Natural killer T cells exacerbate liver injury in a transforming growth factor β receptor II dominant-negative mouse model of primary biliary cirrhosis†
Potential conflict of interest: Nothing to report.
fax: 530-752-4669
Abstract
Primary biliary cirrhosis (PBC) is an organ-specific autoimmune liver disease characterized by the presence of antimitochondrial antibodies and the destruction of small intrahepatic bile ducts with portal inflammation. In previous studies, we reported that both CD1d expression and the frequency of CD1d-restricted natural killer T (NKT) cells were increased in the livers of patients with PBC. To define a specific role of CD1d-restricted NKT cells in the pathogenesis of PBC, particularly early events, we investigated the function of hepatic CD1d-restricted NKT cells in our transforming growth factor β (TGF-β) receptor II dominant-negative (dnTGFβRII) mouse model of PBC. We generated CD1d−/− and CD1d+/− dnTGFβRII mice and performed a comparative study of liver immunopathology. We report herein that these dnTGFβRII mice demonstrate a massive increase of hyperactive CD1d-restricted NKT cells within the hepatic tissues. CD1d−/−dnTGFβRII mice, which lack CD1d-restricted CD1d-restricted NKT cells, exhibit significantly decreased hepatic lymphoid cell infiltrates and milder cholangitis compared with CD1d+/−dnTGFβRII mice. Interestingly, there was a significant increase in the production of interferon-γ in hepatic CD1d-restricted NKT cells activated by α-galactosylceramide in young but not older dnTGFβRII mice, suggesting an age-dependent role of CD1d-restricted NKT cells. Conclusion: These data demonstrate that CD1d-restricted NKT cells in dnTGFβRII mice are a critical factor in liver injury. (HEPATOLOGY 2008.)
Primary biliary cirrhosis (PBC) is an organ-specific autoimmune liver disease characterized by the presence of antimitochondrial antibodies (AMAs) and the destruction of small intrahepatic bile ducts with portal inflammation. Although the pathogenesis of PBC remains unknown, both CD4+ and CD8+ T cell responses play a key role in the destruction of the biliary tract.1-4 Such autoreactive T cells are induced by the CD83+ dendritic cells leading to the generation of autoantibodies against mitochondrial autoantigens, particularly the E2 subunit of the pyruvate dehydrogenase enzyme complex, which is the signature of this disease.5-8 Innate immunity has also been postulated to play a key role in the exacerbation of liver injury.9-14 For example, natural killer T (NKT) cells are increased in number in the liver of PBC patients and recruited more efficiently, resulting in exacerbated hepatic damage which is hypothesized to be due to their higher cytotoxic ability.9 B cells from PBC patients are also abnormal as exemplified by their synthesis of significantly higher levels of polyclonal immunoglobulin M (IgM) and AMAs when stimulated in vitro with oligonucleotides.10, 11, 13
Although CD1d expression and the frequency of CD1d-restricted NKT cells are both increased in PBC liver,15, 16 the involvement of such CD1d-restricted NKT cells in the pathogenesis of PBC is at present not clear. In concanavalin A–induced hepatitis, CD1d-restricted NKT cells are required and sufficient for induction of liver injury by releasing a variety of cytokines, including interleukin-4 (IL-4), IL-5, interferon-γ (IFN-γ), and tumor necrosis factor α (TNF-α).17-19 Depletion of NKT cells ameliorates concanavalin A–induced liver disease,19 and CD1d-deficient mice are highly resistant to concanavalin A–induced hepatitis.18 It is important to note that whereas there is an increase in the hepatic presence of CD1d-restricted NKT cells in PBC patients compared with controls, there is in fact a concomitant decrease of this cell lineage in the peripheral blood of PBC patients compared with controls,15 suggesting a role for selective trafficking of this cell lineage in PBC patients.
We have demonstrated previously that a mouse transgenic for directed expression of a dominant-negative form of TGF-β receptor II (dnTGFβRII), under the influence of the CD4 promoter, mimics a series of pathological and phenotypic features of human PBC, highlighted by significant auto-antibody responses to the same autoantigens as human PBC and an increased number of liver CD1d-restricted NKT cells.20 To more specifically define the functional pathogenic role of this cell lineage, we bred CD1d−/− mice with the dnTGFβRII mice and derived CD1d−/−dnTGFβRII mice. We report herein that CD1d-restricted NKT cells exacerbate liver injury in the early stages of murine PBC by secreting IFN-γ and lymphocyte recruitment.
