Cell culture–produced hepatitis C virus impairs plasmacytoid dendritic cell function†
Potential conflict of interest: Nothing to report.
Abstract
Previous studies suggested a functional impairment of dendritic cells (DCs) in patients with chronic hepatitis C. To investigate whether this effect was mediated by a direct interaction of hepatitis C virus (HCV) with DCs, we studied the effects of infectious cell culture–produced hepatitis C virus (HCVcc) on peripheral blood mononuclear cells (PBMCs), ex vivo isolated plasmacytoid, and myeloid DCs and in vitro generated monocyte-derived DCs of healthy blood donors. HCVcc inhibited toll-like receptor (TLR)-9 (CpG and herpes simples virus)-mediated interferon alpha (IFN-α) production by peripheral blood mononuclear cells (PBMC) and plasmacytoid DCs. This inhibitory effect was also observed in response to ultraviolet (UV)-inactivated, noninfectious HCVcc, and it was not abrogated by neutralizing antibodies, and thus did not appear to require DC infection. Influenza A virus restored maturation and TLR9-mediated IFN-α production. In contrast to its effect on plasmacytoid DCs, HCVcc did not inhibit TLR3-mediated and TLR4-mediated maturation and interleukin (IL)-12, IL-6, IL-10, interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α) production by myeloid DCs and monocyte-derived DCs. Likewise, HCVcc did neither alter the capacity of myeloid DCs nor monocyte-derived DCs to induce CD4 T cell proliferation. Whereas phagocytosis of apoptotic hepatoma cells resulted in DC maturation, this effect was independent of whether the phagocytosed Huh7.5.1 cells were infected with HCVcc. In contrast to HCVcc, vaccinia virus inhibited maturation and TNF-α expression of myeloid DC as well as maturation and IL-6 and IL-10 production of monocyte-derived DC. Conclusion: HCVcc inhibited plasmacytoid DCs but not myeloid-derived and monocytoid-derived DCs via a direct interaction that did not require infection. The response of plasmacytoid DCs to influenza A virus infection was not impaired. (HEPATOLOGY 2007.)
Weak and functionally impaired hepatitis C virus (HCV)-specific T cell responses are a characteristic feature of HCV infection.1 Whereas spontaneously recovered patients maintain strong and broad HCV-specific T cell responses2-5 for decades,6 patients with persistent infection either never mount detectable responses2 or they become rapidly impaired.7, 8 To identify mechanisms that contribute to these insufficient immune responses, investigators have focused on dendritic cells (DCs).
DCs are antigen-presenting cells that operate at the interface between innate and adaptive immune responses.9 DCs express pathogen recognition receptors and display a remarkable capacity to capture antigens in peripheral tissues, process them, and present them to CD4 and CD8 T cell in regional lymph nodes,9 thereby initiating priming and differentiation of virus-specific T cells. Recently, human DCs have been categorized into 2 major subsets, myeloid DCs (mDCs) and plasmacytoid DCs (pDCs).10 Whereas mDCs express high levels of CD11c, pDCs lack CD11c and express CD123 [interleukin-3Rα (IL-3Rα)]. The pDCs are especially important in viral infections because of their capacity to produce high levels of type I interferon.11
DC function in HCV infection has been controversially discussed. Most studies used monocyte-derived DCs (MoDCs) and described reduced CD86 expression12 or impaired allostimulatory capacity of DCs from patients with hepatitis C12-14 as compared with those of healthy subjects12-14 and patients who cleared HCV after treatment.13, 14 Other studies demonstrated that MoDCs from HCV-infected chimpanzees did not differ from those of uninfected chimpanzees with regard to lipopolysaccharide (LPS)-induced maturation,15 cytokine and chemokine production,16 peptide presentation,16 and T cell stimulation.15 Normal maturation in response to TNF-α, normal induction of T cell proliferation, and normal responses to influenza A virus have also been reported for MoDCs from HCV-infected patients.17
When ex vivo isolation techniques became available, the frequency of peripheral blood mDCs18, 19 and pDCs18-22 was reported to be lower in the blood of HCV-infected patients compared with treatment-recovered patients. However, the function of these ex vivo studied DCs remains controversial. Whereas some studies describe that pDCs of HCV-infected patients produce normal levels of interferon alpha (IFN-α) or tumor necrosis factor alpha (TNF-α) when compared with pDCs of healthy controls,18, 22-24 others describe reduced levels.19-21 Multiple factors may contribute to these discrepant results. For example, the specific cytokine milieu in a chronic inflammatory situation may affect the function of DCs isolated from the blood of patients with chronic hepatitis C. Moreover, altered activation and migration patterns of dendritic cells may contribute to redistribution of specific DC population between blood, lymph nodes, and infected liver. Finally, pegylated IFN-α and ribavirin, the standard treatment regimen for chronic HCV infection, are known to affect both number and function of pDCs.25
