Relation between liver progenitor cell expansion and extracellular matrix deposition in a CDE-induced murine model of chronic liver injury†
Potential conflict of interest: Nothing to report.
Abstract
In chronic liver injury, liver progenitor cells (LPCs) proliferate in the periportal area, migrate inside the lobule, and undergo further differentiation. This process is associated with extracellular matrix (ECM) remodeling. We analyzed LPC expansion and matrix accumulation in a choline-deficient, ethionine-supplemented (CDE) model of LPC proliferation. After day 3, CDE induced collagen deposits in the periportal area. Expansion of LPCs as assessed by increased number of cytokeratin 19 (CK19)-positive cells was first observed at day 7, while ECM accumulated 10 times more than in controls. Thereafter, LPCs and ECM increased in parallel. Furthermore, ECM not only accumulates prior to the increase in number of LPCs, but is also found in front of LPCs along the porto-venous gradient of lobular invasion. Double immunostaining revealed that LPCs are embedded in ECM at all times. Moreover, LPCs infiltrating the liver parenchyma are chaperoned by α-smooth muscle actin (α-SMA)–positive cells. Gene expression analyses confirmed these observations. The expression of CK19, α-fetoprotein, E-cadherin, and CD49f messenger RNA (mRNA), largely overexpressed by LPCs, significantly increased between day 7 and day 10. By contrast, at day 3 there was a rapid burst in the expression of components of the ECM, collagen I and laminin, as well as in α-SMA and connective tissue growth factor expression. Conclusion: Our data demonstrate that, in a CDE model, ECM deposition and activation of matrix-producing cells occurred as an initial phase, prior to LPC expansion, and in front of LPCs along the porto-veinous gradient of lobular invasion. Those observations may reveal a fundamental role for the established hepatic microenvironment or niche during the process of activation and differentiation of liver progenitor cells. (HEPATOLOGY 2009.)
In response to liver injury, mature hepatocytes are able to undergo numerous cycles of cell division to compensate for the cell loss endured while maintaining their fully differentiated state.1 However, when proliferative response of mature hepatocytes is overwhelmed or impaired, activation of a dormant compartment of intrahepatic progenitor cells occurs.2 Proliferation of these adult progenitor cells has been identified in many different experimental injury models.3-5 Each of these models exhibit cell loss and parenchymal damage while inhibiting the normal hepatocyte proliferative response. Under these conditions, an increasing number of small cells with a large nuclear-to-cytoplasm ratio and an oval-shaped nucleus was observed.6 These cells, referred to as oval cells in rodents or liver progenitor cells (LPCs), express markers of both biliary and hepatocyte lineages.7 Found in the portal region, once activated and proliferating they migrate into the hepatic lobule, where they presumably undergo further differentiation into first intermediate hepatobiliary cells and next hepatocytes or bile duct cells. This process of LPC proliferation has been suggested in both human and murine studies to be accompanied by matrix remodeling, which could result in fibrosis when unbalanced and uncontrolled.3, 8-11
In humans with various chronic liver diseases such as chronic viral hepatitis, alcohol-induced liver disease, and nonalcoholic steatohepatitis, a ductular reaction often occurs at the portal tract interface.12-14 These proliferating ductules, which are postulated to arise from LPCs, have been shown to be in direct proportion to disease severity.15-19 In addition, in chronic hepatitis B or C, their presence is also related to both the grade of fibrosis and the relative risk for hepatocellular carcinoma.20, 21 In cholestatic liver diseases, portal fibrosis occurs and appears to be induced by the ductular reaction, and it has been shown that ductular epithelium can express cytokines and growth factors that attract and activate stellate cells, leading to collagen deposition.13 Furthermore, LPCs and activated hepatic stellate cells (HSCs) or myofibroblasts producing extracellular matrix (ECM) seem to expand in close proximity and to correlate in terms of numbers, whether in hepatitis C, alcoholic, or nonalcoholic steatohepatitis livers.22-24
In animal models, a correlation has also been found between the magnitude of LPC response and the intensity of fibrosis.25 Connective tissue growth factor (CTGF, also called CNN2), a secreted matricellular protein, and fibronectin, a prominent component of ECM, are up-regulated in the 2-acetylaminofluorene coupled to partial hepatectomy rat model and colocalize with oval cells and stellate cells.26 Cultured primary LPCs isolated from C57Bl/6J fed a choline-deficient, ethionine-supplemented (CDE) diet have a higher proliferative response when more contaminating cells are present in the culture, suggesting a contribution of different cell types to the behavioral response of the progenitor cells.27 Another interesting finding was the expression of Thy-1 in rats, which was mistakenly considered to be expressed by oval cells but in fact was found on the membrane of the HSCs located beside these oval cells.28-30 This colocalization of LPCs and HSCs was also shown in the chimeric livers of alb/uPA transgenic mice, where both cell types were strictly seen in the alb/uPA-expressing and thus diseased zones of the liver parenchyma.31
From these observations, we hypothesize that proliferating LPCs may not only make up for loss of hepatocytes but may also contribute to or promote periportal fibrogenesis. However, the possibility that progenitor cell reaction is dependent on fibrosis or is a by-product of the latter cannot be excluded. Alternatively, a common or related mechanism/stimulus may drive both fibrosis and LPC expansion and differentiation.
