Hepatic AdipoR2 signaling plays a protective role against progression of nonalcoholic steatohepatitis in mice†
Potential conflict of interest: Nothing to report.
Abstract
It is unclear how hepatic adiponectin resistance and sensitivity mediated by the adiponectin receptor, AdipoR2, contributes to the progression of nonalcoholic steatohepatitis (NASH). The aim of this study was to examine the roles of hepatic AdipoR2 in NASH, using an animal model. We fed C57BL/6 mice a methionine-deficient and choline-deficient (MCD) diet for up to 8 weeks and analyzed changes in liver pathology caused by either an AdipoR2 short hairpin RNA–expressing adenovirus or an AdipoR2-overexpressing adenovirus. Inhibition of hepatic AdipoR2 expression aggravated the pathological state of NASH at all stages: fatty changes, inflammation, and fibrosis. In contrast, enhancement of AdipoR2 expression in the liver improved NASH at every stage, from the early stage to the progression of fibrosis. Inhibition of AdipoR2 signaling in the liver diminished hepatic peroxisome proliferator activated receptor (PPAR)-α signaling, with decreased expression of acyl-CoA oxidase (ACO) and catalase, leading to an increase in lipid peroxidation. Hepatic AdipoR2 overexpression had the opposite effect. Reactive oxygen species (ROS) accumulation in liver increases hepatic production of transforming growth factor (TGF)-β1 at all stages of NASH; adiponectin/AdipoR2 signaling ameliorated TGF-β–induced ROS accumulation in primary cultured hepatocytes, by enhancing PPAR-α activity and catalase expression. Conclusion: The adiponectin resistance and sensitivity mediated by AdipoR2 in hepatocytes regulated steatohepatitis progression by changing PPAR-α activity and ROS accumulation, a process in which TGF-β signaling is implicated. Thus, the liver AdipoR2 signaling pathway could be a promising target in treating NASH. (HEPATOLOGY 2008;48:458–473.)
According to a recent report, nonalcoholic fatty liver disease (NAFLD) afflicts as much as 20% of the US adult population. Nonalcoholic steatohepatitis (NASH), part of the spectrum of NAFLD, is the most prevalent liver disease in the United States and is thought to affect approximately 3%-4% of the population.1
In this so called “age of satiation,” NAFLD and NASH are very often accompanied by lifestyle diseases, such as diabetes and obesity, that have insulin resistance as a primary background factor. Thus, these liver diseases can be considered hepatic representations of metabolic syndrome. Given the growing number of metabolic syndrome patients in recent years, the incidences of NAFLD and NASH are expected to increase further, particularly in North America, Europe, Asia, and the western Pacific countries.
NASH is a progressive disease. In a study that followed NASH patients for 10 years, the disease progressed to cirrhosis in 20% of the patients and led to death caused by liver diseases in 8%.2 In a population-based cohort study, approximately 3% of the patients in the community diagnosed with NAFLD developed cirrhosis or a liver-related complication.3 Because NASH is a progressive disease, it is necessary to develop and establish a therapeutic method to treat it. The details of the pathological mechanism of NASH, however, have not yet been clarified.
Obesity is a major risk factor for NASH. The primary factor in the mechanism of the induction of insulin resistance by obesity is abnormal expression and excretion of the adipocytokines, physiologically active substances that are released by adipose tissue. Adiponectin, an adipocytokine that improves insulin resistance and enhances the burning of fatty acids, can be used as an effective therapy against the insulin resistance that accompanies obesity or diabetes.4 In the NAFLD mouse model, adiponectin has anti-inflammatory and insulin resistance-reducing effects, and improves the pathological condition of NAFLD.5 Furthermore, there is an inverse association between serum adiponectin level and transaminase activities in Japanese male workers.6 The genes of the receptors for adiponectin (AdipoR1 and AdipoR2) were recently cloned.7 AdipoR1 is abundantly expressed in skeletal muscles, whereas AdipoR2 is expressed predominantly in the liver, suggesting an association with the pathology of liver diseases. Indeed, decreased expression of AdipoR2 in the livers of patients with NASH has recently been reported,8 suggesting that adiponectin signaling mediated by AdipoR2 might play an important role in the pathogenesis of NASH.
Therefore, in the current study, we examined the role of adiponectin/AdipoR2 signaling during NASH, using an adenovirus and a mouse model. We found that hepatic adiponectin sensitivity and resistance mediated by adiponectin/AdipoR2 signaling plays a significant role in the pathogenesis of NASH. Our findings suggest that the regulation of hepatic sensitivity to adiponectin by AdipoR2 is a promising target in the development of new therapeutic modalities for NASH.
Abbreviations
α-SMA, alpha-smooth muscle actin; ACO, acyl-CoA oxidase; ALT, alanine aminotransferase; AMPK, AMP-activated protein kinase; Col1α1, α1(I) collagen; Col1α2, α2(I) collagen; HE, hematoxylin-eosin; HNE, hydroxynonenal; MCD, methionine-deficient and choline-deficient; MOI, multiplicity of infection; mRNA, messenger RNA; NAC, N-acetylcysteine; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; O/E-R2-adenovirus, Adenovirus producing the mouse adipoR2; PCR, polymerase chain reaction; PPAR, peroxisome proliferator activated receptor; ROS, reactive oxygen species; SEM, standard error of the mean; ShR2-adenovirus, mouse AdipoR2 shRNA-expressing adenovirus; shRNA, short hairpin RNA; SOD, superoxide dismutase; t-BHP, tert-butyl hydroperoxide; TG, triglyceride; TGF, transforming growth factor; TIMP-1, tissue inhibitor of metalloproteinase 1; WT, wild-type.
Materials and Methods
Preparation of Adenovirus.