Abbreviations
α-GalCer, α-galactosylceramide; AMA, antimitochondrial antibody; BSA, bovine serum albumin; dnTGFβRII, transforming growth factor β receptor II dominant-negative; FITC, fluorescein isothiocyanate; IFN-γ, interferon-γ; Ig, immunoglobulin; IL, interleukin; NKT, natural killer T; PBC, primary biliary cirrhosis; PBS, phosphate-buffered saline; PE, phycoerythrin; TCR, T cell receptor; TGFβ, transforming growth factor β.; Th1, T helper 1; Th2, T helper 2; TNF-α, tumor necrosis factor α.
Materials and Methods
Mice.
dnTGFβRII mice were bred onto a C57BL/6 (B6) background (Jackson Laboratory, Bar Harbor, ME) at the University of California animal facility (Davis, CA). CD1d−/− mice on a B6 background were generated as described previously.21 To generate CD1d−/−dnTGFβRII mice, male dnTGFβRII mice were bred with female CD1d−/− mice to obtain CD1d+/−dnTGFβRII mice; male CD1d+/−dnTGFβRII mice were bred with female CD1d−/− mice to obtain CD1d−/−dnTGFβRII and CD1d+/−dnTGFβRII mice. The PBC-like disease described herein occurs equally in male and female mice.20 However, to maintain the homogeneity, the work reported herein only used female mice. In all cases, the dnTGFβRII gene was confirmed via polymerase chain reaction,20 and the expression of CD1d on peripheral blood mononuclear cells was determined by flow cytometry.9 Mice were bled biweekly, and sera were stored at −20°C for assays of AMA and cytokine levels. Mice were fed with the sterile rodent Helicobacter Medical Dosing System (3-drug combination) diets (Bio-Serv, Frenchtown, NJ) and maintained in individual ventilated cages under specific pathogen-free conditions. At 9 weeks and 21 weeks of age, CD1d+/−dnTGFβRII and CD1d−/−dnTGFβRII mice were sacrificed, and livers were removed for phenotypic and histopathological studies. Survival was not considered an endpoint in this work because the animals were manipulated throughout this study; also, the number of animals required for appropriate power calculations were not available. All animal experiments were performed after receiving approval of the Institutional Animal Care and Use Committee of University of California.
Cell Preparation.
Livers were collected from dnTGFβRII, CD1d−/−dnTGFβRII, and for additional purpose of control CD1d+/−dnTGFβRII and wild-type B6 mice. The livers were perfused with phosphate-buffered saline containing 0.2% bovine serum albumin (PBS/0.2% BSA) (EMD Chemicals, Gibbstown, NJ), passed through a nylon mesh, and resuspended in PBS/0.2% BSA.22 The parenchymal cells were removed as pellets after centrifugation at 700 rpm for 1 minute and the nonparenchymal cells were washed in PBS/0.2% BSA 3 times (1500 rpm for 5 minutes) to remove hepatocytes. Lymphocytes were then isolated using Histopaque-1077 (Sigma Chemical Co., St. Louis, MO). After centrifugation, cells were washed with PBS/0.2% BSA and viability was confirmed by trypan blue dye (Sigma Chemical Co.) exclusion. For analyses of Smad2 phosphorylation, CD1d-restricted NKT cells were purified from livers of dnTGFβRII mice by a 10-parameter MoFlo cell sorter (Cytomation, Fort Collins, CO) by staining with CD3 and α-galactosylceramide (α-GalCer)-CD1d tetramer. The purity of cells was usually greater than 97%. Conventional CD4+ T cells were isolated from spleens of B6 and dnTGFβRII mice using MACs (Miltenyi Biotech Inc., Auburn, CA). The purity of cells was greater than 85%. Cells were stimulated with anti-CD3/anti-CD28 monoclonal antibody (5 μg/mL each) and 5 ng/mL of recombinant TGF-β1 (R&D Systems, Minneapolis, MN) for 1 hour.
α-GalCer Injection.
A stock solution of α-GalCer (Alexis Biochemicals Corp., San Diego, CA) was diluted to 0.2 mg/mL in 0.5% polysorbate-20 and stored at −20°C. For cytokine production of CD1d-restricted NKT cells, dnTGFβRII and normal control mice (5 weeks old and 15-20 weeks old) were intraperitoneally injected with 2 μg of α-GalCer, and liver mononuclear cells were isolated 2 hours later. In addition, dnTGFβRII mice (6-8 weeks old) were injected with 4 μg α-GalCer or PBS twice a week for 2 weeks for the isolation and study of CD1d-restricted NKT cells.
Antibodies and Flow Cytometry.
