Therapeutic RNA silencing of Cys-X3-Cys chemokine ligand 1 gene prevents mice from adenovirus vector-induced acute liver injury†
Potential conflict of interest: Nothing to report.
Abstract
Gene therapy using adenovirus vectors may induce acute liver injury. Tissue injury induced by an adenovirus is likely associated with elevated expression of the Cys-X3-Cys chemokine ligand 1 (CX3CL1)/fractalkine (FKN) protein at the site of inflammation. However, the extent to which the actions of FKN contribute to liver injury remains unclear. We induced acute liver injury in mice by a hydrodynamics-based injection of adenovirus vector, which was confirmed to depend on the presence of natural killer (NK) cells and NK-dependent interferon-γ (IFN-γ). When the transferred adenovirus vector was inserted with the FKN gene, the severity of liver injury increased with much more Cys-X3-Cys chemokine receptor 1 (CX3CR1)–positive NK cell recruitment into the liver because of exogenous overproduction of FKN protein. Moreover, when production of endogenous FKN protein was silenced by inserting FKN–small interfering RNA into the adenovirus vector or was neutralized by an FKN-specific antibody, the adenovirus-induced acute severe liver injury was notably prevented with much lower hepatic NK cell infiltration and a significant reduction in the serum levels of IFN-γ. Conclusion: Our findings suggest a strategy to prevent or alleviate adenovirus vector-induced acute liver injury by blocking FKN–CX3CR1 interaction in adenovirus vector–based gene therapy. (HEPATOLOGY 2008.)
Replication-deficient recombinant adenovirus is one of the most advanced and best-studied vector systems in gene therapy1, 2 and is considered an important approach to introducing genetic material into cells, and especially offers a great opportunity to target gene expression to the liver by systemic delivery. However, adenovirus-based gene transfer may elicit an inflammatory response leading to tumor necrosis factor-α–dependent liver injury, which has been verified in mice,3-6 the rhesus macaque,7, 8 and even in clinical trials.9 Therefore, the acute inflammatory response elicited by adenovirus vector results in both loss of gene expression and tissue injury in liver, now believed to be the major limiting factor of adenovirus vector gene therapy. Until now, there have been only limited studies evaluating the safety of this technique, and very few investigations have sought approaches to preventing adenovirus vector–induced liver injury of adenoviral constructs in the context of an ongoing inflammatory process.
Fractalkine (FKN), the only member of the Cys-X3-Cys (CX3C) chemokine that contains both membrane-anchored and soluble forms, has both chemoattractant and cell-adhesive functions, and is believed to be an important regulator of inflammatory response as an inducer of cellular infiltration, including induction of interferon-γ (IFN-γ).10-13 The FKN-CX3C receptor 1 (CX3CR1) system is not only a strong medium in the binding of natural killer (NK) cells to endothelial cells, it is also essential for NK cell–mediated endothelium damage.11, 12
In the present study, after solidly exploring the cellular and molecular factors leading to liver inflammation induced by hydrodynamics-based injection of adenovirus vector, it was supposed that if the up-regulation of FKN could be inhibited by RNA interference in vivo, the liver injury might be improved. Thus, we constructed a novel adenovirus vector by inserting FKN–small interfering RNA (siRNA) to silence FKN messenger RNA (mRNA) and found that the adenovirus-induced acute severe liver injury was strikingly prevented by FKN-siRNA–containing adenovirus vector in an NK cell–dependent manner.
Abbreviations
ALT, alanine aminotransferase; CX3C, Cys-X3-Cys chemokine; CX3CL1, Cys-X3-Cys chemokine ligand 1; CX3CR1, Cys-X3-Cys chemokine receptor 1; EGFP, enhanced green fluorescent protein; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorting; FKN, fractalkine; FQ-PCR, fluorescent quantitative polymerase chain reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HP, hydrodynamics-based; IFN-γ, interferon-γ; IgG, immunoglobulin G; mAb, monoclonal antibody; mRNA, messenger RNA; NK cell, natural killer; NKT cell, natural killer T; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; SEM, standard error of the mean; siRNA, small interfering RNA.
Materials and Methods
Preparation and Use of Replication-Deficient Recombinant Adenovirus.