The nucleotide sequence for the short hairpin RNA (shRNA) against mouse AdipoR2 were as follows: gatccgGCTTAGAGACACCTGTTTGTTttcaagagaAACAAACAGGTGTC TCTAAGCcttttttctcgagg (forward) and aattcctcgagaaaaaagGCTTAGAGACACCTGTTTGTTtctct tgaaAACAAACAGGTGTCTCTAAGCCg (reverse), as previously described.7 The capital letters refer to the hybridizing shRNA-like portion of the hairpin. Vectors that express mouse AdipoR2 shRNAs under the control of the U6 promoter were constructed by inserting the pairs of annealed DNA oligonucleotides into the pSIREN-DNR plasmid (BD Clontech). The U6-driven shRNA cassettes and the CMV-driven DsRed expression cassette were then inserted into the BD-AdenoX expression system (Clontech) to produce the mouse AdipoR2 shRNA-expressing (ShR2)-adenovirus and the DsRed adenovirus (corresponding mock adenovirus).
Adenovirus producing the mouse adipoR2 (O/E-R2-adenovirus) and the corresponding mock adenovirus were constructed with the AdEasy adenoviral system (Stratagene, La Jolla, CA). Recombinant adenoviruses were produced in HEK293 cells and purified on a cesium chloride gradient before use.
Animal Studies.
Male 9-week-old to 10-week-old C57BL/6 mice were housed in cages and maintained under 12-hour light-dark cycles. Mice were fed a methionine-deficient and choline-deficient (MCD) diet (cat NO 960439; ICN, Aurora, Ohio) or standard chow (CE-2; CLEA Japan Inc.) for up to 8 weeks. At various intervals determined by the focus of the specific experimental question, animals were injected intravenously through the tail with either 4 × 109 plaque-forming units of the adenoviruses or saline. In experiments focused on the early stage of NASH, mice were fed the MCD diet for 3 weeks after receiving injections. In experiments for late stage of NASH, we injected mice with adenoviruses or saline after feeding them the MCD diet for 5 weeks; the mice were further fed the MCD diet until 3 weeks after infection.
For tert-butyl hydroperoxide (t-BHP) treatment, animals were treated intraperitoneally with 1.5 mmol t-BHP (Sigma)/kg body weight or vehicle 24 hours before the final sample administration. For N-acetylcysteine (NAC) treatment, animals received intraperitoneally 50 mg/kg doses of NAC or vehicle daily for 1 week or 3 weeks during the feeding period. For vitamin C and vitamin E treatment, animals were treated by gavage with a combined dose of vitamin C (100 mg/kg) and vitamin E (40 mg/kg) or vehicle daily for 4 weeks during the feeding period. All animals received humane care in compliance with the National Research Council's criteria outlined in the “Guide for the Care and Use of Laboratory Animals,” prepared by the US National Academy of Sciences and published by the US National Institutes of Health.
Biochemical and Histological Analysis.
Serum concentrations of alanine aminotransferase (ALT), glucose, and insulin were measured as previously described.9-11 A commercially available enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN) was used to determine serum adiponectin. Hepatic triglyceride (TG) content and liver hydroxyproline concentrations were measured as previously described.9-11 Liver tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and stained with hematoxylin-eosin (HE) and a Masson-trichrome solution. For protein or RNA analysis, tissues were frozen in liquid nitrogen and stored at −80°C until needed.
Western Blot Analysis.
Western blotting was performed as described,10 using the following antibodies: peroxisome proliferator-activated receptor (PPAR)-α (Santa Cruz Biotechnology, Santa Cruz, CA), AdipoR1 and AdipoR2 (Immuno-Biological Laboratories Co., Ltd., Gumma, Japan), catalase and acyl-CoA oxidase (ACO) (Abcam Ltd., Cambridge, United Kingdom), adenosine monophosphate–activated protein kinase (AMPK)-α and phospho-AMPK-α (Thr172) (Cell Signaling Technology Inc., Beverly, MA), and β-actin (Sigma Aldrich Inc., St Louis, MO).
Immunohistochemistry.
Alpha-smooth muscle actin (α-SMA) and 4-hydroxynonenal (4-HNE) were detected in paraffin-embedded liver sections as previously described.9-11 For transforming growth factor (TGF)-β1 immunostaining, anti–TGF-β1 antibody (Santa Cruz Biotechnology) was used.
Real-Time Quantitative and Reverse Transcription Polymerase Chain Reaction Analysis.
Total RNA was extracted from total liver homogenates or hepatocytes using Isogen (Nippon Gene, Tokyo, Japan), as previously described.9-11 Reverse transcription and real-time polymerase chain reaction (PCR) amplification were performed as previously described, using Taqman assay reagents (Applied Biosystems, Foster City, CA).9-11
Hepatocyte Isolation and Cell Culture.
Heterozygous AdipoR2 gene knockout mice (C57BL/6 background) were purchased from Jackson Laboratories (Bar Harbor, ME). Male wild-type (WT) and homozygous (AdipoR2 KO) littermates were obtained by breeding heterozygous mice.
Primary cultured hepatocytes were prepared from livers of WT mice or AdipoR2 KO mice, and plated on six-well collagen-coated culture plates, as previously described.10, 11 After a 16-hour culture period, the culture medium was replaced with fresh medium with or without 10 μg/mL recombinant full-length mouse adiponectin (BioVendor Laboratory Medicine Inc., Czech Republic). Six hours later, cells were exposed to 1 nM recombinant TGF-β (R & D Systems, Minneapolis, MN), or vehicle, and further cultured for 12 hours.
Measuring Hepatic Protein Carbonyls.
The concentration of hepatic proteins containing carbonyl groups (those that react with 2,4-dinitrophenylhydrazine to form the corresponding hydrazone) was determined spectrophotometrically with a protein carbonyl assay kit (Cayman Chemical, Ann Arbor, MI).
Statistical Analysis.
All data are expressed as means ± standard error of the mean (SEM). Statistical analyses were performed using the unpaired Student test or one-way analysis of variance. When the analysis of variance analyses were applied, differences in mean values among groups were examined by Fisher's multiple comparison test.
Results
Adenovirus-Mediated Expression of AdipoR2 in Primary Cultures of Hepatocytes, and in the Livers of Mice Fed MCD Diet.
In primary cultures of hepatocytes, the ShR2-adenovirus we created significantly inhibited AdipoR2 expression, and the O/E-R2-adenovirus significantly increased it (Fig. 1A).