To address this, we exposed C57Bl/6J mice to a CDE diet and followed the establishment of liver progenitor cell reaction and its correlation to the extracellular matrix deposition and activation of hepatic stellate cells and myofibroblasts.
Abbreviations
CDE, choline-deficient ethionine-supplemented; CK19, cytokeratin 19; CTGF, connective tissue growth factor; ECM, extracellular matrix; HSC, hepatic stellate cell; LPC, liver progenitor cell; mRNA, messenger RNA; PCR, polymerase chain reaction; α-SMA, α-smooth muscle actin.
Materials and Methods
Animal Models.
Five-week-old male C57Bl/6J mice obtained from Janvier-Breeding Center (Le Genest St. Isle, France) were housed five per cage under pathogen-free conditions following a 12-hour light/dark cycle. At all times, mice were allowed unrestricted access to drinks and diet. The animals were handled according to guidelines for humane care for laboratory animals established by the Université Catholique de Louvain in accordance with European Union Regulation, and the study protocol was approved by the local ethics committee. After 1 week of acclimatization, mice were randomly assigned to one experimental group and received normal chow (control) or a diet deficient in choline (MP Biomedicals, Irvine, CA) together with drinking water supplemented with 0.15% (wt/vol) ethionine (Sigma, Bornem, Belgium) (CDE group).32 In the first experiment, control and CDE mice were sacrificed at 3 weeks (n = 5). For the timeline study, CDE mice were sacrificed at 3, 7, 10, 14, 18, or 21 days after initiation of the diet, and control mice were sacrificed at 21 days. Five animals were analyzed per time point. Livers were excised immediately after sacrifice. Part of the liver was fixed in 4% formalin or frozen in optimal cutting temperature (OCT) medium for histological analyses; the remaining liver was immediately snap-frozen in liquid nitrogen and kept at −80°C until use.
Histology and Immunohistochemistry.
Hematoxylin-eosin and Sirius red stainings were performed using standard protocols. For immunohistochemical detections, 5-μm liver sections were deparaffined and rehydrated in graded alcohol. Endogenous peroxidases were blocked by immersion in 3% H2O2 in methanol for 15 minutes. To ensure optimal antigen retrieval, the slides were submitted to heat-induced epitope retrieval in citrate buffer (pH 8.0) for 45 minutes at 100°C. After incubation with 1% bovine serum albumin for 30 minutes (room temperature) to block nonspecific binding sites, sections were incubated for 1 hour at 37°C with specific primary antibodies directed against cytokeratin 19 (CK19) (dilution 1:50; DSHB, University of Iowa, Iowa City, IA), α-smooth muscle actin (α-SMA) (dilution 1:50; Dako, Glostrup, Denmark), collagen I (dilution 1:500; Abcam, Cambridge, UK), E-cadherin (dilution 1:500; BD, Erembodegem, Belgium), or CTGF (dilution 1:3,000; Abcam, Cambridge UK). Detection was performed using anti-rat peroxidase-coupled secondary antibody (dilution 1:200) or anti-rabbit or anti-mouse Envision (Dako, Glostrup, Denmark) and the peroxidase activity was revealed by immersion of the sections for 5 minutes in a solution of diaminobenzidine (Dako, Glostrup, Denmark). Slides were then counterstained with hematoxylin for one minute. For each antibody, all slides were treated simultaneously to ensure homogeneity of the technique.
Immunofluorescence.
For immunofluorescence labeling, sections were costained with antibodies directed against CK19 (dilution 1:50; DSHB, University of Iowa) and collagen I (dilution 1:50; Abcam, Cambridge, UK). Secondary antibodies were either goat anti-rat/AlexaFluor 594 or donkey anti-rabbit/AlexaFluor 488 (dilution 1:1,000, Invitrogen, Merelbeke, Belgium). Hoechst (dilution 1:10,000) was used to reveal the nuclei.