The recombinant replication-deficient E1, E3-deleted type 5 Adeno-X expression system was purchased from BD Biosciences Clontech. The complementary DNA encoding mouse FKN-CX3 chemokine ligand 1 (CX3CL1) or enhanced green fluorescent protein (EGFP) was used to prepare recombinant adenovirus AdFKN and AdEGFP following the instruction provided by the supplier, respectively. A virus containing the bacterial-galactosidase gene (AdLacZ) was used as a control. A small interfering RNA (siRNA) sequence corresponding to nucleotides 2290-2309 of the Mus musculus chemokine (C-X3-C motif) CX3CL1 gene (NCBI accession number: NM_009142) was introduced into the pshuttle-SIREN vector (BD Biosciences Clontech, CA) using the sense and antisense strand oligonucleotides 5′ GATCCGGACAAGCCACATAGGAAATTCAAGAGATTTCCTATGTGGCTTGTCCTTTTT-TACGCGTA 3′ and 5′ AGCTTACGCGTAAAAAAGGACAAGCCACATAGGAAATCTCTTGAATTTCC-TATGTGGCTTGTCCG 3′, respectively and used for construction of AdsiFKN recombinant adenoviruses. Adenovirus containing a random siRNA sequence (GAGACCCTATCCGTGATTA) (AdsiNeg) was used as a control. All viruses were propagated in HEK293 cells and purified by CsCl discontinued density gradient centrifugation. The typical titers of the vectors were in the range of 1.3–2.2 × 1012 particles/mL or 1–2 × 1010 plaque-forming units (pfu)/mL as determined via spectrophotometry or Adeno-X Rapid Titer Kit (BD Biosciences Clontech). A sterile carrier solution [phosphate-buffered saline (PBS)] was used for control injections and dilution of the viruses.
Mice and Intravenous Injection.
Five-week-old male C57BL/6 mice and severe combined immunodeficient (SCID) mice (15-20 g) were purchased from Shanghai Experimental Center, Chinese Academy of Science. All animals received care in compliance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals. In our study, mice received intravenous adenovirus injection in a volume of 200 μL (conventional injection) or 1.6 mL (hydrodynamics-based injection) as described previously.14 In all experiments, animals were injected with 4 × 109 pfu virus into the tail vein. All injections were performed within 5 seconds using a 26-gauge needle.
Antibody Treatments, Serum Alanine Aminotransferase Assay, and Enzyme-Linked Immunosorbent Assay for Serum IFN-γ.
To deplete NKs or natural killer T (NKT) cells, mice were injected intravenously with 50 μg/day of anti-AsGM1 (Wako Pure Chemicals Industries, Osaka, Japan) starting on day −1 until termination of the experiment. To deplete αβTCR+T cells, mice were injected intravenously every other day with 50 μg of TCR alpha+ TCR beta antibody (H57-597) (Abcam) starting on day −1 until termination of the experiment. Anti-NK1.1 monoclonal antibodies (mAb) (PK136) were obtained from the American Type Culture Collection (Manassas, VA) from partially purified hybridoma culture supernatant via ammonium sulfate precipitation. Mice received an injection of anti-NK1.1 mAb (50 μg/day) intraperitoneally starting on day −1. This protocol resulted in a ≥90% decrease in the number of indicated cells. Control mouse immunoglobulin G (IgG) 2a antibody (for anti-NK1.1 treatment) was purchased from PharMingen (San Diego, CA), and control rabbit IgG (for anti-AsGM1 treatment) was purchased from Calbiochem (La Jolla, CA). Control antibodies were injected at equivalent doses and schedules. To determine the effects of FKN-CX3CR1 blockade, animals received a single intravenous injection of polyclonal rabbit anti-rat CX3CR1 antibody (50 μg) (Torrey Pines Biolabs, San Diego, CA), polyclonal rabbit anti-mouse CX3CL1 antibody (50 μg) (eBioscience, San Diego, CA), or rabbit IgG (50 μg) every other day starting on day −1.
To identify liver injury, mice were anesthetized and bled via the retro-orbital venous plexus. Serum alanine aminotransferase (ALT) activities were determined using a serum aminotransferase test kit (Rong Sheng, Shanghai, China) following the manufacturer's instructions.
For enzyme-linked immunosorbent assay (ELISA), sera from mice were harvested. IFN-γ concentrations were analyzed using a mouse IFN-γ sandwich ELISA kit (Jingmei Biotech, Shenzhen, China).