Adenovirus-mediated expression of AdipoR2 in primary cultures of hepatocytes, and in the livers of mice fed an MCD diet. (A) Levels of AdipoR2 mRNA in primary cultures of mice hepatocytes 48 hours after infection with 100 MOI of mock-adenovirus or ShR2-adenovirus (upper panel), and mock-adenovirus or O/E-R2-adenovirus (lower panel). (B) Levels of hepatic AdipoR2 mRNA in mice fed control diet or MCD diet for 1 week (left figure) or 3 weeks (right figure), with treatment of saline, mock-adenovirus, or ShR2-adenovirus. (C) Levels of hepatic AdipoR2 mRNA in mice fed control diet or MCD diet for 1 week (left figure) or 3 weeks (right figure), with treatment of saline, mock-adenovirus, or O/E-R2-adenovirus. Real-time PCR analysis was used to quantitate the mRNA levels of AdipoR2. The results (mean ± SEM) of four individual experiments are shown. All real-time PCRs were performed in duplicate. *P < 0.05 compared with the mock group. (D) Western blot analysis of expression of hepatic AdipoR2 in mice fed control diet or MCD diet for 1 week (left figure) or 3 weeks (right figure), with treatment of mock-adenovirus or ShR2-adenovirus. (E) Western blot analysis of expression of hepatic AdipoR2 in mice fed control diet or MCD diet for 1 week (left figure) or 3 weeks (right figure), with treatment of mock-adenovirus or O/E-R2-adenovirus. Cont, mice fed the control diet; MCD, mice fed the MCD diet; N/S, mice that received saline; Mock, mice that received the mock adenovirus; ShR2, mice that received the ShR2-adenovirus; O/E-R2, mice that received the O/E-R2-adenovirus.
Mice fed an MCD diet are widely used as a model for NASH because they demonstrate similar hepatic histology to that observed in human cases of NASH.9, 12 In the current study, we administered ShR2-adenovirus, O/E-R2-adenovirus, or each corresponding mock adenovirus to male C57BL/6 mice, and then fed them an MCD diet. One week after each adenovirus administration, we found that primarily the parenchymal cells of the liver had been infected; adenovirus infection was confirmed in more than 80% of parenchymal cells but in less than 25% of the nonparenchymal cells (data not shown).
Until 3 weeks after treatment, the ShR2-adenovirus significantly suppressed hepatic AdipoR2 messenger RNA (mRNA) and protein expression (Fig. 1B,D), whereas the O/E-R2-adenovirus significantly increased hepatic AdipoR2 mRNA and protein expression (Fig. 1C,E).
AdipoR2 Signaling in the Liver Regulates the Progression of the Early Stage of Nutritional Steatohepatitis.
After 3 weeks of MCD feeding, the group receiving the ShR2-adenovirus exhibited significant increases in accumulation of lipid droplets in hepatocytes, infiltration of inflammatory cells in the liver, and hepatic TG concentrations, particularly in the perivenular areas, relative to the group receiving the corresponding mock adenovirus (Fig. 2B,C). MCD feeding elevated serum ALT levels to a greater extent in the ShR2-adenovirus group than in the mock adenovirus group (Fig. 2A).
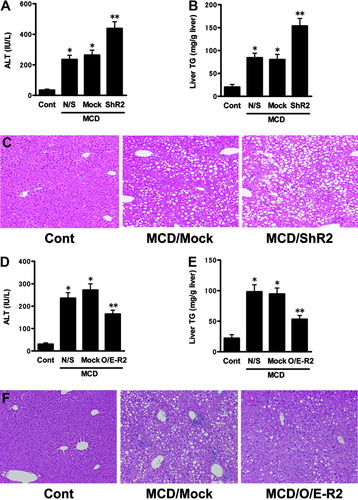
AdipoR2 signaling in the liver, controlled by the adenoviruses, regulates the progression of the early stage of steatohepatitis. The effect of hepatic AdipoR2 down-regulation on (A) serum ALT activities, (B) hepatic TG levels, and (C) HE-stained sections of representative liver samples, in each treated group that received 3 weeks of MCD or control feeding. Values are means ± SEM (n = 6-9/group). *P < 0.05 compared with the control diet group. **P < 0.05 compared with the mock group. Cont, mice fed the control diet; MCD, mice fed the MCD diet; N/S, mice that received saline; Mock, mice that received the mock adenovirus; ShR2, mice that received the ShR2-adenovirus. The effects of increased hepatic AdipoR2 expression on (D) serum ALT activities, (E) hepatic TG levels, and (F) HE-stained sections of representative liver samples from each treated group that received 3 weeks of MCD or control feeding. Values are means ± SEM (n = 6-9/group). *P < 0.05 compared with the control diet group. **P < 0.05 compared with the mock group. Cont, mice fed the control diet; MCD, mice fed the MCD diet; N/S, mice that received saline; Mock, mice that received the mock adenovirus; O/E-R2, mice that received the O/E-R2-adenovirus.
Conversely, the administration of O/E-R2-adenovirus inhibited the increase in the accumulation of lipid droplets in hepatocytes and the infiltration of inflammatory cells, which occurred after 3 weeks of MCD feeding (Fig. 2F). Consistent with the histological findings, hepatic TG concentrations were significantly lower in the O/E-R2-adenovirus group than in the mock adenovirus group (Fig. 2E). The administration of O/E-R2-adenovirus also significantly decreased the serum ALT levels (Fig. 2D).
Neither ShR2-adenovirus nor O/E-R2-adenovirus treatment had any significant effect on body weight, food intake, and serum levels of glucose, insulin, or adiponectin, in comparison with the corresponding mock adenovirus treatment (data not shown). Moreover, the effects of adenovirus treatment were tissue specific, because there were no differences in AdipoR2 mRNA levels in muscle, fat, kidney, or brain between the saline-treated mice and the mice treated with ShR2-adenovirus or O/E-R2-adenovirus (data not shown).
AdipoR2 Signaling in the Liver Regulates Hepatic Reactive Oxygen Species Accumulation in Early Stage of NASH, Through Activation of PPAR-α and Catalase.
We performed immunostaining of 4-HNE–adducted protein as a product of the lipid peroxidation reaction. In the early stage of nutritional steatohepatitis induced by 3 weeks of MCD feeding, lipid peroxidation in the liver was significantly enhanced, relative to the liver of control-fed mice (Fig. 3A). The ShR2-adenovirus group demonstrated more intense staining of hepatocytes after MCD feeding, centering around the centrilobular region of the liver, than did the mock adenovirus group (Fig. 3A). Conversely, 4-HNE–adducted protein in hepatocytes around the centrilobular region was significantly less intense in the livers of the O/E-R2-adenovirus group than in those of the mock adenovirus group (Fig. 3B).