Morphometrical Quantification.
Sirius red and CK19 stainings were performed on serial sections. For each liver section, 10 images were taken at random at 10× magnification and digitized through a Zeiss microscope coupled to an Axiocam camera (MR3, Carl Zeiss, Munich, Germany) and analyzed using Axiovision software (Zeiss). The ratio of stained area to the surface of reference (total area, leaving out the white areas) was expressed as the area percentage of the liver. The percentage of stained area for each image was then averaged to give a mean score per liver section.
RNA Extraction, Reverse Transcription, and Quantitative Polymerase Chain Reaction.
Total RNA was extracted using TRIpure Isolation Reagent (Roche Diagnostics, Vilvoorde, Belgium). Reverse transcription and quantitative real-time polymerase chain reaction (PCR) analysis were performed as described.33 Primer pairs for transcripts of interest were designed using the Primer Express design software (Applied Biosystems, Lennik, Belgium) and listed in Table 1. RPL19 RNA was chosen as an invariant standard. Results are expressed as fold expression relative to expression in the control group (value set at 1) using the ΔCt method.
| Target | Primer Sequence | |
|---|---|---|
| Forward | Reverse | |
| α-Fetoprotein | 5′-CACACCCGCTTCCCTCAT-3′ | 5′-TTTTCGTGCAATGCTTTGGA-3′ |
| α-SMA | 5′-TCCTGACGCTGAAGTATCCGATA-3′ | 5′-GGTGCCAGATCTTTTCCATGTC-3′ |
| Albumin | 5′-GCTGCGCTGAAGCCAATC-3′ | 5′-GCTGAAATTCAGCAAGCACTGT-3′ |
| CD49f | 5′-GGAGTAGCTTGGTGGATCATCCT-3′ | 5′-ACACTAATAGAGCCATCAGAATC-3′ |
| CK19 | 5′-AGCGTGATCAGCGGTTTTG-3′ | 5′-CCTGGTTCTGGCGCTCTATG-3′ |
| CTGF | 5′-CGCCAACCGCAAGATTG-3′ | 5′-ACACGGACCCACCGAAGAC-3′ |
| E-cadherin | 5′-CTTTTCGGAAGACTCCCGATT-3′ | 5′-GCTTTAGATGCCGCTTCACTGT-3′ |
| Laminin | 5′-TTTGATAGACGCGTGAACGATAA-3′ | 5′-TGGCGGGAATTCTCCTTAGA-3′ |
| Procollagen I | 5′-GACTGGAAGAGCGGAGAGTACTG-3′ | 5′-CAGGTCTGACCTGTCTCCATGTT-3′ |
| RPL19 | 5′-GAAGGTCAAAGGGAATGTGTTCA-3′ | 5′-CCTTGTCTGCCTTCAGCTTGT-3′ |
Statistical Analysis.
All data are presented as the mean ± standard deviation. Differences between CDE and control groups were analyzed using the unpaired Student t test or analysis of variance (with appropriate post hoc analysis) for multiple comparisons. Correlation was assessed via linear regression and Pearson correlation. Statistical significance was assumed for P < 0.05.
Results
Evaluation of Oval Cell Expansion After 3 Weeks of CDE Diet
A strong proliferative response of progenitor (oval) cells was observed when C57Bl/6J mice were exposed to the CDE diet. In normal liver, CK19, a commonly used biliary epithelium and oval cell marker, stained one or two bile ducts together with a few isolated cells surrounding the portal tract, representing less than 0.3% of the area of the liver section (Fig. 1A). After 21 days of a CDE diet, a dramatic expansion of CK19-positive, E-cadherin–overexpressing cells was observed (Fig. 1B,E). Those cells covered up to 5.6% of the liver section (P < 0.001 versus controls), and irradiated from the periportal zone into the parenchyma. Oval cells were principally organized in small nests (of three to four cells) or arborizing, ductlike structures (Fig. 1B), whereas isolated cells could be found at some distance from the portal tract.