The recently developed HCV culture system26-28 enabled us to test the effect of HCV on DC subpopulations under controlled in vitro conditions. Specifically, we asked whether exposure to HCV affected cytokine production and maturation of DCs from healthy control persons that had never been exposed to HCV. Peripheral blood mononuclear cells (PBMCs) and DC subpopulations were exposed to cell culture–produced hepatitis C virus (HCVcc) in an “acute setting,” that is, for up to 42 hours, and at HCV RNA and core protein concentrations similar to those observed in the blood of acute HCV infection.20 The study also provided the opportunity to assess the effect of HCV structural proteins in the context of their configuration in infectious viral particles rather than as recombinant proteins as in previous studies.12, 29
Abbreviations
CFSE, 5-, 6-carboxyfluorescien diacetate succinimidyl ester; DC, dendritic cell; EIA, enzyme immunoassay; FITC, fluorescein isothiocyanate; HCV, hepatitis C virus; HCVcc, cell culture–produced hepatitis C virus; HSV, herpes simplex virus; IFN-α, interferon alpha; IFN-γ, interferon gamma; IL, interleukin; LPS, lipopolysaccharide; mDC, myeloid DC; MFI, mean fluorescence intensity; MoDC, monocyte-derived DC; PBMC, peripheral blood mononuclear cell; pDC, plasmacytoid DC; RPMI, Roswell Park Memorial Institute; TLR9, toll-like receptor 9; TNF-α, tumor necrosis factor alpha; UV, ultraviolet.
Materials and Methods
Cells and Media.
The human hepatoma cell lines Huh7.5 (Apath, St. Louis, MO) and Huh7.5.1 (provided by Dr. Francis V. Chisari, Scripps Research Institute, La Jolla, CA) were maintained in Dulbecco's modified Eagle's medium, 10% fetal bovine serum (FBS, US Bio-Technologies, Pottstown, PA), 10 mM HEPES, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine (Mediatech, Herndon, VA) and trypsinized and passaged every 3.5 days.
Preparation of HCVcc.
HCV of the Japanese Fulminant Hepatitis Strain (JFH-1) was produced as described26-28 and used as a well-characterized and unlimited stock of HCV. The plasmid pJFH-1 (provided by Dr. T. Wakita, National Institute of Infectious Diseases, Tokyo, Japan) was digested with XbaI. Purified and linearized DNA served as a template for in vitro transcription using the MEGAscript T7 Kit (Ambion, Austin, TX). Ten micrograms per milliliter in vitro transcribed JFH-1 RNA were transfected into naïve Huh7.5 cells using DMRIE-C (Invitrogen, Carlsbad, CA) and Opti-MEM-I reduced serum medium (Invitrogen). Supernatants of days 12 through 16 cultures were used to infect naive Huh7.5.1 cells at a multiplicity of infection of 0.01.
Quantification of HCVcc RNA Concentration, Infectious Titer, and Core Protein.
RNA was extracted from Huh7.5.1 culture supernatant using the QIAamp Viral RNA Kit (Qiagen, Valencia, CA). HCV RNA was quantitated by real-time reverse transciption polymerase chain reaction using TaqMan EZ RT-PCR Core Reagents (Applied Biosystems, Foster City, CA) according to manufacturer's protocol with published primers and probe.30 The infectious HCV titer was determined as described.31 HCVcore was quantitated by enzyme immunoassay (EIA) (Ortho HCVcore Ag ELISA test kit [Ortho-Clinical Diagnostics, Tokyo, Japan]). The viability of HCV-infected Huh7.5.1 cultures was assessed by trypan blue staining and lactate dehydrogenase release using the CytoTox96 assay (Promega Corp., Madison, WI) (Fig. 1). Filtered (0.45 μm) supernatant of HCV-infected Huh-7.5.1 cells containing either 5 × 108 HCV RNA copies/mL (equivalent to 4.9 × 104 focus-forming units [ffu]/mL and an HCVcore concentration of 300,000 fmol/L) or 108 HCV RNA copies/mL (equivalent to 104 ffu/mL and an HCVcore concentration of 60,000 fmol/L) was used for DC experiments. Because Huh7.5.1 cultures were apoptotic at the time of HCVcc harvest, supernatant from uninfected Huh7.5.1 cultures that were rendered apoptotic by ultraviolet (UV) irradiation was included as a negative control in all experiments, but the results did not significantly differ from those with supernatant from nonirradiated, uninfected Huh7.5.1 cultures.