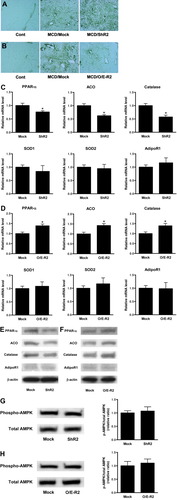
Hepatic AdipoR2 signaling controls ROS accumulation in the early stage of steatohepatitis, through regulation of the activities of PPAR-α and catalase. (A) The effect of hepatic AdipoR2 down-regulation on immunohistochemical staining for 4-HNE in the livers of each treated group that received 3 weeks of MCD feeding or control feeding. (B) The effects of increased hepatic AdipoR2 expression on immunohistochemical staining for 4-HNE in the livers from each treated group that received 3 weeks of MCD feeding or control feeding. (C, D) Real-time PCR was used to analyze each treated group that received 3 weeks of MCD feeding, and to quantitate the hepatic mRNA levels of PPAR-α, ACO, catalase, SOD1, SOD2, and AdipoR1. (C) Mock-adenovirus or ShR2-adenovirus treated group. (D) Mock-adenovirus or O/E-R2-adenovirus treated group. All real-time PCR reactions were performed in duplicate. Values are means ± SEM (n = 5-7/group). *P < 0.05 compared with the mock group. (E, F) Western blot analysis of expression of hepatic PPAR-α, ACO, catalase, and AdipoR1 in mice fed MCD diet for 3 weeks. (E) Mock-adenovirus or ShR2-adenovirus treated group. (F) Mock-adenovirus or O/E-R2-adenovirus treated group. (G, H) Western blot analysis of phosphorylated and total AMPK-α of liver lysates in mice fed MCD diet for 3 weeks. Representative blots are shown (left figure). Ratio of phosphorylated versus total AMPK-α was calculated by densitometric quantification, and expressed as a ratio of the values in mock-adenovirus–treated mice (right figure). Results are means ± SEM (n = 5-7/group). (G) Mock-adenovirus or ShR2-adenovirus treated group. (H) Mock-adenovirus or O/E-R2-adenovirus treated group. Cont, mice fed the control diet; MCD, mice fed the MCD diet; Mock, mice that received the mock adenovirus; ShR2, mice that received the ShR2-adenovirus; O/E-R2, mice that received the O/E-R2 adenovirus.
In the livers of the ShR2-adenovirus group, the expression of hepatic PPAR-α and the PPAR-α target gene ACO, was significantly reduced relative to the livers of the mock adenovirus group (Fig. 3C,E). The expression of catalase, an antioxidant enzyme, was also significantly reduced (Fig. 3C,E). Conversely, in the livers of the O/E-R2-adenovirus group, the expression of hepatic PPAR-α, ACO, and catalase was significantly increased relative to the livers of the mock adenovirus group (Fig. 3D,F). The alteration of AdipoR2 expression did not significantly change hepatic AMPK activity (Fig. 3G,H).
AdipoR2 Signaling in the Liver Plays a Key Role in the Progression of the Late Fibrotic Stage of Nutritional Steatohepatitis.
To examine how AdipoR2 deficiency in the liver affects the progression of fibrosis in steatohepatitis, we injected mice with mock or ShR2-adenovirus after feeding them an MCD diet for 5 weeks. Three weeks after infection, more extensive fibrosis was caused by steatohepatitis in the livers of the ShR2-adenovirus group than those of the mock adenovirus group (Fig. 4D). Fibrosis was conspicuously localized around the central vein and throughout the lobule in a pericellular distribution. Extraction and quantitation of liver hydroxyproline confirmed the microscopic results. The hydroxyproline content of the livers of the group infected with the ShR2-adenovirus was significantly higher than the content in those of the group infected with the mock adenovirus (Fig. 4A). Real-time PCR analysis of whole liver homogenates from ShR2-adenovirus-infected mice after MCD feeding showed a significant increase in α1(I) collagen (Col1α1), α2 (I) collagen (Col1α2), and tissue inhibitor of metalloproteinase 1 (TIMP-1) mRNA levels relative to mock adenovirus-infected mice (Fig. 4B). The livers of MCD-fed ShR2-adenovirus-infected mice also exhibited increased staining for α-SMA, a marker of stellate cell activation in areas of damage, in comparison with mock adenovirus-infected mice (Fig. 4C).
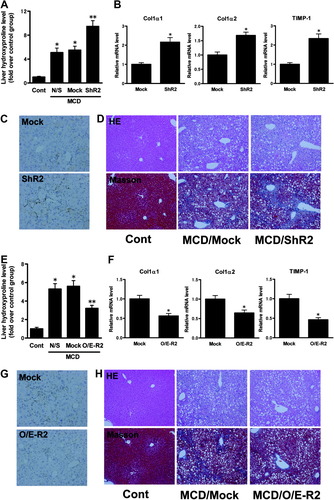
AdipoR2 signaling in the liver regulated the progression of late fibrotic stage of steatohepatitis. (A) The effect of hepatic AdipoR2 down-regulation on liver hydroxyproline concentrations in each treated group that received 8 weeks of MCD or control feeding. The data are expressed as fold over the control diet group. Values are means ± SEM (n = 5-7/group). *P < 0.05 compared with the control diet group. **P < 0.05 compared with the mock group. (B) Real-time PCR analysis was used to quantitate hepatic mRNA levels of Col1α1, Col1α2, and TIMP-1 in each treated group, infected with mock-adenovirus or ShR2-adenovirus. Here, all animals received 8 weeks of MCD feeding. All real-time PCR reactions were performed in duplicate. Values are means ± SEM (n = 5-7/group). *P < 0.05 compared with the mock group. (C) Immunohistochemical staining for α-SMA in the livers of each treated group that received 8 weeks of MCD feeding. (D) HE-stained sections and Masson trichrome (Masson)-stained sections of representative liver samples from each treated group that received 8 weeks of MCD or control feeding. (E) The effect of increased hepatic AdipoR2 expression on liver hydroxyproline concentrations in each treated group that received 8 weeks of MCD or control feeding. The data are expressed as fold over the control diet group. Values are means ± SEM (n = 5-7/group). *P < 0.05 compared with the control diet group. **P < 0.05 compared with the mock group. (F) Real-time PCR analysis was used to quantitate hepatic mRNA levels of Col1α1, Col1α2, and TIMP-1 in each treated group, infected with mock-adenovirus or O/E-R2-adenovirus. Here, all animals received 8 weeks of MCD feeding. All real-time PCR reactions were performed in duplicate. Values are means ± SEM (n = 5-7/group). *P < 0.05 compared with the mock group. (G) Immunohistochemical staining for α-SMA in the livers of each treated group that received 8 weeks of MCD feeding. (H) HE-stained sections and Masson trichrome (Masson)-stained sections of representative liver samples from each treated group that received 8 weeks of MCD or control feeding. Cont, mice fed the control diet; MCD, mice fed the MCD diet; N/S, mice that received saline; Mock, mice that received the mock adenovirus; ShR2, mice that received the ShR2-adenovirus; O/E-R2, mice that received the O/E-R2-adenovirus.