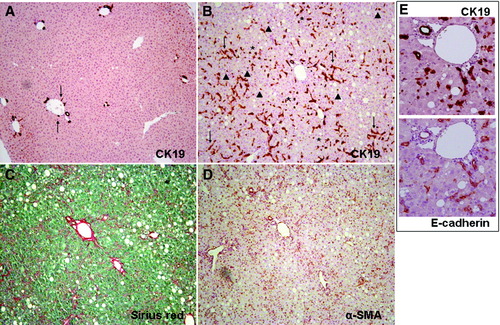
Liver sections obtained from control mice (A) and mice receiving the CDE diet for 21 days (B-E) were stained with antibodies specifically directed against CK19 (A, B and E upper panel), α-SMA (D), or E-cadherin (E, or lower panel) or with Sirius red (C) to show extracellular collagen deposits. In control liver, CK19-positive staining is restricted to bile ducts and isolated cells of the periportal tract (arrows). In CDE livers, beside bile ducts, CK19-positive cells are dramatically increased in number, forming arborescence inside the lobule. Those cells are organized in small clusters (asterisk) or ductlike structures (arrows). Isolated cells (arrowheads) are found at a distance from the periportal tract. Those CK19-positives cells (including bile duct cells) express E-cadherin. The pattern of collagen deposition and α-SMA expression parallels that of CK19-positive staining. The pictures display representative photomicrograph (magnification ×10).
Together with the expansion of LPCs, an increased deposition of ECM was observed as demonstrated by Sirius red staining (Fig. 1C). Matrix deposition occurred around CK19-positive cells and surrounded the arborizing or ductlike structures. An increased number of α-SMA–positive cells was also found, and the topography of those cells paralleled that of LPCs and ECM deposition (Fig. 1D).
Timeline-Based Study
To follow the course of LPC expansion and ECM deposition, livers were analyzed after 0, 3, 7, 10, 14, 18, or 21 days of a CDE diet.
Expansion of LPCs.
As shown in Fig. 2, after administration of CDE for 3 days, rare CK19-positive cells were seen attached to the portal tract, either isolated or forming small clusters consisting of two to three cells (Fig. 2B). At day 7, there were seemingly more clusters of oval cells around the portal tract elongating and expanding inside the parenchyma or organized in small nests (Fig. 2C). At the same time, small clusters and solitary oval cells were seen distant from the portal tract. From day 10 on, CK19-positive cells were more numerous, forming clusters that contained an increasing number of cells and expanded inside the lobule. In addition to these clusters, two types of isolated cells were observed, both solitary and strongly CK19-positive oval cells and isolated small hepatocyte-like cells could be seen (Fig. 2D, arrow). The latter, in comparison with the oval cells, expressed CK19 in a lighter shade of brown, were bigger with a rounder nucleus, a lower nuclear-to-cytoplasm ratio, and resembled hepatocytes (Fig. 2D, inset). From day 14 to day 21, CK19-positive cells continued to rise in number, and by the end of the treatment infiltrated the parenchyma forming bridges between portal tracts and the centrilobular veins, which until then were spared from oval cells (Fig. 1B). The number of solitary cells still augmented, while the quantity of small hepatocyte-like cells (or intermediate hepatobiliary cells) appeared to remain stable.17 Morphometrical analysis confirmed that the number of CK19-positive cells significantly increased at day 7 and further at day 10. Thereafter, their number incrementally became highest by the end of the experiment (Fig. 3A).
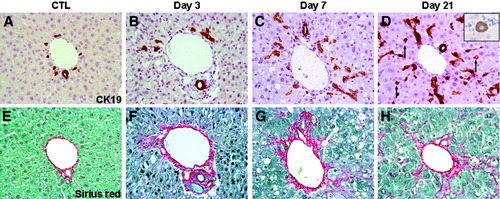
Liver sections obtained from mice receiving the control diet (A, E) or the CDE diet for 3 days (B, F), 7 days (C, G), or 21 days (D, H) were stained with antibodies specific for CK19 (A-D) or with Sirius red (E-H). Representative photomicrographs on consecutive sections and centered on the same area for the two stainings are shown and demonstrate the parallel expansion of progenitor cells and collagen meshwork with time on the CDE diet. Inset in panel D shows a large, CK19-positive cell with a round nucleus and a low nuclear-to-cytoplasm index resembling a hepatocyte (magnification ×40).