Cell surface phenotype was determined by multicolor flow cytometry.9 Before staining cells with previously defined optimal dilution of monoclonal antibodies, the cells were preincubated with anti-CD16/32 (clone 93) to block nonspecific FcRγ binding. The following antibodies were used in this study: anti-CD3 allophycocyanin, anti-CD4 allophycocyanin/Cy7 (Biolegend, San Diego, CA), anti-NK1.1 fluorescein isothiocyanate (FITC), anti-CD44 FITC, anti-CD69 FITC, anti-CD8α phycoerythrin (PE)/Cy5 (eBioscience, San Diego, CA), and R-PE–conjugated mouse CD1d tetramers loaded with α-GalCer (α-GalCer–CD1d tetramer). For intracellular staining, liver mononuclear cells were incubated with brefeldin A (10 μg/mL) (eBioscience) at 37°C for 2 hours, then incubated with anti-CD16/32 antibodies, followed by staining with PE-conjugated α-GalCer–CD1d tetramer and PE/Cy5-conjugated CD3, permeabilized with Cytofix/Cytoperm reagent (BD Biosciences, San Diego, CA), and stained with FITC-conjugated anti-IFN-γ (clone XMG1.2), IL-4 (clone 11B11), or rat IgG1 isotype control (clone R3-34) (eBioscience). Stained cells were assessed on a 5-color FACScan flow cytometer (BD Immunocytometry Systems, San Jose, CA) upgraded by Cytec Development (Fremont, CA). Acquired data were analyzed with CELLQUEST Pro (BD Immunocytometry Systems) and FlowJo softwares (Tree Star, Inc., Ashland, OR).
Immunoblot Analysis.
Cells were lysed in RIPA buffer [50 mM Tris HCl (pH 8.0), 0.5% sodium deoxycholate, 1% NP-40, 150 mM NaCl, 0.1% sodium dodecyl sulfate] containing complete protease inhibitor cocktail (Boehringer-Manheim), and lysates were collected after centrifugation. Total cell lysates of indicated cell populations were separated on precast 4%-12% Bis-Tris polyacrylamide mini-gel electrophoresis (NuPAGE System; Invitrogen, Carlsbad, CA), transferred to a nitrocellulose membrane, and probed with antibodies to total Smad2 (Transduction Laboratories) and anti–phospho-Smad2 (Ser465/467; Upstate Biotechnology). The amounts of protein were determined using a Bio-Rad protein assay to ensure equal protein loading for the analysis.
Determination of Serum AMA and Cytokine Levels.
Serum IgM, IgA, and IgG AMA titers were measured by enzyme-linked immunosorbent assay as described previously.23 Briefly, purified recombinant E2 subunit of the pyruvate dehydrogenase enzyme complex at 5 μg/mL in carbonate buffer (pH 9.6) was coated onto enzyme-linked immunosorbent assay plates at 4°C overnight and blocked with 1% BSA for 1 hour. Sera diluted 1:500 were added for 2 hours at room temperature. The microtiter plate was washed with PBS ×4 followed by the addition of horseradish peroxidase–conjugated goat anti-mouse G, A, and M (1:10,000) (Zymed, San Francisco, CA). The microtiter plate was incubated for another 1 hour and immunoreactivity was detected by measuring the optical density at 450 nm after exposure for 15 minutes to tetramethylbenzidine substrate (R&D Systems). Known positive and negative samples were included in each assay.
Sera cytokine levels were analyzed using the BD Cytometric Bead Array kit (BD Immunocytometry Systems). Briefly, samples were mixed for 2 hours at room temperature with florescence-labeled capture beads with the PE detection reagents to measure the relative quantities of IL-12 p70, TNF-α, IL-10, IL-6, IL-1β, IL-8, IFN-γ, IL-4, IL-2, and IL-5. Samples were then washed with washing buffer and analyzed on a FACScan flow cytometer (BD Immunocytometry Systems); data were analyzed using BD Cytometric Bead Array Analysis software (BD Immunocytometry Systems).
Histopathology.
Livers were fixed in a 1:1 solution of formalin (18.75%) and methanol (100%) for 1 day at room temperature. Paraffin-embedded tissue sections were then cut into 5-μm slices for routine hematoxylin (DakoCytomation, Carpinteria, CA) and eosin (American Master Tech Scientific, Lodi, CA) staining. The sections were numbered and blindly interpreted by a pathologist who was unaware of the protocol, and the data were recorded according to the coded numbers of the slides.
Statistical Analysis.
All results are expressed as the mean ± standard error of the mean and were evaluated with 1-way analysis of variance, followed by an unpaired 2-tailed Student t test for comparison between 2 groups.
Results
Phenotype Analysis of Hepatic Lymphoid Cells.