Next, to examine the influence of liver AdipoR2 overexpression on the progression of fibrosis in steatohepatitis, we infected mice with mock or O/E-R2-adenovirus after feeding them an MCD diet for 5 weeks. As shown in Fig. 4H, fibrosis progressed to a lesser extent in the livers of the O/E-R2-adenovirus group than in those of the mock adenovirus group, 3 weeks after infection. The hydroxyproline content of the livers of O/E-R2-adenovirus-infected mice was significantly lower than in the livers of mock adenovirus-infected mice (Fig. 4E). Real-time PCR analysis of whole liver homogenates of O/E-R2-adenovirus-infected mice following MCD feeding revealed a significant decrease in Col1α1, Col1α2, and TIMP-1 mRNA levels relative to mock adenovirus-infected mice (Fig. 4F). The livers of MCD-fed, O/E-R2-adenovirus-infected mice also exhibited decreased staining for α-smooth muscle actin in comparison with mock adenovirus-infected mice (Fig. 4G).
AdipoR2 Signaling in the Liver Regulates Hepatic Reactive Oxygen Species Accumulation in the Late Fibrotic Stage of NASH, Through Activation of PPAR-α and Catalase.
In the late stage of nutritional steatohepatitis induced by 8-week MCD feeding, lipid peroxidation in the liver was significantly enhanced, relative to the liver of control-fed mice (Fig. 5A). Immunostaining of 4-HNE-adducted protein demonstrated increased lipid peroxidation in the livers of ShR2-adenovirus-infected mice relative to those of mock adenovirus-infected mice (Fig. 5A). In the livers of the ShR2-adenovirus group, the expression of hepatic PPAR-α, ACO, and catalase were significantly reduced relative to the livers of the mock adenovirus group (Fig. 5B,C).
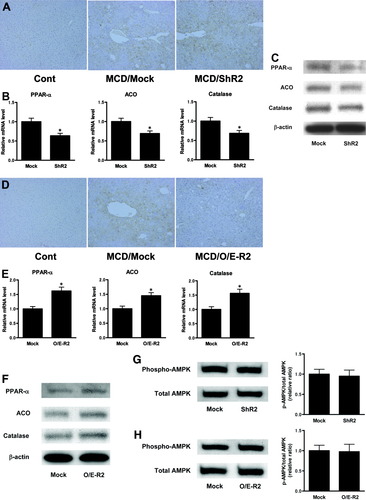
AdipoR2 signaling in the liver regulates hepatic ROS accumulation in the late fibrotic stage of NASH, through activation of PPAR-α and catalase. (A) The effect of hepatic AdipoR2 down-regulation on 4-HNE staining in the livers of each treated group; animals received 8 weeks of MCD feeding or control feeding. (B) Real-time PCR was used to quantitate the hepatic mRNA levels of PPAR-α, ACO, and catalase in each treated group, infected with mock-adenovirus or ShR2-adenovirus. Here, all animals received 8 weeks of MCD feeding. All real-time PCR reactions were performed in duplicate. Values are means ± SEM (n = 5-7/group). *P < 0.05 compared with the mock group. (C) Western blot analysis of expression of hepatic PPAR-α, ACO, and catalase in mice fed MCD diet for 8 weeks, with treatment of mock-adenovirus or ShR2-adenovirus. (D) The effect of increased hepatic AdipoR2 expression on 4-HNE staining in the livers of each treated group; animals received 8 weeks of MCD feeding or control feeding. (E) Real-time PCR was used to quantitate the hepatic mRNA levels of PPAR-α, ACO, and catalase in each treated group, infected with mock-adenovirus or O/E-R2-adenovirus. All animals received 8 weeks of MCD feeding. All real-time PCR reactions were performed in duplicate. Values are means ± SEM (n = 5-7/group). *P < 0.05 compared with the mock group. (F) Western blot analysis of expression of hepatic PPAR-α, ACO, and catalase in mice fed MCD diet for 8 weeks, with treatment of mock-adenovirus or O/E-R2-adenovirus. (G, H) Western blot analysis of phosphorylated and total AMPK-α of liver lysates in mice fed MCD diet for 8 weeks. Representative blots are shown (left figure), and ratio of phosphorylated versus total AMPK-α was calculated by densitometric quantification, and expressed as a ratio of the values in mock-adenovirus–treated mice (right figure). Results are means ± SEM (n = 5-7/group). (G) Mock-adenovirus or ShR2-adenovirus treated group. (H) Mock-adenovirus or O/E-R2-adenovirus treated group. Cont, mice fed the control diet; MCD, mice fed the MCD diet; Mock, mice that received the mock adenovirus; ShR2, mice that received the ShR2-adenovirus; O/E-R2, mice that received the O/E-R2-adenovirus.