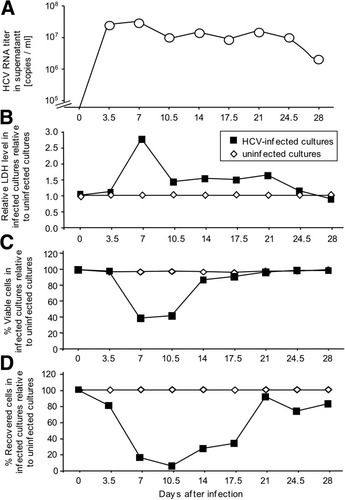
Generation of HCVcc from the supernatant of infected Huh 7.5.1 cells. Huh 7.5.1 cells were infected at a multiplicity of infection of 0.01 with HCV. HCV RNA (A) and lactate dehydrogenase (B) were quantitated in culture supernatants, and the total number (C) and percentage of viable, that is, trypan-blue negative (D) Huh7.5.1 cells was assessed at each cell passage at the indicated day after infection.
Isolation of PBMC, DCs, and Monocytes.
Buffy coats were collected from uninfected donors under a protocol approved by the Institutional Review Board of the NIH Clinical Center. PBMCs were isolated as described.6 The mDCs and pDCs were isolated at >90% purity from 1 to 4 × 108 fresh PBMCs with the CD1c DC Isolation Kit (Miltenyi Biotec, Auburn, CA) and the BDCA-4 Cell Isolation Kit (Miltenyi Biotec), respectively. To maintain an immature phenotype, DCs were cultured overnight in complete Roswell Park Memorial Institute (RPMI) medium [RPMI-1640 with 100 IU/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine and 10% fetal bovine serum (FBS) unless otherwise indicated] with (for pDCs) or without (for mDCs) 10 ng/mL interleukin (IL)-3 (Peprotech, Rocky Hill, NJ).
To generate MoDCs, monocytes were isolated from PBMCs with the Monocyte Isolation Kit II (Miltenyi Biotec) and cultured at 2 × 106/mL in complete RPMI medium with 50 ng/mL granulocyte–macrophage colony stimulating factor and 20 ng/mL IL-4 (both from Peprotech) for 6 days. More than 99% were immature DCs, as determined by high forward and side scatter and the absence of CD14 expression by flow cytometry.
Analysis of Maturation and Cytokine Production of DCs.
Immature mDCs and pDCs (5 × 105/mL) were cultured overnight, and MoDCs for 3 hours with 107 RNA copies/mL HCVcc (HCVcore concentration 6,000 fmol/L) or 5 × 107 RNA copies/mL HCVcc (HCVcore concentration 30,000 fmol/L) or 5 × 107 RNA copies/mL UV-inactivated (1 J/cm2), noninfectious HCVcc. Controls consisted of supernatant from uninfected Huh7.5.1 cells and supernatant from UV-irradiated (40,000 μJ/cm2), apoptotic Huh7.5.1 cells.
After the preincubation time, 2 μM CpG2216 (Coley Pharmaceutical Canada, Ottawa, Canada), 2 μg/mL lipopolysaccharide (LPS, Invivogen, San Diego, CA), 20 μg/mL polyinosinic-polycytidylic acid (Poly I:C, Invivogen) or 107 copies/mL influenza A virus PR8 (provided by Drs. J. Yewdell and J. Benninck, NIAID, NIH, Bethesda, MD) were added (without removing HCVcc) for 24 hours. For vaccinia virus experiments, 4 × 104/mL mDCs or 2 × 105/mL MoDCs were incubated for 24 hours with 2.5 × 106 pfu/mL vaccinia WR strain and 2 μg/mL LPS.
After the respective incubation, culture supernatants were spun (300g, 5 minutes) to remove cell debris and quantitated for IL-12p70, IL-10, IL-6, TNF-α, and interferon gamma (IFN-γ) using the BD CBA Kit (BD Biosciences) and the FACSCalibur according to manufacturer's protocol. IFN-α and IFN-β were quantitated by EIA (PBL Biomedical Laboratories, Piscataway, NJ; and TFB, Tokyo, Japan). Cells were washed with phosphate-buffered saline, 3% FBS, and 0.09% sodium azide, stained with combinations of fluorescein isothiocyanate (FITC)-HLA-DR, FITC-CD80, PE-CD83, PE-CD86, APC-CD11c (BD Biosciences, San Diego, CA), and FITC-CCR7 antibodies (R&D Systems, Minneapolis, MN) and analyzed on an LSRII (BD Biosciences) or FACSCalibur (BD Biosciences) with CellQuest, FACSDiva (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR).
Analysis of Cytokine Production by PBMC and Monocytes.