We evaluated the absolute number of CD1d-restricted NKT cells as a function of age in the liver of dnTGFβRII and for comparison control mice using α-GalCer–loaded CD1d tetramer reagent and flow cytometry. Significantly increased hepatic CD1d-restricted NKT cells were observed in dnTGFβRII mice compared with littermate control mice at the 2 ages studied (P < 0.05) (Fig. 1A -B). To investigate whether the dnTGFβRII gene acted on hepatic CD1d-restricted NKT cells, we measured the phosphorylation of Smad2, a TGF-β signaling molecule, in cells cultured with recombinant TGF-β via western blotting. Phosphorylated Smad2 was undetectable in CD1d-restricted NKT cells with or without addition of TGF-β1 to the culture medium (Fig. 1C). This result suggests that the increased number of CD1d-restricted NKT cells in dnTGFβRII mice is due to the direct effect of the dnTGFβRII gene. The activation status of hepatic CD1d-restricted NKT cells in dnTGFβRII mice was evaluated by measuring the expression of NK1.1, CD69 (an early activation marker), and CD44 (a late activation marker) on CD3+α-GalCer–CD1d tetramer+ cells. All hepatic CD1d-restricted NKT cells in dnTGFβRII and normal mice express an activated (memory) phenotype (CD62L−CD69+CD44hiIL-2Rβhi),24 however, the expression levels of NK1.1, CD44, and CD69 on hepatic CD1d-restricted NKT cells in dnTGFβRII mice were significantly higher than control mice (P < 0.005 for NK1.1; P < 0.005 for CD69; and P < 0.05 for CD44) (Fig. 1D).
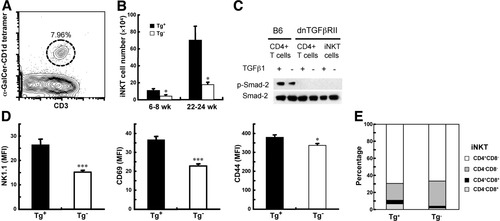
Increased number, activation markers and the CD4−CD8+ subpopulation of CD1d-restricted NKT cells in the livers of dnTGFβRII mice. (A) Representative flow cytometry analysis of CD1d-restricted NKT cells (CD3+ α-GalCer–CD1d tetramer+). (B) Absolute numbers of CD1d-restricted NKT cells in dnTGFβRII mice (Tg+) or littermate control mice (Tg−). The absolute number of CD1d-restricted NKT cells was calculated by multiplying the frequency of CD1d-restricted NKT cells by the absolute number of hepatic lymphoid cells (n = 5-9 per group). (C) The expression of Smad2 and phosphorylated Smad2 in the indicated cells was measured via western blotting. Splenic CD4+ cells from B6 mice or dnTGFβRII mice were loaded parallel to serve as positive and negative control, respectively. (D) The expression of NK1.1, CD69, and CD44 on hepatic CD1d-restricted NKT cells was analyzed via flow cytometry. The levels of surface molecules indicated by the mean fluorescence intensity (MFI) for each group were calculated (n = 6 per group). *P < 0.05; ***P < 0.0005. (E) Subsets of CD1d-restricted NKT cells in the liver (n = 5-9 per group). Tg+, dnTGFβRII mice; Tg−, littermate controls.
We used multiparameter flow cytometry analysis to evaluate the expression of CD4 and CD8 on hepatic CD1d-restricted NKT cells. The frequency of CD4+ and CD8+ cells on the gated population of CD3+α-GalCer–CD1d tetramer+ cells was determined. In addition to the dominant populations of CD4−CD8− and CD4+CD8− CD1d-restricted NKT subsets, an obvious subset of CD4−CD8+ CD1d-restricted NKT cells was noted in the dnTGFβRII mice (Fig. 1E).
Cytokine Expression by Hepatic CD1d-Restricted NKT Cells.
We examined the functional properties of CD1d-restricted NKT cells by assessing their capacity for cytokine production at the single-cell level by intracellular staining. Hepatic CD1d-restricted NKT cells from 5 week-old dnTGFβRII mice that were injected with α-GalCer produced increased levels of IFN-γ (1.43-fold) and IL-4 (1.14-fold) compared with that of control littermate mice (P < 0.001 for IFN-γ production, P < 0.01 for IL-4 production, and P < 0.01 for IFN-γ/IL-4) (Fig. 2A -B). An additional assessment of cytokine production resulting from activation of CD1d-restricted NKT cells in mice was conducted by measuring serum cytokine levels 2 hours after in vivo administration of α-GalCer. Serum IL-2, IL-4, IFN-γ, and TNF-α levels were significantly augmented in dnTGFβRII mice (Fig. 2C). These results suggest that hepatic CD1d-restricted NKT cells in dnTGFβRII mice were more responsive to α-GalCer than the normal mice.