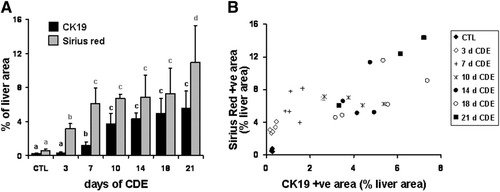
(A) Morphometrical quantification of the area of the liver section occupied by CK19-positive cells (black bars) or Sirius red staining (gray bars) in control mice and mice receiving the CDE diet for the periods indicated. Data are expressed as the mean ± standard deviation of the percentage of liver section area related to the total area of the section minus vascular structures for n = 5/group. For each parameter, data of bars identified by a different letter (a, b, c, d) are significantly different. (B) CK19 morphometrical data area plotted against a Sirius red–stained area for each individual liver from control mice (black diamonds) and mice receiving the CDE diet for 3 (white diamonds), 7 (plus signs), 10 (asterisks), 14 (black circles), 18 (white circles), or 21 days (black squares). Note that there is a significant increase in Sirius red staining at day 3 of the CDE diet (white diamonds), whereas the CK19-positive area remains similar to that found in controls (black diamonds). The relationship between Sirius red and CK19 morphometrical data is thereafter linear for mice fed the CDE diet between 7 and 21 days.
Matrix Deposition.
In normal liver, Sirius red staining strictly surrounded ductular and vascular structures (Fig. 2E). Matrix deposition increased with duration of CDE administration. At day 3, Sirius red staining was more intense around the portal tract, where collagen fibers got thicker and longer and tended to expand into the lobule between the hepatic cords (Fig. 2F). From day 7 on, the fibers bundled up, formed a meshwork around the portal tracts and the progenitor cells, and extended into the lobule (Fig. 2G). At day 10, these growing networks expanded toward one another, and stretching fiber extremities from neighboring portal tracts merged. This collagen accumulation proceeded to day 21, where ultimately an extended collagen network widened over the entire lobule (Fig. 1C, 2H). As assessed via morphometrical analysis, a significant five-fold increase in ECM deposition had occurred by day 3, then doubled at day 7 (Fig. 3A). The amount of deposited matrix then appeared to stabilize up to day 18, with a further increase at day 21.
Thus, between day 10 and day 21, the amount of ECM and the number of CK19 progenitor cells grew slowly in parallel. This is in sharp contrast with earlier time points where these two processes were totally unbalanced: at day 3, the increase in LPCs was negligible compared with the five-fold increase of ECM deposition. At day 7, matrix deposition was 10 times higher than in controls, while LPCs initiated their expansion (Fig. 3B).
Source of Extracellular Matrix.
During the wound-healing process, matrix-producing cells are mainly portal myofibroblasts and activated HSCs, both of which are α-SMA–positive cells. In normal liver, α-SMA–positive cells are perivascular. The number of α-SMA–positive cells increased with time in the CDE model. At day 3, only periportally located myofibroblasts stained positive (Fig. 4A). At day 7, α-SMA–positive cells represented a dense cell population located mainly in the periportal area (Fig. 4B). At later time points, α-SMA–positive cells also infiltrated the lobule, where they appeared intermingled with the LPC cords (Fig. 4C,F). At all time points, α-SMA–positive cells were found in the direct vicinity of LPCs, whether those were in clusters or isolated.
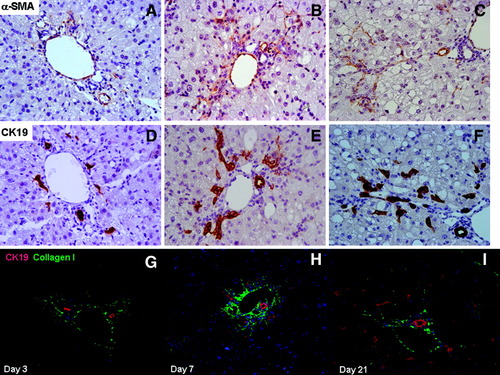
(A-F) Consecutive liver sections stained with specific antibodies against α-SMA (A-C) or CK19 (D-F) from mice receiving the CDE diet for 3 (A, D), 7 (B, E), and 21 days (C,F). (Magnification ×40.) (G-I) Liver sections from mice receiving the CDE diet for 3 (G), 7 (H), and 21 days (I) labeled with antibodies to CK19 (red) and collagen I (green) showing that CK19-positive cells are surrounded by collagen and that collagen fibers progress ahead of CK19-positive cells along a portal central axis of lobular invasion. Nuclei are visualized in blue. (Magnification ×40.)
Double immunostaining for CK19 and collagen I evidenced that LPCs, whether isolated, in cluster, or displaying ductlike structures, were surrounded by ECM. Moreover, collagen I containing ECM expanded inside the lobule ahead and in front of LPCs (Fig. 4G-I).
Thus, α-SMA–positive cells were in the direct vicinity of LPCs, and LPCs appeared embedded into newly deposited ECM. When looking at the porto-central progression, the first elements to penetrate the lobule were ECM and α-SMA–positive cells, in front of LPCs.
Gene Expression.
The results for the gene expression profile study are assembled in Fig. 5.