PBMCs (2 × 106/mL) or monocytes (2 × 106/mL) were incubated for 48 hours with 107 RNA copies/mL or 5 × 107 RNA copies/mL HCVcc or 5 × 107 RNA copies/mL UV-inactivated HCVcc or supernatant from UV-irradiated, apoptotic Huh7.5.1 cells with 2 μM CpG2216 or 5 × 106 pfu/mL UV-inactivated herpes simplex virus (HSV) 2 strain 33 (a gift of Dr. Jeffrey Cohen, National Institute of Allergy and Infectious Diseases, National Institutes of Health). In selected experiments, AB serum was replaced with serum from a treatment-recovered, previously HCV genotype 2a infected patient. This serum blocked HCVcc infectivity to Huh7.5.1 cells by 95% (not shown). Cell culture supernatants were collected and analyzed as described.
Allogeneic Mixed Lymphocyte Reaction.
Immature DCs were incubated for 1 hour with or without 107 RNA copies/mL HCVcc as described, then washed with RPMI. Freshly isolated or thawed allogeneic PBMCs were resuspended at 4 × 106/mL in phosphate-buffered saline, labeled for 10 minutes with 200 nM carboxyfluorescein diacetate succinimidyl ester (5-,6-carboxy fluorescein diacetate succinimidyl ester [CFSE], Molecular Probes, Eugene, OR), washed with ice-cold FBS, and subsequently with complete RPMI medium and seeded at 5 × 105/well into a 96-well flat bottom plate (Nunc, Rochester, NY) at PBMC:DC ratios of 25:1, 100:1, and 500:1. After 6 days, cells were harvested and stained with 5 μg /mL ethidium monoazide (Molecular Probes) and subsequently with phycoerythrin (PE)-CD4, PE-Cy5-CD16, PE-Cy5-CD19, allophycocyanin (APC)-CD3, APC-Cy7-CD8 (BD Biosciences), PE-Cy5-CD14 antibodies (Serotec, Raleigh, NC). The percentage of CFSE-low CD4 T cells was determined on an LSR II flow cytometer.
Induction of Apoptosis of Huh7.5.1 and Uptake by MoDCs.
Huh 7.5.1 cells were infected with HCVcc or not infected. After 5 days of culture, 5 × 105 cells/mL were UV-irradiated with either 0, 40,000, or 120,000 μJ/cm2using the Stratalinker UV Crosslinker Model 1800 (Stratagene, La Jolla, CA), washed and cultured for another 24 hours before assessment of apoptosis with the apoptosis bromodeoxyuridine terminal deoxynucleotidyl transferase-mediated nick-end labeling (APO-BrdU-TUNEL) Assay Kit (Molecular Probes). MoDCs were then co-cultured at a 2:1 ratio with irradiated or nonirradiated, HCVcc-infected or noninfected Huh7.5.1 cells. After 24 hours, cells were stained with FITC-HLA-DR, FITC-CD80, FITC-CD40, PE-CD83, PE-CD86, or APC-CD11c antibodies (all from BD Biosciences) and analyzed by flow cytometry. MoDCs were identified by high forward scatter and side scatter and CD11c expression.
Statistics.
Data were analyzed by paired t tests or by analysis of variance followed by post-hoc Dunnett or Newmann-Keuls test using GraphPad Prism (San Diego, CA). A 2-sided p value of < 0.05 was considered significant.
Results
HCVcc Inhibited TLR9-Induced IFN-α Production of PBMCs and pDCs.
As a first assessment of the effect of HCVcc, PBMCs of healthy HCV-uninfected blood donors were exposed to HCVcc with or without stimulation with the toll-like receptor 9 (TLR9) ligand CpG (cytosine-phosphate-guanine sequences). As shown in Fig. 2A, HCVcc did not induce IFN-α, IL-10, IL-12, or TNF-α, but blocked TLR9-induced IFN-α production. This was attributable to a time-dependent and dose-dependent inhibition of TLR9-mediated IFN-α production by pDCs (Fig. 2B), with inhibition reaching a plateau approximately 24 hours after the start of the incubation. Inhibition by 5 × 107 HCV RNA copies/mL HCVcc (equivalent to an infectious titer of 4.9 × 103 ffu/mL and 30,000 fmol/L HCVcore) exceeded inhibition by 107 HCV RNA copies/mL HCVcc (equivalent to an infectious titer of 103 ffu/mL and 6,000 fmol/L HCVcore). This effect was clearly HCVcc-related, because supernatant from apoptotic, uninfected Huh7.5.1 cells had no effect (Fig. 2B).