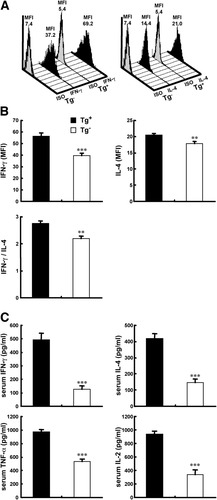
Increased cytokine production in young dnTGFβRII mice injected with α-GalCer. Five-week-old dnTGFβRII mice and control mice were intraperitoneally injected with 2 μg of α-GalCer. Cytokine expression was measured 2 hours after injection. (A, B) IFN-γ and IL-4 expression in hepatic CD1d-restricted NKT cells were measured via intracellular flow cytometry. (A) Representative flow cytometry analysis of cytokine expression in CD1d-restricted NKT cells. (B) The amounts of IFN-γ and IL-4 production per cell indicated by the mean fluorescence intensity (MFI) for each group were calculated. (C) Serum levels of IFN-γ, TNF-α, IL-2, and IL-4 were measured using cytometric bead array (n = 6 per group). *P < 0.05; **P < 0.01; ***P < 0.0005.
In Vivo Injection with α-GalCer–Induced Cell Infiltration and Serum IFN-γ Production.
α-GalCer is a potent immunostimulator for CD1d-restricted NKT cells both in vivo and in vitro.25 To examine the effect of activated CD1d-restricted NKT cells in the development of PBC, α-GalCer was injected into young dnTGFβRII mice, and disease progress was monitored. A hallmark of the dnTGFβRII mouse model for PBC is the progressive infiltration of cells into the liver and increased levels of serum cytokines such as IFN-γ.20 As seen in Fig. 3, an increased number of liver cellular infiltrates, including CD1d-restricted NKT cells, NK (CD3− NK1.1+) cells, and NKT (CD3+ NK1.1+ α-GalCer-CD1d tetramer−) cells accompanied by augmented serum IFN-γ production was observed in dnTGFβRII mice injected with α-GalCer compared with control mice (Fig. 3A-B). Serum IFN-γ levels increased massively within hours after a single α-GalCer injection as shown in Fig. 2C, but increased only slightly 3 days later as shown in Fig. 3C. In addition, multiple injections of α-GalCer maintained serum levels of IFN-γ but did not increase them (Fig. 3C).
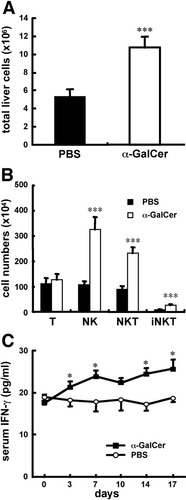
In vivo injection with α-GalCer induces increased cell infiltration and serum IFN-γ production in dnTGFβRII mice. dnTGFβRII mice (6-8 weeks old) were injected with 4 μg α-GalCer or PBS twice a week for 2 weeks. The numbers of (A) total liver cells, and (B) T (CD3+NK1.1−) cells, NK (CD3− NK1.1+) cells, NKT (CD3+ NK1.1+ α-GalCer–CD1d tetramer−) cells, and iNKT (CD3+ α-GalCer–CD1d tetramer+) cells were measured 3 days after the last injection. (C) Sera were collected at indicated time points and IFN-γ production was measured via cytometric bead array (n = 5-6 per group). *P < 0.05; ***P < 0.0005.
Reduced Serum IFN-γ and Liver Cell Infiltrates in 9-Week-Old CD1d−/−dnTGFβRII Mice.
To investigate the functional contribution of CD1d-restricted NKT cells in the induction of liver cell injury in dnTGFβRII mice, a CD1d-deficient genotype was crossed onto dnTGFβRII mice. As determined by fluorescence-activated cell sorting analysis, the expression of CD1d on peripheral blood mononuclear cells and of CD1d-restricted NKT cells in the livers and spleens of CD1d−/−dnTGFβRII mice were undetectable (data not shown). CD1d+/−dnTGFβRII mice, as control mice, develop autoimmune cholangitis, including moderate cell infiltration in biliary ducts, increased serum AMAs, elevated CD3+ T cells, reversed ratio of CD4 to CD8, and increased serum IFN-γ, TNF-α, and IL-6 production20 (Figs. 4-6). At 9 weeks of age, serum IFN-γ production in CD1d−/−dnTGFβRII mice was significantly lower than in CD1d+/−dnTGFβRII mice (Fig. 4A). In addition, the number of hepatic lymphocytes, excluding CD1d-restricted NKT cells, was significantly decreased in CD1d−/−dnTGFβRII mice compared with CD1d+/−dnTGFβRII mice (Fig. 4B). There was a milder lymphoid cell infiltrate in the livers of CD1d−/−dnTGFβRII mice compared with CD1d+/−dnTGFβRII mice (Fig. 4C).