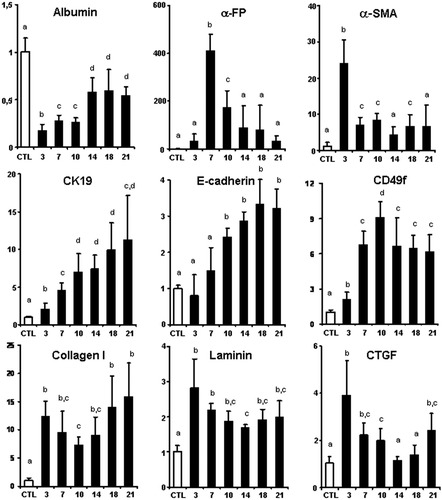
Effects of CDE diet on relative expression of albumin, α-fetoprotein (α-FP), α-SMA, CK19, E-cadherin, CD49f, collagen type I, laminin, and CTGF mRNA determined via quantitative real-time PCR in the liver of control mice (open bars) or mice fed the CDE diet for 3 to 21 days (black bars). Values were normalized to the expression of RPL19 mRNA regarded as an internal control and expressed in relation to the mean value in untreated controls arbitrarily set at 1. All data are expressed as the mean ± standard deviation for n = 5/group. In all graphs, the X axis represents the number of days on the CDE diet and the Y axis represents the ratio of mRNA expression for the gene of interest on mRNA expression of RPL19, with value of this ratio arbitrarily set at 1 in the control group. Data identified by a different letter (a, b, c, d) are significantly different.
Albumin messenger RNA (mRNA), expressed by mature hepatocytes, fell rapidly after the introduction of the CDE diet to 20% of control levels, compatible with the loss of functional hepatocytes. While liver insult persisted, albumin expression rose at day 14 and stabilized at 60% of control levels up to the end of the experiment.
Compared with controls, CK19 mRNA quadrupled after day 7 of a CDE diet and then gradually rose to reach, after day 21, levels more than 10 times higher than in controls. mRNA for E-cadherin, a general marker of epithelial cells that is strongly overexpressed by oval cells, significantly increased after 10 days of a CDE diet.34 The maximal expression (3.5-fold over control values) was seen at late time points of the experiments (days 18 and 21). Also, the hepatic expression of CD49f (integrin-α6), carried by oval cells, increased significantly after 7 days of a CDE diet.35 α-Fetoprotein mRNA expressed by immature hepatocytes was induced dramatically (400-fold) in CDE mice after 7 days. Thereafter, the magnitude of overexpression decreased but remained higher than in controls at all time points (40-fold increase at day 21 compared with controls).
When we analyzed gene expression for components of the ECM, we saw a rapid burst in the expression of collagen I and laminin mRNA at day 3. Later, those levels slightly decreased, and either a second peak of expression was seen (collagen I) or a stabilization occurred (laminin). The level of expression of those ECM genes remained at all time points significantly higher than in controls. This reinforced the observation drawn from the immunohistochemistry analyses that active deposition of ECM occurred before expansion of LPCs. Even more, observation at day 3 showed an increase in gene expression of both α-SMA (by a factor 25) and CTGF/CNN2 (by a factor 4), a secreted matricellular protein also involved in the activation of HSCs, supporting that activation of matrix-producing cells occurred as an initial phase in the process. As assessed via immunohistochemistry, hepatocytes in normal liver express low levels of CTFG. After 3 days on a CDE diet, a significant proportion of hepatocytes (≈30%, mainly in zone 2) become strongly CTGF-positive (Fig. 6A). After 7 days, fewer hepatocytes stained positive (paralleling mRNA data) and were preferentially found in the periportal area (Fig. 6B). This expression remained stable in intensity and localization up to the end of the experiment. CTGF expression was not found in LPCs or bile ducts (Fig. 6B,D).
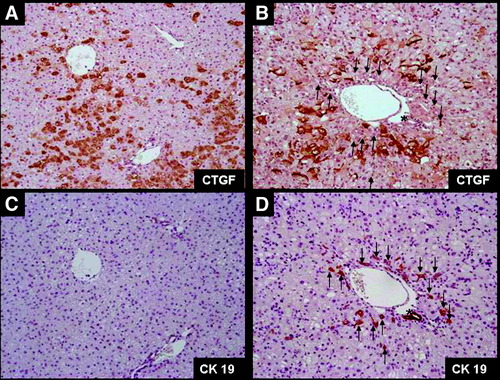
Consecutive liver sections stained with CTGF (A-B) or CK19 (C-D) from mice receiving the CDE diet for 3 days (A-C) or 7 days (B-D) show that hepatocytes appear to be the main cellular source for CTGF in the CDE model. Asterisks (*) denote a CTGF-negative and CK19-positive bile duct. Arrows indicate CK19-positive LPCs. Note that no CTGF staining was found in this area.