Preparation of Liver Lymphocytes and Fluorometric Analysis.
Mononuclear cells were isolated from livers by passing tissue through a 200-gauge stainless steel mesh in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco BRL). The cell suspension was centrifuged at 500g for 5 minutes, and the supernatant was discarded. The cell pellet was resuspended in 40% percoll (Sigma) in RPMI 1640 medium via vigorous vortexing. The cell suspension was gently overlaid onto 70% percoll and centrifuged for 20 minutes at 750g at room temperature. Mononuclear cells (MNCs) were collected from the interphase and then washed twice in PBS containing 5% fetal bovine serum. The degree of contamination by Kupffer cells and hepatocytes was minimal. Livers from groups of 3 mice were pooled for cell isolation and fluorescence-activated cell sorting (FACS) analysis.
For FACS analysis, 106 MNCs were stained with saturating amounts of mAb for 30 minutes on ice. The following antibodies were used: polyclonal rabbit anti-rat CX3CR1 antibody, phycoerythrin-conjugated goat anti-rabbit IgG (H+L), fluorescein isothiocyanate–conjugated NK1.1 (PK136), phycoerythrin-conjugated CD69 (H1.2F3), and cy-chrome–conjugated CD3 (145-2C11), all purchased from PharMingen (San Diego, CA). Cells were acquired using FACSCalibur (Becton Dickinson) and were analyzed with WinMDI version 2.8 software.
Fluorescent Quantitative Polymerase Chain Reaction Assay and Western Blotting.
Total RNA was extracted from liver tissue via the phenol/chloroform method using TRIzol reagent (Invitrogen, CA). Cellular RNA (1 μg) was used for complementary DNA synthesis. Transcripts were quantified via TaqMan polymerase chain reaction (PCR) on an ABI-Prism 7000 Sequence Detection System (Applied Biosystems, Weiterstadt, Germany) with a Premix Ex Taq Perfect Real Time Kit (Takara). For detection of mouse FKN, IFN-γ, and the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH), we established detections systems with primers and probes synthesized by Takara. Expressions were calculated relative to the data for GAPDH obtained with every matching assay.
The liver specimens harvested from mice after 48 hours of treatment with AdsiFKN or AdFKN were frozen in liquid nitrogen. After being carefully powdered, the specimens were lysed in radioimmunoprecipitation assay buffer and protease inhibitors (Roche Molecular Biochemicals, Indianapolis, IN). After determination of protein concentration using the Bio-Rad Bradford protein assay (Bio-Rad Laboratories Inc., Hercules, CA), 30 μg of total protein was loaded per lane on a 10% Bis-Tris Gel (Invitrogen) and transferred to a polyvinylidene fluoride membrane (Invitrogen). After blocking with 5% milk in Tris buffer/saline containing 0.1% Tween-20 at 4°C overnight, the membrane was incubated with goat anti-mouse FKN polyclonal antibody (R&D Systems, Minneapolis, MN) for 60 minutes at room temperature. Blots were stained with horseradish peroxidase–conjugated secondary antibodies (Promega) for 45 minutes at room temperature. The detection of specific signal was performed using the enhanced chemiluminescence (ECL) detection system (Pierce Biotechnology).
Histology, X-Gal Staining, and Immunostaining.
For histological analysis, the livers of mice were removed at the indicated time intervals and embedded in paraffin. Three-micrometer-thick sections were cut from each paraffin block. For 2-color immunofluorescence staining, after blocking of nonspecific staining, deparaffinized sections were immunostained with optimal dilutions of the primary antibodies, including goat anti-mouse CX3CL1 polyclonal antibody (R&D Systems, Minneapolis, MN), rabbit anti-mouse CX3CR1 polyclonal antibody, fluorescein isothiocyanate–conjugated NK1.1 (PK136; BD PharMingen), and isotype-matched IgG at 37°C for 60 minutes. The sections were then incubated with fluorescein isothiocyanate–conjugated swine anti-goat mAb (BD PharMingen), phycoerythrin-conjugated goat anti-rabbit mAb (BD PharMingen) secondary antibody at 37°C for 30 minutes. As negative controls, nonimmunized rabbit IgG was used as a primary antibody. Sections were analyzed under a confocal laser scanning microscope (Zeiss). The paraffin sections were also used for hematoxylin-eosin staining. Sections were then analyzed via light microscopy.
