 versus controls. ▴P < 0.001 versus cyclosporin A. #P < 0.001 versus FK506. §P < 0.05 versus isoproterenol.
sAC Is Involved in Regulation of Cholangiocyte Secretion.
sAC is not regulated by G-proteins but rather responds to increases in intracellular HCO3− concentrations.34 The dependence of liver cell secretory properties on the presence of HCO3− in the media is a well-known phenomenon, thought to be a consequence of HCO3− transport by Cl−/HCO3− exchange or CFTR.8, 9 Earlier studies have shown that in the presence of HCO3− or cAMP in the culture media, vesicular trafficking to the apical membrane was dramatically increased by cAMP.35 Thus, we studied if sAC played a role in the regulation of cholangiocyte fluid secretion. By inducing isohydric increases in intracellular HCO3− concentrations (i.e., changing HCO3− without changing intracellular pHi), we stimulated fluid secretion in IBDUs, as measured using video-optical planimetry from the changes in ductular area over time.25 As shown in Fig. 4, changing from HEPES-buffered media to KRB 25 mM/5% CO2 increased fluid secretion by 76 ± 34%, whereas changing from 25 mM/5% CO2 to 50 mM/10% CO2 stimulated fluid secretion by 101 ± 34%. The stimulation of secretion achieved by increasing [HCO3−] was almost equal to that stimulated by administration of secretin or forskolin (Fig. 1A). As expected for sAC, these changes were inhibited by H89 (10 μM) by preventing the generation of HCO3− from CO2 with acetazolamide (100 μM) and by the specific sAC inhibitor KH7 (30 μM).19 Moreover, silencing sAC gene expression with siRNA significantly inhibited HCO3-stimulated fluid secretion (increase over 30 minutes = 22 ± 15% and 10 ± 6%, respectively) compared with IBDUs treated with scramble siRNA (Fig. 4). These findings are consistent with the involvement of sAC in the regulation of cholangiocyte fluid secretion.
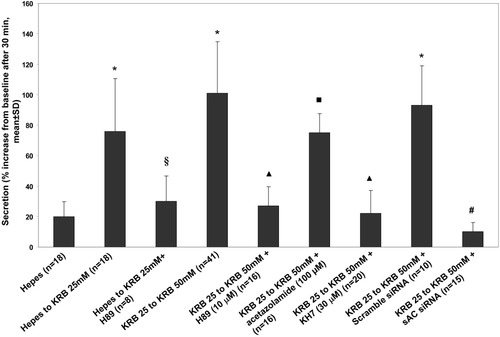
Increases in intracellular bicarbonate concentrations stimulated fluid secretion in normal rat IBDUs. Secretion was measured as in Figs. 1 and 3. To avoid changes in pHi, these maneuvers were conducted in isohydric conditions buffering with 5% CO2 (in 25 mM KRB) or 10% CO2 (in 50 mM KRB). HCO3−-stimulated fluid secretion was inhibited by H89 (10 μM), by the carbonic anhydrase inhibitor acetazolamide (100 μM), by the specific sAC inhibitor KH7 (30 μM), and by gene silencing of sAC with siRNA (50 nM). *P < 0.001 versus HEPES. §P < 0.001 versus HEPES to 25 mM KRB. ▴P < 0.001 versus 25 mM KRB to 50 mM KRB. ▪P < 0.01 versus 25 mM KRB to 50 mM KRB. #P < 0.001 versus 25 mM KRB to 50 mM KRB + scramble siRNA.
Gene Expression of ACs in Cholangiocytes from Cholestatic Rats.
Using real-time PCR, we studied AC isoform gene expression in SDCs and LDCs isolated from rats treated with ANIT or with LPS. LPS causes a cholestasis associated with reduced secretin-stimulated cholangiocyte secretion,13, 14, 36 whereas in ANIT-treated rats, cholestasis results from toxic injury to cholangiocytes and is associated with proliferation of both SDCs and LDCs.20 As shown in Fig. 5, expression of the Ca2+-insensitive, protein kinase C (PKC)-stimulated AC4 and AC7 was up-regulated by ANIT in both SDCs and LDCs; expression of the Ca2+-inhibitable isoforms AC5, AC6, and AC9 was down-regulated in SDCs and LDCs after both LPS and ANIT, while the Ca2+-calmodulin stimulated AC8 was decreased by LPS in both SDCs and LDCs. Finally, the gene expression of sAC was up-regulated after ANIT and LPS treatments, but only in SDCs.