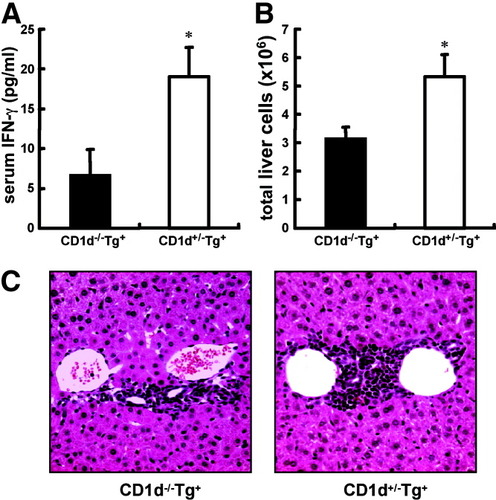
Reduced serum IFN-γ production and liver cell infiltrates in the livers of 9-week-old CD1−/−dnTGFβRII mice. (A) Serum IFN-γ production was measured using cytometric bead array. (B) The number of total liver cells excluding CD1d-restricted NKT cells was measured. (C) Representative results of histopathologic examination of livers in 9-week-old CD1d−/−dnTGFβRII mice (left panel) and CD1d+/−dnTGFβRII littermates (right panel) (n = 5 per group). *P < 0.05. (Original magnification ×200.)
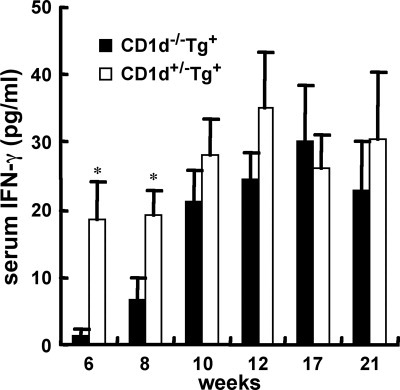
Serum levels of IFN-γ at different ages of CD1d−/−dnTGFβRII and heterozygous controls were measured (n = 5-9 per group). *P < 0.05.
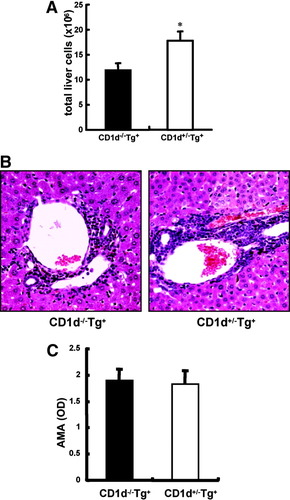
Reduced liver cell infiltrates in the livers of 21-week-old CD1d-restricted NKT-deficient dnTGFβRII mice. (A) The number of total liver cells excluding CD1d-restricted NKT cells was measured. (B) Representative results of histopathological examination of livers in 21-week-old CD1d−/−dnTGFβRII mice and CD1d+/−dnTGFβRII littermates. (Original magnification ×200). (C) Serum AMAs were measured via enzyme-linked immunosorbent assay (n = 5 per group). OD, optical density. *P < 0.05.
Reduced Liver Cell Infiltrates in Livers of 21-Week-Old CD1d−/−dnTGFβRII Mice.
We measured serum levels of IFN-γ from CD1d−/−dnTGFβRII and heterozygous littermate control mice of varying ages. Unexpectedly, the serum level of IFN-γ in CD1d−/−dnTGFβRII mice >10 weeks old showed no significant difference compared with age-matched CD1d+/−dnTGFβRII mice (Fig. 5). At 21 weeks of age, the number of liver cell infiltrates, excluding CD1d-restricted NKT cells, was still significantly lower in CD1d−/−dnTGFβRII mice than in CD1d+/−dnTGFβRII mice (Fig. 6A). In addition, 21-week-old CD1d−/−dnTGFβRII mice still demonstrate a milder cell infiltration in the biliary duct of the liver compared with that of CD1d+/−dnTGFβRII mice (Fig. 6B). However, serum AMA levels were similar between the 2 groups of mice (Fig. 6C).
CD1d-Restricted NKT Cells in Older dnTGFβRII Mice Do Not Secrete High Levels of IFN-γ and IL-4.