Lipid peroxidation in the livers of O/E-R2-adenovirus-infected mice was significantly reduced, relative to those of mock adenovirus-infected mice (Fig. 5D). In the livers of the O/E-R2-adenovirus group, the expression of hepatic PPAR-α, ACO, and catalase was significantly increased relative to the livers of the mock adenovirus group (Fig. 5E,F). The alteration of AdipoR2 expression did not significantly change hepatic AMPK activity (Fig. 5G,H).
Both Reactive Oxygen Species Accumulation and AdipoR2 Signaling in the Liver Regulate Hepatic TGF-β1 Levels at All Stages of NASH Pathology.
Hepatic TGF-β1 expression level in MCD-fed mice significantly increased 1 week after treatment, and continued to rise until 8 weeks after treatment, compared with the level in control-fed mice (Fig. 6A). Co-administration of NAC, an antioxidant agent known to eliminate reactive oxygen species (ROS),13 significantly reduced the elevation of hepatic TGF-β1 expression in mice fed the MCD diet for 1 week, 3 weeks, or 8 weeks, in comparison with each corresponding group administered vehicle (Fig. 6A). This observation was confirmed by immunostaining for TGF-β1 (Fig. 6B,C). Co-administration of combined doses of vitamin C and vitamin E, other antioxidant agents known to eliminate hepatic ROS accumulation,14 also significantly reduced the elevation of hepatic TGF-β1 expression in mice fed an MCD diet for 4 weeks (Fig. 6D). Acute administration of t-BHP, which is reported to induce hepatic ROS accumulation,15-17 also increased hepatic TGF-β1 expression in mice (Fig. 6E). Real-time PCR analysis of whole liver homogenates from ShR2-adenovirus-infected mice after 3 weeks' MCD feeding showed a significant increase in TGF-β1 mRNA levels relative to mock adenovirus-infected mice (Fig. 7A), whereas hepatic TGF-β1 mRNA levels in O/E-R2-adenovirus-infected mice after 3 weeks' MCD feeding was significantly lower than in the livers of mock adenovirus-infected mice (Fig. 7B). This observation was confirmed by immunostaining for TGF-β1 (Fig. 7C,D). Similarly, in experiments focused on the late fibrotic stage of NASH induced by 8-week MCD feeding, TGF-β1 mRNA and protein expression in the livers of ShR2-adenovirus-infected mice was significantly higher than in the livers of mock adenovirus-infected mice (Fig. 7E,G), whereas hepatic TGF-β1 expression in the O/E-R2-adenovirus was significantly lower than in the livers of mock adenovirus-infected mice (Fig. 7F,H).
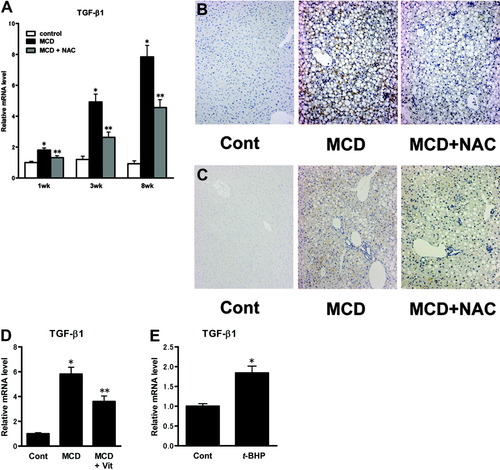
ROS accumulation in the liver enhances hepatic TGF-β1 levels. (A) Mice were fed an MCD diet or control diet for 1 week, 3 weeks, or 8 weeks, and co-treated with NAC or vehicle. In the 1-week or 3-week treated groups, animals were given NAC or vehicle daily during the feeding period. In the 8-week treated group, animals were given NAC or vehicle daily for the last 3 weeks of the feeding period. Real-time PCR was used to analyze each group, and to quantitate the hepatic mRNA levels of TGF-β1. Values are means ± SEM (n = 5-7/group). *P < 0.05 compared with the corresponding control diet group in the same feeding period. **P < 0.05 compared with the corresponding MCD diet group in the same feeding period. Control: animals fed control diet with vehicle treatment. MCD: animals fed MCD diet with vehicle treatment. MCD+NAC: animals fed MCD diet with NAC treatment. (B, C) Immunohistochemical staining for TGF-β1. Representative liver samples from mice fed control diet or MCD diet for 3 weeks (B) and for 8 weeks (C), with or without co-administration of NAC. Cont: animals fed control diet with vehicle treatment; MCD: animals fed MCD diet with vehicle treatment; MCD+NAC: animals fed MCD diet with NAC treatment; (D) Mice were fed an MCD diet or control diet for 4 weeks, along with co-administration of both vitamin C and vitamin E, or vehicle. Real-time PCR was used to quantitate the hepatic mRNA levels of TGF-β1. Values are means ± SEM (n = 5–7/group). *P < 0.05 compared with the control diet group. **P < 0.05 compared with the MCD diet group with vehicle treatment. Cont, animals fed control diet with vehicle treatment; MCD, animals fed MCD diet with vehicle treatment; MCD+Vit, animals fed MCD diet with combined doses of vitamin C and vitamin E. (E) The amount of hepatic TGF-β1 mRNA in mice treated with t-BHP or vehicle. Values are means ± SEM (n = 5–7/group). *P < 0.05 compared with the control group. Cont, mice given the vehicle; t-BHP, mice given the t-BHP.