Discussion
Previous analyses of chronically diseased human livers have demonstrated a correlation between the scale of ductular reaction and the severity of fibrosis.12, 13, 15, 22, 24 Further investigations in murine models reported the presence of ECM in the vicinity of LPCs and a close proximity between HSCs and LPCs.3, 25, 36 Our data confirm the correlation between the magnitude of LPC expansion and the importance of ECM deposition. More importantly, the present study strongly supports that deposition of ECM precedes the expansion of LPCs. Furthermore, ECM not only accumulates prior to the increase in number of LPCs, but is also found in front of LPCs along the porto-veinous gradient of lobular invasion. Moreover, LPCs infiltrating the liver parenchyma are chaperoned by α-SMA–positive cells, suggesting that those myofibroblastic cells may represent the main cellular source of ECM required for LPC proliferation and lobular invasion.
The evaluation of both LPC proliferation and ECM deposition during the time course of CDE-induced liver injury elucidates that in the early phase of the process, ECM deposition occurs prior to LPC amplification. Excess matrix deposition, as revealed by Sirius red staining, first occurs after 3 days of a CDE diet with collagen fibers thickening in the periportal area, while oval cell response is on hold. Morphometrical analyses of Sirius red and CK19 immunostaining sustain these observations. Quantitative real-time PCR provides further confirmation demonstrating peaks in the expression of genes encoding for matrix protein (collagen I, laminin, CTGF) prior to increased expression of CK19, E-cadherin, or CD49f mRNA. In addition to the presence of ECM deposition before the expansion of LPCs, we made the clear observation that LPCs are embedded in matrix and that matrix is deposited ahead of the LPCs during penetration into the lobule.
Accumulating data support the hypothesis of a fundamental role for the established hepatic microenvironment in the activation, proliferation, and survival of LPCs in a hostile environment.5, 31, 37 The ECM is a structural network consisting of collagens (principally collagen I in the wound-healing context), glycoproteins, and basement membranes (including laminin which increases cellular motility and migration).7 It creates a micro-environment withholding all kinds of secreted factors functioning as motogens, mitogens, and morphogens.38 Matrix deposition seems to chaperone and escort the oval cells, paving the way from the portal tract to the parenchyma (Fig. 2H) and allowing the progenitor cells to invade the deposited matrix in the direction it determined. The manner in which LPCs and ECM interact remains to be elucidated, but collagen receptors on the LPCs, as suggested by the detection of CD49f/integrin-α6,35 sensing the migratory direction could be one possibility. In addition to support and anchor, ECM probably also provides nurture and protection against damaging secreted cytokines and toxic necrotic cell contents. Only very few oval cells are found outside this protective cocoon. The rather rare LPCs detected outside this ECM environment express CK19 less strongly, have a rounder nucleus, and have a lower nucleus-to-cytoplasm ratio, suggesting that they are small hepatocyte-like cells. This evokes the possibility that the matrix tract may be left by cells when they reach their endpoint and are ready to differentiate or are already differentiating. CTGF, a matricellular protein produced under the control of transforming growth factor β1, is implicated in fibrogenesis.10, 11 Upon initiation of a CDE diet, a significant proportion of hepatocytes overexpressed CTGF. With time, this number decreased, and hepatocytes expressing and secreting CTGF were preferentially found around the portal tract in proximity to the LPC reaction. Interestingly, in the acetylaminofluorene and partial hepatectomy rat model, inhibition of CTGF by Iloprost resulted in decreased Thy1+ cells, now recognized as mesenchymal cells with characteristics of myofibroblasts/activated stellate cells, and was associated with a significant decrease of oval cells.26, 30 This reinforces the concept of interdependence between deposited matrix and progenitor cells.
In this study, we observed, with time, an increased number of α-SMA–positive cells found in the vicinity of LPCs. The colocalization of myofibroblast-like cells and LPCs has been documented in the acetylaminofluorene and partial hepatectomy rat model.9, 39 In the same model, it has been recently demonstrated that Thy-1, firstly introduced as a marker of oval cells, is in fact not expressed by the LPCs themselves but by the neighboring HSCs or progenitors of the mesenchymal lineage.28, 29, 40 In addition to colocalization, we show that α-SMA–positive cells and ECM deposition penetrate into the lobule prior to and in front of LPCs.