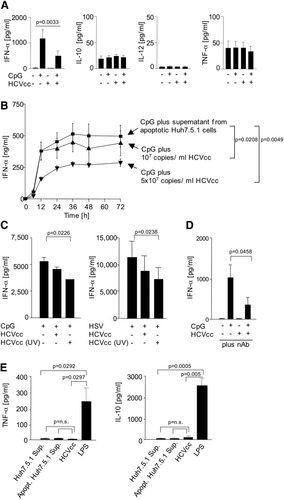
HCVcc inhibited TLR9-induced IFN-α production of PBMC and pDCs. (A) 5 × 107 copies/mL HCVcc inhibited IFN-α production, but not IL-10, IL-12, or TNF-α production by PBMC that were stimulated with the TLR9 ligand CpG. Mean and standard deviation of the results of 7 healthy blood donors are shown. (B) HCVcc inhibited IFN-α production of CpG-stimulated pDCs in a concentration-dependent manner. Mean and standard deviation of the results of 3 healthy blood donors are shown. (C) 5 × 107 copies/mL HCVcc inhibited IFN-α production of CpG-stimulated and HSV-stimulated PBMCs irrespective of whether HCVcc was infectious or rendered noninfectious to Huh7.5.1 cells by UV-inactivation. Mean and standard deviation of the results of 5 healthy blood donors are shown. (D) Human serum, which blocked HCVcc infectivity to Huh7.5.1 cells by 95%, did not abrogate HCVcc inhibitory effect on IFN-α production of CpG-stimulated pDCs. Mean and standard deviation of the results of 6 healthy blood donors are shown. (E) 5 × 107 copies/mL HCVcc induced neither TNF-α nor IL-10 production by monocytes. Mean and standard deviation of the results of 3 healthy blood donors are shown.
In addition to inhibition of CpG-mediated IFN-α production, HCVcc did also inhibit the IFN-α response to a second TLR9 ligand, HSV (Fig. 2C). Inactivated HCVcc that was rendered noninfectious to Huh7.5.1 cells by UV irradiation had the same inhibitory effect (Fig. 2C). Inhibition was not abrogated by human sera that contained HCVcc-neutralizing antibodies (Fig. 2D), suggesting it did not require pDC infection. It also did not require IL-10 or TNF-α production by monocytes (Fig. 2E).
HCVcc Does Not Inhibit Influenza A Virus–Induced Cytokine Production and Maturation of pDCs.
Because IFN-α production by DCs can also be elicited by influenza A virus and because influenza-specific immune responses are not impaired in HCV infection, we asked whether influenza A virus may be able to override an HCV-specific impairment of pDC function.
Isolated pDCs were cultured overnight with or without HCVcc before addition of influenza A virus the next day. As shown in Fig. 3A, influenza A virus stimulated pDCs to produce significant levels of IFN-α/β and TNF-α, and HCVcc did not inhibit this response. Moreover, HCVcc did neither impair the up-regulation of maturation markers HLA-DR, CD80, CD83, and CD86 nor the expression of the lymph node homing marker CCR7 in response to influenza A virus.
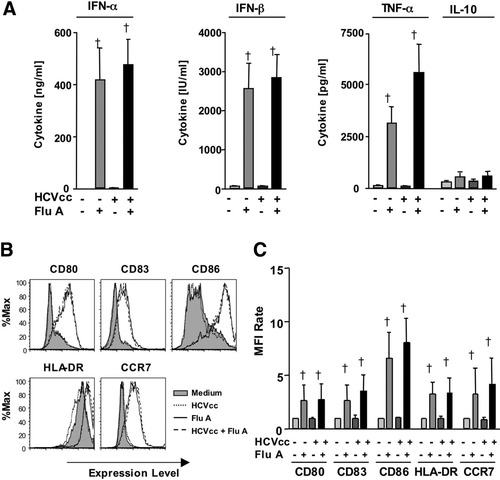
HCVcc did not inhibit influenza A virus–induced maturation and cytokine production of pDC. Freshly isolated pDC were cultured with or without HCVcc or 107 influenza A virus. Secretion of the indicated cytokines was determined by cytometric bead array (CBA) and EIA assays (A). Expression of the indicated cell surface markers was evaluated by flow cytometry (B, C). Data are shown as histograms for a representative subject (B) or as bar graphs displaying mean and standard deviation of the results of 6 subjects (A, C). The mean fluorescence intensity (MFI) rate reflects the MFI of a sample divided by that of the negative control (supernatant from uninfected Huh7.5.1 cells). †: Significant induction of the respective cell surface marker by FluA as compared with the corresponding control without FluA (P < 0.05). FluA, influenza A virus.
Vaccinia Virus But Not HCVcc Inhibited LPS-Induced Maturation and Cytokine Production of mDCs.