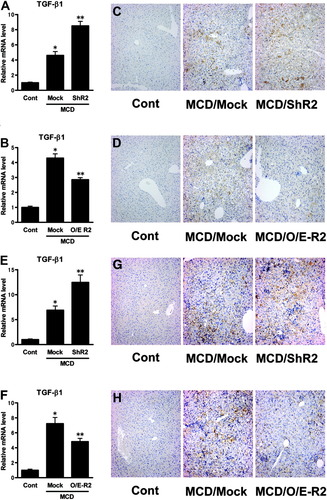
AdipoR2 signaling in the liver also regulates hepatic TGF-β1 level in NASH pathology. (A, B) The amount of hepatic TGF-β1 mRNA in mice fed control diet or MCD diet for 3 weeks, with treatment of mock, ShR2, or O/E-R2 adenovirus. (A) Mock-adenovirus or ShR2-adenovirus treated group. (B) Mock-adenovirus or O/E-R2-adenovirus treated group. Real-time PCR was used to quantitate the hepatic mRNA levels of TGF-β1. Values are means ± SEM (n = 5-7/group). *P < 0.05 compared with the control diet group. **P < 0.05 compared with the mock group. (C, D) Immunohistochemical staining for TGF-β1. Representative liver samples from mice fed control diet or MCD diet for 3 weeks, with treatment of mock, ShR2, or O/E-R2 adenovirus. (C) Mock-adenovirus or ShR2-adenovirus treated group. (D) Mock-adenovirus or O/E-R2-adenovirus treated group. (E, F) The amount of hepatic TGF-β1 mRNA in mice fed control diet or MCD diet for 8 weeks, with treatment of mock, ShR2, or O/E-R2 adenovirus. (E) Mock-adenovirus or ShR2-adenovirus treated group. (F) Mock-adenovirus or O/E-R2-adenovirus treated group. Real-time PCR was used to quantitate the hepatic mRNA levels of TGF-β1. Values are means ± SEM (n = 5-7/group). *P < 0.05 compared with the control diet group. **P < 0.05 compared with the mock group. (G, H) Immunohistochemical staining for TGF-β1. Representative liver samples from mice fed control diet or MCD diet for 8 weeks, with treatment of mock, ShR2, or O/E-R2 adenovirus. (G) Mock-adenovirus or ShR2-adenovirus treated group. (H) Mock-adenovirus or O/E-R2-adenovirus treated group. Cont, mice fed the control diet; MCD, mice fed the MCD diet; Mock, mice that received the mock adenovirus; ShR2; mice that received the ShR2-adenovirus; O/E-R2, mice that received the O/E-R2-adenovirus.
AdipoR2 Signaling Suppresses TGF-β–Induced ROS Accumulation in Hepatocytes Through Activation of PPAR-α and Catalase.
TGF-β treatment significantly increased ROS accumulation in both WT hepatocytes and AdipoR2 KO hepatocytes (Fig. 8A). Addition of adiponectin significantly suppressed TGF-β–induced ROS accumulation in WT hepatocytes, but had no effect in AdipoR2 KO hepatocytes (Fig. 8A). At the same time, TGF-β treatment significantly decreased the mRNA level of PPAR-α, ACO, and catalase in both WT hepatocytes and AdipoR2 KO hepatocytes (Fig. 8B). Cotreatment of adiponectin significantly reversed this effect only in WT hepatocytes. In AdipoR2 KO hepatocytes, addition of adiponectin had no effect (Fig. 8B).
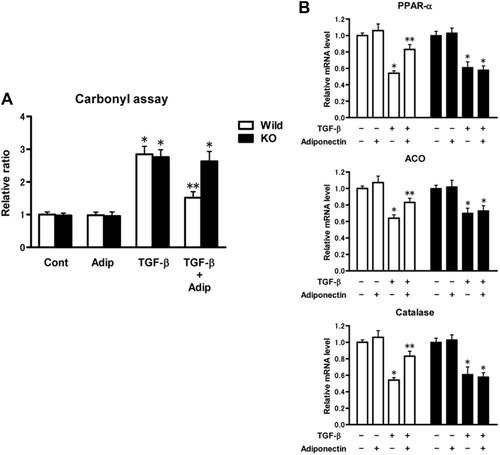
AdipoR2 signaling suppresses TGF-β–induced ROS accumulation in primary cultured hepatocytes. (A) Protein carbonyl content in primary cultured wild-type (white bar) or AdipoR2 KO (black bar) hepatocytes, after incubation with TGF-β with addition of adiponectin, or not. Quantifications were normalized for the content from the wild-type hepatocytes cultures without adiponectin or TGF-β. The results (mean ± SEM) of four individual experiments are shown. *P < 0.05 compared with the control group. **P < 0.05 compared with the content from the AdipoR2 KO hepatocytes after incubation of both TGF-β and adiponectin. (B) The amount of PPAR-α, ACO, and catalase mRNA in primary cultured wild-type (white bar) or AdipoR2 KO (black bar) hepatocytes, after incubation with TGF-β with addition of adiponectin, or not. Real time PCR was used to quantitate the hepatic mRNA level, and quantifications were normalized for RNA from the corresponding control cultures without addition of TGF-β or adiponectin. All real-time PCR reactions were performed in duplicate. The results (mean ± SEM) of four individual experiments are shown. *P < 0.05 compared with the cultures without addition of TGF-β. **P < 0.05 compared with the corresponding hepatocyte cultures with TGF-β without addition of adiponectin.
Discussion
We have elucidated the importance of hepatic AdipoR2 signaling in the pathology of NASH, using a mouse model of NASH induced by an MCD diet. At all stages of NASH pathology in the liver (fat accumulation, inflammation, and fibrosis), inhibition of AdipoR2 expression in the liver significantly exacerbated the pathological state of NASH. Conversely, increased AdipoR2 expression in the liver improved NASH at every stage.
Adiponectin activates adenosine monophosphate-activated protein kinase and PPAR-α through its receptors AdipoR1 and AdipoR2; regulation of these genes is thought to be a primary mechanism by which adiponectin acts against insulin resistance and diabetes.4 Recently, by controlling AdipoR1, AdipoR2, or both genes with knockout mice or adenovirus, Yamauchi et al.18 proved that the two receptors differ in the details of their downstream signaling pathways. AdipoR1 is more tightly linked to the activation of the AMPK pathway and couples the inhibition of hepatic glucose production to increased activation of fatty acid oxidation, whereas AdipoR2 is mainly involved in the activation of the PPAR-α pathway. In agreement with their findings, alteration of AdipoR2 expression did not change hepatic AMPK activity in our NASH animal model. They also reported that the expression levels of AdipoR1 and AdipoR2 change according to pathology, thereby regulating the adiponectin sensitivity and resistance of each organ. In fact, the expression levels of AdipoR1 and AdipoR2 are reportedly controlled by insulin/PI3 kinase/Foxo1,19 suggesting an important role for this pathway in the control of adiponectin sensitivity. These reports suggest that the possibility that AdipoR2, which is expressed predominantly in the liver, regulates the adiponectin sensitivity and resistance of the liver, mediating PPAR-α activity. The results of our current study support this possibility.