To test the possibility of whether individual CD1d-restricted NKT cells in young and older dnTGFβRII mice have differences in their ability to secrete cytokines, we measured IFN-γ and IL-4 expression in hepatic CD1d-restricted NKT cells from 15- to 20-week-old mice injected with α-GalCer. In contrast to the significantly increased expression of IFN-γ and IL-4—especially IFN-γ in hepatic CD1d-restricted NKT cells in young dnTGFβRII mice (Fig. 2B)—the expression levels of IFN-γ and IL-4 in CD1d-restricted NKT cells in older dnTGFβRII mice was similar to age-matched normal controls (Fig. 7).
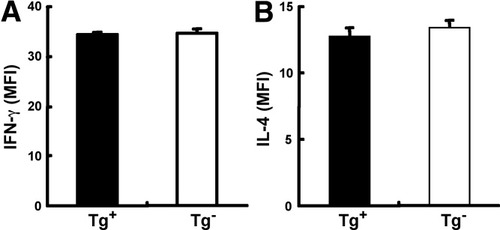
CD1d-restricted NKT IFN-γ and IL-4 expression in older dnTGFβRII mice. Fifteen- to 20-week-old dnTGFβRII mice and control mice were intraperitoneally injected with 2 μg of α-GalCer and IFN-γ, and IL-4 expression in hepatic CD1d-restricted NKT cells was measured 2 hours later via intracellular flow cytometry (n = 5 per group).
Discussion
Previous work has demonstrated that while the levels of CD1d-restricted NKT cells in the peripheral blood of PBC patients is lower than those of healthy individuals, the frequency of CD1d-restricted NKT cells in PBC liver is significantly higher than in blood. In addition, the frequency of CD1d-restricted NKT cells in the liver is significantly higher in PBC than in controls.15 These results imply that CD1d-restricted NKT cells may play a contributory role in the liver injury of PBC. However, it is impossible to examine this in humans, particularly as an early event—hence our use of the unique murine model herein. In the present study, we examined the functional characteristics of CD1d-restricted NKT cells and their role in the pathogenesis of murine PBC by using our dnTGFβRII mouse model. We report an increased number of CD1d-restricted NKT cells in the livers of dnTGFβRII mice compared with controls in each of the age groups studied. The activation markers on naïve CD1d-restricted NKT cells and cytokine expression in α-GalCer–induced CD1d-restricted NKT cells were significantly increased in dnTGFβRII mice. Moreover, injection with α-GalCer in young dnTGFβRII mice enhanced liver cell infiltration and IFN-γ production. Using dnTGFβRII mice, which were bred to be CD1d-deficient, we demonstrated decreased mononuclear cell infiltration in the livers of young and older CD1d-deficient dnTGFβRII mice. Serum levels of IFN-γ were significantly lower in young CD1d-deficient dnTGFβRII mice than heterozygous littermate mice. These results suggest CD1d-restricted NKT cells could promote the development of PBC in early stages by secreting IFN-γ.
The most studied CD1d-restricted NKT cell, the type I NKT cell (also referred to as invariant NKT cell) expresses a semi-invariant T cell receptor (TCR) composed of a canonical Vα14-Jα18 rearrangement in mice and Vα24-Jα18 rearrangement in humans paired with limited TCRβ chain repertoires, as well as NK markers, including NK1.1, the IL-2/IL-15Rβ chain (CD122), and various Ly49 molecules. These cells are almost uniformly reactive to the marine sponge–derived glycolipid α-GalCer presented by the nonclassical class I antigen-presenting molecule, CD1d, and they can be readily identified by their specificity using α-GalCer–CD1d tetramer. CD1d-restricted NKT cells activated by α-GalCer presented by CD1d produce a mixture of T helper 1 (Th1) cytokines, such as IFN-γ and TNF-α, and other T helper 2 (Th2) cytokines, including IL-4 and IL-13.26-28 CD1d-restricted NKT cells have been implicated in the recognition of bacterial glycosphingolipids29-32 and regulation of immune responses associated with a broad range of diseases, including autoimmunity, infectious disease, and cancer,33-38 and a number of investigators have reported increased or decreased CD1d-restricted NKT cells in a variety of human autoimmune diseases, including type I diabetes, systemic sclerosis, rheumatoid arthritis, multiple sclerosis, and PBC.15, 39-41 Several animal models indicate that CD1d-restricted NKT cells prevent autoimmunity and inflammation—either when activated naturally or when using α-GalCer—by increasing Th2 immune responses or inhibiting Th1 responses in some diabetes models, or by inducing IL-10 in experimental autoimmune encephalomyelitis.37, 42-44 However, other studies suggest that CD1d-restricted NKT cells exacerbate autoimmune disease by stimulating secretion of either Th1 or Th2 cytokines.38, 43, 45