To examine whether HCVcc affected cytokine production or maturation of other DC populations, mDCs were preincubated with HCVcc overnight before addition of maturation stimuli. As maturation stimulus, LPS, a TLR4 ligand, was used because in contrast to pDCs,32 mDCs express TLR4. LPS stimulated TNF-α, IL-10, and IL-6 production by mDCs, but HCVcc did neither induce nor inhibit the production of IL-12, TNF-α, IFN-γ, IL-10, and IL-6 (Fig. 4A). Consistent with these findings, HCVcc neither induced nor inhibited the expression of maturation markers CD80, CD86, and DR. To ensure that the experimental system allowed for a negative impact to be seen, vaccinia virus was included based on a previous report that it inhibits human dendritic cells.33 Vaccinia virus inhibited TNF-α production as well as CD80 and CD86 expression by mDCs stimulated with LPS (Fig. 4A).
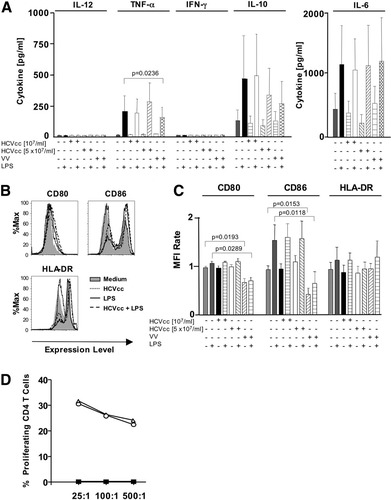
Vaccinia virus but not HCVcc inhibited LPS-induced maturation and cytokine production of mDCs. (A-C) Freshly isolated mDCs were cultured with or without HCVcc, vaccinia virus, and LPS. Secreted cytokines were quantitated by CBA and EIA assays (A). Expression of the indicated cell surface markers was evaluated by flow cytometry (B, C). Data are shown as histograms for a representative subject (B) or as bar graphs displaying mean and standard deviation of the results of 6 subjects (A, C). The mean fluorescence intensity (MFI) rate reflects the MFI of a sample divided by that of the negative control (supernatant from Huh7.5.1 cells). (D) HCVcc did not influence the capacity of mDC to induce alloreactive CD4 T cell proliferation. DCs were preincubated without (open circles) or with (open triangles) 107 HCV RNA copies/mL HCVcc, washed, and cultured with CFSE-labeled allogeneic PBMC for 6 days at the indicated effector:DC ratios. Filled squares indicate CD4 T cell proliferation in the absence of DCs. A representative experiment is shown.
Because mDCs are potent inducers of allogeneic T cell proliferation, the effect of HCVcc was also examined on this function. As shown in Fig. 4D for a representative subject, preincubation of mDCs with HCVcc did not affect their capacity to stimulate CD4 T cell proliferation. The effect of vaccinia virus on the allostimulatory capacity of mDCs could not be assessed, because the duration of the culture would result in lysis of T cells because of a cytopathic effect of vaccinia virus.
Vaccinia Virus But Not HCVcc Inhibited LPS-Induced Maturation and Cytokine Production of MoDCs.
To allow comparison of our results with previously published studies,12, 29 we also exposed MoDCs to HCVcc. HCVcc neither induced cytokine production (Fig. 5A) and maturation of MoDCs (Fig. 5B,C) nor inhibited responses to the TLR4 ligand LPS (Fig. 5A-C) nor affected the capacity of MoDCs to induce allogeneic T cell proliferation (Fig. 5D). Similar effects were observed when MoDCs were stimulated with TLR4 ligands such as poly I:C (not shown). In contrast to HCVcc, vaccinia virus inhibited IL-10 and IL-6 secretion of LPS-stimulated MoDCs (Fig. 5A) and impaired their maturation, that is, CD80, CD86, and HLA-DR expression (Fig. 5B,C).
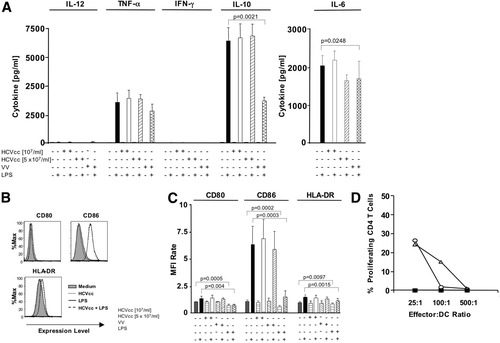
Vaccinia virus but not HCVcc inhibited LPS-induced maturation and cytokine production of MoDCs. (A-C) Freshly isolated MoDCs were cultured with or without HCVcc, vaccinia virus and LPS. Secretion of the indicated cytokines was determined by CBA and EIA assays (A). Expression of the indicated cell surface markers was evaluated by flow cytometry (B, C). Data are shown as histograms for a representative subject (B) or as bar graphs displaying mean and standard deviation of the results of 6 subjects (A, C). The mean fluorescence intensity (MFI) rate reflects the MFI of a sample divided by that of the negative control (supernatant from Huh7.5.1 cells). (D) HCVcc did not influence the capacity of MoDCs to induce alloreactive CD4 T cell proliferation. DCs were preincubated without (open circles) or with (open triangles) 107 HCV RNA copies/mL HCVcc, washed, and cultured with CFSE-labeled allogeneic PBMC for 6 days at the indicated effector:DC ratios. Filled squares indicate CD4 T cell proliferation in the absence of DCs. A representative experiment is shown.