In this study, we first demonstrated that the inhibition of AdipoR2 signaling in the liver aggravated NASH at every stage, from the early stage to the progression of fibrosis, by inhibiting PPAR-α activity in hepatocytes. We also proved that hepatic AdipoR2 overexpression had the opposite effect.
PPAR-α controls the expression of a number of genes involved in peroxisomal and mitochondrial β-oxidation. The inhibition of AdipoR2 signaling in the liver caused the inhibition of PPAR-α signaling in hepatocytes, increasing the TG accumulation in hepatocytes in livers with steatohepatitis and also inhibiting the expression of ACO, a hepatic enzyme that contributes to fatty acid oxidation. Meanwhile, the inhibition of AdipoR2 signaling induced lipid peroxidation and oxidative stress in livers with steatohepatitis, and it also reduced the expression of the antioxidant enzyme catalase. Toyama et al.20 recently reported that PPAR-α ligand activated hepatic catalase,20 which is also consistent with our results. They showed that PPAR-α ligand also activated hepatic superoxide dismutase (SOD), although the enhancement of expression was more specific and striking for catalase than SOD. These results suggest that alteration of PPAR-α activity, regulated by AdipoR2 signaling, would not be severe enough to significantly change hepatic SOD expression in our NASH mice model. In our NASH model, hepatic expression of antioxidant enzymes, such as catalase, significantly decreased (Table 1), similar to the situation in NASH patients.21 Future studies will be required to determine the role of regulation of hepatic antioxidant molecules other than those in the PPAR-α and AdipoR2 signaling pathways in the pathogenesis of NASH.
| Gene | MCD (3 weeks) | MCD (8 weeks) |
|---|---|---|
| PPAR-α | 1.31 ± 0.18 | 0.46 ± 0.02* |
| ACO | 0.79 ± 0.12 | 0.69 ± 0.03* |
| Catalase | 0.72 ± 0.04* | 0.43 ± 0.04* |
| SOD1 | 1.03 ± 0.06 | 0.68 ± 0.09* |
| SOD2 | 1.09 ± 0.12 | 0.62 ± 0.05* |
| AdipoR1 | 1.18 ± 0.17 | 1.19 ± 0.14 |
| AdipoR2 | 0.93 ± 0.13 | 0.73 ± 0.11 |
- Results (and standard error of the mean) from mice fed the MCD diet (n = 4-5/group). Quantifications were normalized for RNA from the liver in mice fed the control diet for the same total period of time.
- * P < 0.05 versus the corresponding control group.
Accumulated ROS and TGF-β signaling in liver are thought to play a key role in the pathogenesis of nonalcoholic steatohepatitis.22 In the current study, we found that the ROS production and TGF-β expression in liver were significantly enhanced at every stage of nutritional steatohepatitis. Accumulated ROS in liver activates hepatic nonparenchymal cells, including Kupffer cells and hepatic stellate cells.23 Activated Kupffer cells are reported to release TGF-β, a known pro-fibrotic factor that has been implicated in the activation of other surrounding liver cells, including hepatic stellate cells, through a paracrine mechanism.24 Hepatic stellate cells are known to be key players in liver fibrosis; once activated by multiple stimuli such as ROS and TGF-β, they produce collagen and additional TGF-β, leading to the further activation of hepatic stellate cells via a paracrine or autocrine mechanism.25 In the current study, hepatic expression of TGF-β1 was enhanced at both the early stage and late fibrotic stage of NASH. We also found that elimination of ROS accumulation in liver decreased hepatic TGF-β1 levels, whereas enhancement of ROS in liver increased TGF-β1 mRNA levels. Our in vitro study showed that the treatment of TGF-β exaggerated ROS accumulation in hepatocytes, along with decreased expression of PPAR-α, ACO, and catalase. These results suggest that a vicious cycle of ROS and TGF-β could play a key role in liver injury in the early stage of NASH as well as in the late fibrotic stage. Recent reports also showed that silencing of TGF-β signaling in hepatocytes, rather than in nonparenchymal cells, could be effective in treating acute liver injury.26 Our in vitro study also proved that AdipoR2 signaling suppressed TGF-β–induced ROS accumulation in hepatocytes, through activation of PPAR-α and catalase. Because AdipoR2 signaling was controlled mainly in hepatocytes in our in vivo study, we hypothesize that ROS accumulation in hepatocytes, which is controlled by multiple mediators including TGF-β, could be regulated by AdipoR2 signaling. This mechanism could play a key role in the pathogenesis of NASH. This exaggerated accumulation of ROS led to parenchymal cell damage and nonparenchymal cell activation with enhanced release of TGF-β in the liver, thus establishing the malignant cycle of NASH progression.
In hepatocytes in our in vitro study, adiponectin/AdipoR2 signaling did not have any direct effect on the mRNA level of PPAR-α, ACO, and catalase, but reversed the decrease in expression levels that resulted from TGF-β treatment. These results suggest that adiponectin/AdipoR2 signaling in hepatocytes is involved in the activation of the PPAR-α pathway, mainly by antagonizing the actions of various molecules, such as TGF-β, on PPAR-α activities.
In summary, we demonstrated that an increase or decrease in AdipoR2 signaling in hepatocytes regulates the level of progression of nutritional steatohepatitis. AdipoR2 signaling plays a pivotal role in the pathology-regulating mechanism, which is initiated by ROS that accumulate due to dysregulation of PPAR-α and catalase activity in hepatocytes.
The two-hit theory proposed by Day and James27 is widely advocated as a pathogenic mechanism for NASH. This theory claims that, in addition to steatosis (the first hit), the development of steatohepatitis requires the presence of some other factor or factors (the second hit). The current study revealed that decreased AdipoR2 signaling in the liver can serve as the second hit in NASH progression. Because AdipoR2 genes could regulate susceptibility to NASH, relevant genetic and proteomic analysis will prove insightful in the future.
There are no established therapeutic modalities for NASH, and it is urgent to develop such modalities. The enhancement of the AdipoR2 signaling pathway in the liver will serve as a promising target in the treatment of NASH.
Acknowledgements
The authors thank T. Yokoyama, S. Tanaka, J. Amano, and T. Chiyo for technical assistance.