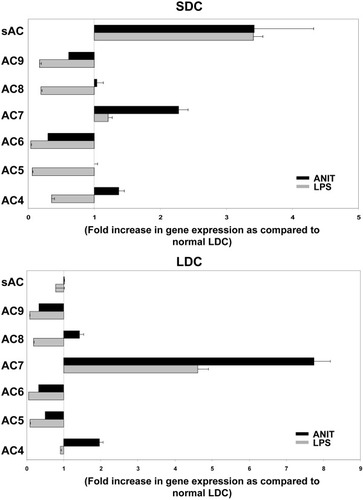
Expression levels of different AC isoforms in small and large cholangiocytes isolated from LPS-treated and ANIT-fed rats. Plot A shows the results for SDCs, while the results for LDCs are shown in plot B. Columns represent the mean ± standard deviations of the ratio between AC copy number/β-actin copy number in ANIT or LPS-treated animals versus AC copy number/β-actin copy number in untreated controls (cholangiocyte subpopulations isolated from normal rats) on two different isolations. Each isolation represent the pools of 16 rats for normal cholangiocytes and eight rats for ANIT or LPS treatments.
Discussion
This study demostrates that the intrahepatic biliary epithelium expresses multiple AC isoforms with a heterogenous pattern of expression among different cholangiocyte subpopulations. The Ca2+-insensitive ACs are more expressed in small cholangiocytes, whereas the Ca2+-activated ACs are more expressed in large cholangiocytes. The study also demonstrates that specific AC isoforms are functionally linked to certain secretory stimuli. In fact, AC8 and AC9 mediate cholangiocyte secretion induced by secretin and β2-adrenergic receptor agonists, respectively, whereas sAC couples secretion to the metabolic status of the cell.
Evaluation of the gene expression profile of AC isoforms in hepatocytes and cholangiocytes from different segments of the intrahepatic biliary tree of normal and cholestatic rat livers indicated that liver cells express all ACs except AC1 and AC2. Compared with hepatocytes, cholangiocytes do not express AC3, but do express AC8. Quantitative differences in AC gene expression between LDCs and SDCs were found. The Ca2+-activated AC isoforms (AC5, AC6, AC9) and the HCO3−-activated isoform, sAC,16 were more expressed in large cholangiocytes. The expression of AC isoforms positively (AC8)37 or negatively (AC5, AC6, and AC9)37 regulated by Ca2+ and of isoforms that are regulated by intracellular HCO3− (sAC)16 is consistent with the secretory function of large cholangiocytes and its regulation by intracellular Ca2+and HCO3−.12, 38 The Ca2+-insensitive AC4 and AC739, 40 were instead preferentially expressed by small cholangiocytes. Expression of these PKC-regulated ACs in small cholangiocytes is consistent with their ability to proliferate and participate in the repair of liver damage.
Intrahepatic rat cholangiocytes expressed AC8. Previous studies have identified AC8 in nervous tissue41 and pancreatic islets.42 Thus, AC8 expression by cholangiocytes further supports the concept that similarities exist between neuroendocrine tissue and the intrahepatic biliary tree.43 Higher expression of AC8 in LDCs compared with SDCs suggests that AC8 is involved in secretin-stimulated bile secretion. Consistent with this hypothesis, we showed that in LCD models, secretin-stimulated secretion and cAMP generation were inhibited by two different calmodulin inhibitors and by silencing AC8 expression. When secretion was induced by forskolin, a general stimulator of AC isoforms except for AC9 and sAC,30, 31 administration of calmodulin inhibitors and of AC8 siRNA did not cause a significant inhibitory effect. AC8 is the only known calmodulin-activated AC in cholangiocytes; in fact, AC9 activity is inhibited by calmodulin. Consistent with the data on secretion, secretin-stimulated cAMP production was inhibited by calmodulin inhibitors (Fig. 1B). Furthermore, silencing of AC8 gene expression in NRC cells23, 24 blocked secretin-stimulated but not forskolin-stimulated cAMP production and fluid secretion, consistent with a functional relationship between secretin and AC8.
Previous studies in isolated perfused liver have led to the speculation that a Ca2+-activated adenylyl cyclase isoform was activated by secretin.44 Alvaro et al.38 showed that cholinergic stimulation was synergistic with secretin in promoting Cl−/HCO3− exchange, and that this synergism occurred at the level of a Ca2+-dependent stimulation of cAMP production. The Ca2+/calmodulin system is activated by capacitative Ca2+ entry into the cell.44 In our study, we documented that acetylcholine stimulated cholangiocyte secretion in a calmodulin-dependent way, suggesting that the secretin and cholinergic agonists converge at the level of AC8. This mechanism is important because during digestion, secretin targets the biliary epithelium during parasympathetic predominance.38
Cholangiocytes also expressed AC9, a Ca2+-inhibitable AC typically unresponsive to forskolin. AC9 activity is stimulated by β2-adrenergic receptor agonists and inhibited by calcineurin, a protein phosphatase activated by Ca2+/calmodulin. AC9 is more expressed in LDCs, suggesting that it may also regulate bile secretion. In fact, administration of the β2-agonist isoproterenol32 stimulated secretion in IBDUs. This effect was inhibited by GABA, an inhibitor of AC9.33 Administration of FK506 and cyclosporin stimulated an H89-inhibitable secretion in IBDUs. This is consistent with the concept that AC9 in cholangiocytes is tonically inhibited by calcineurin and may be derepressed by β-adrenergic stimulation.