Apoptotic Cells Induce DC Maturation Independently of HCV Infection.
DCs are characterized by a unique capacity to phagocytose and process external antigens from virus-infected cells or dying cells. Phagocytosis of apoptotic or necrotic cells stimulates DC maturation,34, 35 resulting in subsequent antigen presentation. Because HCVcc did not directly affect mDC and MoDC function, we considered the possibility that it might do so indirectly, as a result of phagocytosis of HCVcc-infected cells. To examine whether phagocytosis of HCVcc-infected and noninfected human hepatoma cells differentially affected DC function, MoDCs were cocultured with either HCVcc-infected or noninfected Huh7.5.1 cells. Control experiments demonstrated that more than 90% of MoDCs phagocytosed fluorescence dye-labeled Huh 7.5.1 cells within 3 hours of culture at 37°C (not shown). Interestingly, MoDCs that phagocytosed HCVcc-infected Huh7.5.1 cells displayed significantly higher CD80, CD83, and CD86 levels than those MoDCs that phagocytosed uninfected Huh7.5.1 cells (Fig. 6A). Because more HCVcc-infected than uninfected Huh7.5 cells were apoptotic (Fig. 6B), we wondered whether HCV infection or apoptosis of the phagocytosed cells was responsible for the maturation of MoDCs. To differentiate between both scenarios, HCVcc-infected and noninfected Huh7.5 cells were triggered to undergo apoptosis by UV irradiation. Induction of apoptosis was confirmed by the APO-BrdU-TUNEL assay, and the respective cells were then cocultured with MoDC. CD80 and CD83 expression on MoDCs correlated to the percentage of apoptotic Huh7.5.1 cells in the culture and were independent of HCV infection (Fig. 6C,D). Thus, the presence of HCVcc in apoptotic and phagocytosed Huh7.5.1 cells did not affect DC maturation.
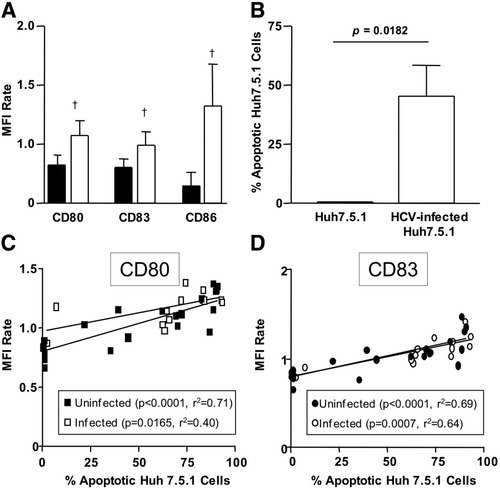
Apoptotic cells induced DC maturation independently of HCV infection. (A) CD80, CD83, and CD86 expression was evaluated on MoDC, which had been co-cultured with HCVcc-infected Huh 7.5.1 cells (open bars) or noninfected Huh7.5.1 cells (filled bars). More than 90% of the MoDCs had phagocytosed Huh7.5 cells at the time of analysis. (B) The percentage of apoptotic cells within the HCVcc-infected or uninfected Huh 7.5.1 cell culture was assessed by terminal deoxynucleotidyl transferase-mediated nick-end labeling assay. (C-D) HCVcc-infected Huh 7.5.1 cells (open symbols) or noninfected Huh7.5.1 cells (filled symbols) were UV-irradiated and cocultured with MoDCs. CD80 (C) and CD83 (D) expression levels on MoDCs were correlated with the percentage of apoptotic Huh 7.5.1 cells in the culture.
Discussion
For a number of reasons, it has proved difficult to reach a consensus on the effect of HCV on DCs. First, most published reports focus on DCs from patients with chronic hepatitis C, who differ in their viral titer, genotype, and duration of infection. Second, most earlier studies assessed MoDCs that were generated by stimulating peripheral blood monocytes for several days in vitro with granulocyte–macrophage colony-stimulating factor and IL-4.12-15, 17, 29 Finally, studies that describe an effect of recombinant proteins on DC function typically use high-protein concentrations between 1 and 10 μg/mL and do not provide the proteins in the context of the complete virus particle.