The observations of both LPC–ECM and LPC–α-SMA–positive cell colocalization support two propositions. First, the matrix is likely to be produced, at least partly, by those α-SMA–positive myofibroblasts. Whether those are activated HSCs or periportal myofibroblasts or arise from mesenchymal progenitor or epithelial mesenchymal transition between LPCs and HSCs remains to be determined.9-11, 30, 41, 42 Second, in addition to interactions with ECM, cross-talks between myofibroblastic cells and LPCs are probably necessary for proliferation, migration, and phenotypic differentiation of LPCs.
HSCs or myofibroblasts produce a large array of growth factors and signaling molecules.10, 11 Primary oval cells from CDE-treated mice were shown to proliferate more in culture when more contaminating cells were present.27 Furthermore, it has been shown that in fetal mouse liver, cell–cell contact between Thy-1–positive cells and CD49f (integrin-α6)–expressing LPCs are essential for the maturation of the latter into morphologically mature hepatocyte-like cells in vitro.35, 43 These findings favor the hypothesis that the fate of LPCs is determined by interactions with specific ECM and requires the cooperation of other cell types, in particular chaperon α-SMA–positive cells.
Whether LPCs can produce or influence the production of ECM components is unclear but has been suggested. HPPL, a liver progenitor cell line derived from mouse hepatoblasts, has been shown to produce ECM proteins, including laminin-α5, -β1, -β2, -γ1, and -γ2 subunits as detected via real-time PCR.44 Also, Balb/c mice, a strain highly susceptible to fibrosis induced by various stimuli, paradoxically developed a weaker fibrosis in response to CDE than fibroresistant C57 mice.45 Importantly, in this context, Balb/c mice also developed a lesser expansion of LPCs. We made similar paradoxical observations in adiponectin-deficient mice, which are prone to develop severe fibrosis. Conversely, in fibroresistant ob/ob mice fed a CDE diet, both LPC expansion and ECM deposition occurred as in controls (data not shown). This suggests that, in the CDE model, the fibrotic reaction was associated with, dependent upon, or controlled by progenitor cells.25
In the CDE model, proliferation and differentiation of LPCs is believed to make up for cell loss induced by toxic agents and an inability of remaining mature cells to proliferate. Very recently, convincing evidence has been provided supporting that panCK-positive cells in the canal of Hering differentiate into peribiliary hepatocytes in a mouse model of sublethal acetaminophen intoxication.46 From studies on liver development, it is well known that CK19 expression is lost during terminal hepatocyte differentiation but is maintained while differentiation progresses along the biliary lineage.47 In our model, we observed a deep fall in albumin expression concomitant to the initial hepatic damage. In a second phase, while LPCs were expanding, α-fetoprotein expression dramatically increased, whereas albumin expression returned to subnormal levels. In parallel, we observed CK19-positive, hepatocyte-like cells at the intrabolular extremities of the trabecular formations of LPCs. Our interpretation is that a CDE diet induces a significant hepatotoxic damage, impairing hepatocyte function as assessed by a significant drop in albumin expression. As a response, LPCs proliferate and undergo an at least partial hepatocytic differentiation with massive expression of early hepatoblastic marker α-fetoprotein. Some of those cells transform into a more differentiated phenotype that is able to partly restore albumin expression. The CK19-positive hepatocyte-like cells observed in the parenchyma may represent a transitional step in this phenotypic transformation. CK19-positive cells strongly overexpress E-cadherin, and hepatic E-cadherin mRNA expression progressively increases with increased numbers of LPCs. This might be compatible with the requirement of some cellular coherence for pseudoductular formation and for penetration into the parenchyma prior to integration of the differentiated cells into the epithelial sheet in the periportal area and deeper into the parenchyma.34
Altogether, our results support the hypothesis that LPCs need a support matrix, presumably provided by myofibroblasts, for migration and anchor in order to differentiate for restitution of the damaged liver. We show with our data that already at day 3, such a matrix is produced, putting the liver in a state of readiness for the upcoming proliferation of the LPCs. The observation that ECM is required prior to proliferation of LPCs and in front of LPCs during penetration into the parenchyma reinforces the hypothesis of a fundamental role for the established specific hepatic microenvironment or niche in LPC proliferation and differentiation. Further studies are needed to understand the mechanisms for the interactive cross-talk between LPCs, associated myofibroblasts, and the ECM.
Acknowledgements
The TROMA-III antibody developed by Rolf Kemler was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.