When activated, CD1d-restricted NKT cells produce a mixture of Th1 and Th2 cytokines within 1-2 hours of TCR ligation. However, they can polarize the immune response in either a Th1 or Th2 direction.26-28 In this study, hepatic CD1d-restricted NKT cells from 5-week-old dnTGFβRII mice injected with α-GalCer produced a 1.43-fold increase of IFN-γ compared with control mice but only a 1.14-fold increase in the production of IL-4 (Fig. 2B). This result suggests that CD1d-restricted NKT cells in dnTGFβRII mice favor IFN-γ over IL-4 production. The increased expression of IFN-γ in the liver is critical to the development of PBC.46, 47 IFN-γ promotes the production of chemokines, IFN-γ–inducible protein 10, and monokine induced by IFN-γ, which in turn recruits autoreactive CD4+ T cells expressing the CXCR3 chemokine receptor and leads to the destruction of the biliary tract.48
In CD1d−/−dnTGFβRII mice, the absolute number of mononuclear cell infiltrates including T cells and NK cells was significantly decreased compared with CD1d+/−dnTGFβRII mice (Figs. 4A, 6A). Studies have shown that following activation, CD1d-restricted NKT cells rapidly secrete IFN-γ that leads to the activation of NK cells, and, subsequently, other cell types, including T cells and B cells.49-51 In PBC patients, NK cells are recruited to the liver more efficiently, resulting in exacerbated hepatic damage by their higher cytotoxic ability.9 Taken together, these results suggest that the onset of PBC is correlated with the abnormal CD1d-restricted NKT cells in patients and diseased animals. CD1d-restricted NKT cells in PBC express higher levels of activation markers and secrete higher levels of IFN-γ when activated. By secreting IFN-γ, they induce the activation of NK cells and/or recruit autoreactive CD4 T cells and lead to the destruction of the bile duct. In this regard, we note that there were no significant differences in levels of AMAs. This point is interesting in light of our theories on the orchestrated response that leads to biliary destruction, which involve not only autoantibodies, but in particular CD8 cells and limbs of both innate and adaptive responses.2, 3
The serum level of IFN-γ was significantly decreased in young CD1d−/−dnTGFβRII mice; however, it was slightly decreased in older CD1d−/−dnTGFβRII mice compared with that of CD1d+/−dnTGFβRII mice. We suggest that the serum level of IFN-γ in early stage disease is primarily produced by CD1d-restricted NKT cells, whereas NK cells, T cells, and other cell types may contribute to the production of IFN-γ in late stages in dnTGFβRII mice. As noted herein, IFN-γ and IL-4 expression in α-GalCer–activated, CD1d-restricted NKT cells in young dnTGFβRII mice were significantly higher than that of controls, whereas no differences were noted in older mice (Figs. 2A, 7). This suggests a pathogenic role of CD1d-restricted NKT cells in dnTGFβRII mice in early stage disease and perhaps a potentially protective role in the later stage of the disease. In contrast to PBC-prone dnTGFβRII mice, young lupus-prone (NZB × NZW) F1 mice do not display numerical or functional deficiencies in CD1d-restricted NKT cells. However, massive expansion of CD1d-restricted NKT cells and markedly enhanced cytokine production in aged (NZB × NZW) F1 mice have been observed.45 The functions of NKT cells, including cytokine production, are increased in an age-dependent fashion in both diseased and normal mice.52 One limitation of this study is the absence of survival data. In addition, it is not possible to measure serum alkaline phosphatase in mice. Other liver enzymes were not measured due to sera quantity limitations. However, the cell numbers and histological features are clearly different between dnTGFβRII and CD1d−/−dnTGFβRII mice.
It has also been reported that NKT cells are activated during microbial infections through the recognition of exogenous and endogeneous glycolipid antigens.29-32 In the present study, we used α-GalCer for activation of NKT cells, which allowed us to address this issue in the dnTGFβRII model. This is clearly a multifactorial issue, and future work will likewise need to focus on the role of microbial and environmental agents in infection-prone dnTGFβRII mice.53 However, we should emphasize that our colony is maintained under specific pathogen-free conditions. Finally, we should also emphasize that there are multiple functions of NKT cells that relate not only to cytokine production, but also regulatory function. In future experiments, serial studies—including measurement of additional cytokines such as IL-21—should be performed to dissect these events, because they will be important for understanding the natural history of PBC in patients. With the establishment of this model, we should also emphasize the need to study multiple other organs in addition to the data reported in earlier work.20
Acknowledgements
We thank Archana Khurana for preparation of the CD1d tetramers.