The recent establishment of the HCV culture system26-28 now allows the generation of a well-characterized HCV strain (JFH-1) in vitro. In this study we generated HCVcc, confirmed its robust infectivity in human hepatoma cells, and studied its effects on freshly isolated PBMCs and DCs subpopulations of healthy blood donors who had never been exposed to HCV previously. This approach allowed us to detect a dose-dependent inhibitory effect of HCVcc on TLR9, that is, CpG- and HSV-mediated IFN-α production by pDCs, suggesting that HCVcc impairs the pDC response in an acute setting within as little as 42 hours of exposure. Infection with influenza A virus, which stimulates pDCs by binding to TLR7 and by direct infection,36 restored IFN-α production, consistent with the clinical observation that the influenza A virus immune response is not impaired in HCV infection.8
The question of whether HCV replication in DCs is required to mediate this inhibitory effect is difficult to address directly because a low-level, positive polymerase chain reaction signal does not necessarily indicate that HCV replicates in DCs and produces new infectious virus. Attempts to passage HCVcc in DC cultures failed, which is consistent with the observations that human dendritic cells do not express Claudin-1, an essential factor for HCV entry and that pseudoparticles bearing HCV E1 and E2 do not enter dendritic cells (L.B. Dustin, S. Marukian, and C.M. Rice, personal communication). We have therefore used the following approaches to address this question: As shown in Fig. 2C and D, the inhibitory effect of HCVcc on pDCs was also observed in response to UV-inactivated, noninfectious HCV, and it was not abrogated by neutralizing antibodies, indicating that it did not require replicating virus in pDCs. The mechanism underlying the inhibitory effect that HCVcc, but not influenza A virus, exert on TLR9-stimulated pDCs suggests a protein or viral–receptor interaction that can now be investigated. HCVcc, for example, may directly bind to TLR9 in endosomes and thereby compete with TLR9 agonists CpG and HSV. Alternatively, dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin (DC-SIGN), a type II membrane-spanning C-type lectin, on the surface of DCs that is known to bind HCV37-39 or other receptors may be involved.
Whether these effects extend from tissue culture–grown HCVcc used in our experiments to the large variety of natural HCV sequences infected patients needs to be determined. Although HCVcc shares physical characteristics with serum-derived HCV particles26 and has been shown to be infectious in vivo27 and after recovery from infected animals it can be regrown in tissue culture.40, 41 It should be noted that HCVcc contains the sequence of a rare and unique genotype 2 isolate from a Japanese patient with fulminant hepatitis (JFH-1). We have attempted to test whether natural HCV (that is, HCV genotype 2a–containing patient serum) has inhibitory effects on DCs. However, these studies remained inconclusive, with greatly variable results among different patient sera (data not shown). The reasons are likely differences among patient sera to sustain DC culture and activation and possibly sequence differences among natural HCV isolates.
In contrast to its effect on pDCs, HCVcc did not inhibit TLR3-mediated and TLR4-mediated maturation (CD80, CD83, and DR expression) and IL-12, IL-6, IL-10, IFN-γ, and TNF-α production by mDCs and MoDCs, even at the higher concentration. HCV RNA and HCVcore concentrations in these experiments (5 × 107 RNA copies/mL and 30,000 fmol HCVcore/L, respectively) were comparable to those in the sera of HCV-infected patients20, 42 and to those used in in vitro experiments with recombinant HCVcore (4,500-22,500 fmol/L), in which an inhibitory effect was observed.20
The lack of any HCVcc effect on mDCs and MoDCs is in agreement with previous publications showing that incubation of DCs with HCV proteins in acute settings does not affect LPS-induced maturation of DCs.29 They are, however, in disagreement with other publications that show impaired function of MoDCs that were derived from patients with chronic hepatitis C12, 13, 20 or MoDCs that were cultured in vitro with recombinant HCV proteins for prolonged periods during their generation from monocytes.12, 29 Although it is possible that the unique JFH1 strain that was used for our in vitro experiments may not reflect the full impact of HCV that is seen in human infection, we favor the possibility that the specific inflammatory micromilieu in the HCV-infected liver contributes to the functional alterations that are observed for DCs from HCV-infected patients. Changes in the function of intrahepatic NK cells, which modulate DC activity,43 may also play a role. These factors are likely most prominent in a chronic, established HCV infection and may complement the direct, “acute” effect of HCV on pDC function that is described in this study.