In addition to the transmembrane AC isoforms that generate cAMP in response to G-protein–coupled receptors, an important source of cAMP is provided by sAC.30, 34 Soluble AC is not stimulated by forskolin and G proteins, but it is uniquely dependent on [HCO3−] and therefore can act as a cellular metabolic sensor. Here we show that sAC is expressed in cholangiocytes and that cholangiocyte secretion stimulation is sensitive to increases in HCO3−. This effect was inhibited by H89, acetazolamide, KH7, and sAC gene silencing with siRNA. These data demonstrate the role of sAC30, 34 in the regulation of cholangiocyte secretion. Likely, in cholangiocyte sAC maintains a tonic level of cAMP and fluid secretion during the interdigestive phase.
As shown in Fig. 6, based on the expression of AC isoforms, their different regulatory mechanisms and functional interactions with specific agonists, we propose that the level of cAMP stimulating CFTR-dependent secretion is the result of the integration of different stimuli acting on Ca2+-dependent, Ca2+-independent, and HCO3-dependent ACs. Thus, the intracellular Ca2+ concentration resulting from the flow-dependent bending of apical cilia, and the intracellular concentration of [HCO3]i, resulting from the cellular metabolic status determine a resting level of cAMP that eventually will be further modulated by gastrointestinal hormones, cholinergic agonists, and β-adrenergic agonists, acting via AC8 or AC9. Our studies therefore highlight an important cross-talk between the Ca2+- and cAMP-dependent pathways at the level of ACs.
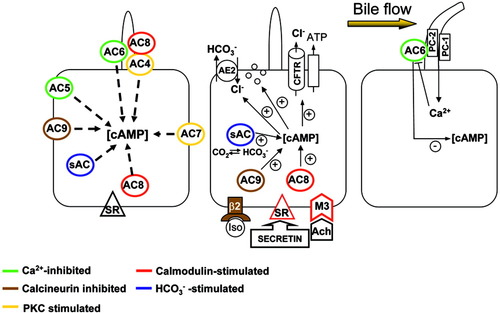
Diagram showing AC isoform expression in LDC subpopulations, with the proposed integration of cAMP signaling (see Discussion for details). ACs are color-coded to indicate their mechanisms of activation/inhibition. The membrane topography of the ACs is hypothetical except for the ciliary location of AC6, AC4, and AC8.12, 18 AC8 is also functionally linked to stimulation of the basolateral secretin receptor (SR) and cholinergic stimuli. Stimulation of secretin receptor and activation of AC8 increases [cAMP] and apical CFTR-dependent secretion, including vesicular transport and AE2 activity. Increased bile flow will bend cholangiocyte primary cilia, triggering Ca2+ influx via policystin 1 and 2 (PC1, PC2).12 Ca2+ influx will in turn inhibit AC6, counteracting the effect of secretin.12 β-Adrenergic agonist (isoproterenol) stimulates the calcineurin-inhibitable AC9 while modulated by calcineurin. sAC transduces the intracellular HCO3 concentration into cAMP levels. All these mechanisms combine to determine the level of cellular cAMP responsible for CFTR-dependent secretion.
Other studies indicate that the InsP3 receptor-mediated Ca2+ pathway is inhibited in cholestatic conditions,45 leaving cAMP/PKA as the main regulatory pathway. Our study uncovered the existence of a previously unrecognized modulation of AC gene expression in cholangiocytes during experimental cholestasis. While calcium-activated ACs were down-regulated in both cholangiocyte subpopulations, calcium-insensitive ACs and sAC become up-regulated. The PKC-stimulated AC7 was strongly up-regulated, particularly in conditions in which cholangiocyte proliferation prevails (ANIT). Expression of the [HCO3]i-activated sAC increased in SDCs, likely compensating for the metabolic disturbance induced by cholestasis.
In conclusion, our findings indicate that AC regulation is a fundamental regulatory node in the complex array of stimuli targeting cholangiocytes. Differential expression of AC isoforms with different regulatory properties is an important strategy to achieve specificity. Together with our previous studies,13, 14 the present study further indicates that modulation of different ACs plays an important pathophysiological role in biliary tree diseases.
Acknowledgements
We are indebted to Drs. J. Buck and L.R. Levin (Weill Cornell Medical College, New York, NY) for providing the KH7 sAC inhibitor.