X-gal staining was performed to determine transduction efficiency after AdLacZ exposure. In brief, frozen liver sections were prepared and fixed in 4% paraformaldehyde for 10 minutes and incubated in a solution of 50 mM Tris (pH 8.0), 2 mM MgCl2, 15 mM NaCl, 2 mM K3Fe(CN)6, and 2 mM K4Fe(CN)6 · 3H2O, with 12.5 mg/mL X-gal in N′,N-dimethylformamide for 30 minutes. For immunohistochemistry staining of Hexon, the Adeno-X Rapid Titer Kit (BD Biosciences Clontech) was used.
Statistical Analysis.
Data are given as the mean and standard error of the mean (SEM) or standard deviation as appropriate. Differences between groups were analyzed via Student t test. A P value of < 0.05 was considered statistically significant. All calculations were performed using the Origin 7.0 software package.
Results
Hydrodynamics-Based Injection of Adenovirus Vector Induces Acute Liver Injury.
Three type V adenovirus vectors—AdLacZ (Escherichia coli β-galactosidase), AdEGFP, and AdsiNeg (random siRNA sequence)—with deletions in the E1 and E3 regions and carrying different expressing cassette were chosen for infection. Hydrodynamics-based (HP) injection of the adenoviruses (4 × 109 pfu, each virus) exhibited much higher infection efficiency, by which more than 50% hepatocytes were infected 24 hours after injection, whereas the infection efficiency was much lower in the conventional injection (Fig. 1A). HP injection of adenoviruses caused acute liver injury with a dramatic increase in serum ALT levels (Fig. 1B) and tissue injury (Fig. 1C). It has been reported that HP injection may cause transient liver damage with relatively high serum ALT levels, but with rapid return to normal.14 This finding was confirmed by our observation that HP injection of PBS/control induced only mild liver injury with slightly elevated ALT levels (412.6 ± 44.5 U/L) on day 1, and returning to normal within 3 days (Fig. 1B). It was also observed that lymphocytes infiltrated the liver extensively (Fig. 1C), and that the total amount of hepatic MNCs was significantly increased (Fig. 1D), suggesting that adenovirus vectors caused severe inflammation.
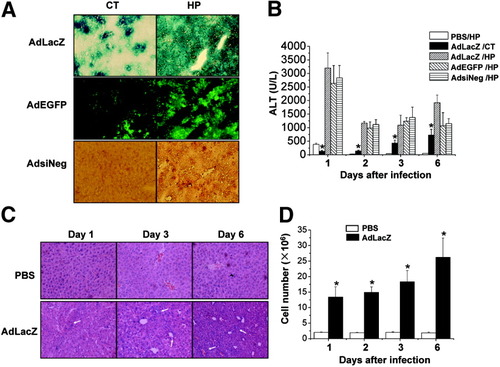
Hydrodynamic vein injection of adenovirus vector induces acute liver injury. (A) Exogenous gene expression was detected via histological examination. C57BL/6 mice (B6) were injected with distinct adenovirus vectors (AdLacZ, AdEGFP, or AdsiNeg; 4 × 109 pfu) via conventional (CT) or HP injection. Twenty-four hours after injection, mice were sacrificed and frozen liver sections were made. The difference of viral infectious efficiencies between the 2 methods of injection was identified by detecting expression of β-galactosidase, EGFP, or Hexon viral protein in AdLacZ, AdEGFP, or AdsiNeg, respectively. (B) Serum ALT levels of B6 mice HP-injected with PBS or 4 × 109 pfu AdLacZ, AdEGFP, or AdsiNeg virus and mice conventionally injected with 4 × 109 pfu AdLacZ virus were measured. Values are expressed as the means ± SEM of 6 mice at each time point. *P < 0.05 versus mice HP-injected with adenovirus. (C) B6 mice were HP-injected with AdLacZ (4 × 109 pfu) virus or PBS for various time periods, and liver paraffin sections were prepared and stained with hematoxylin-eosin (n = 3) (original magnification ×100). The arrows indicate the heavy infiltrated lymphocytes. (D) Absolute numbers of MNCs per liver were counted at various time points after HP injection of PBS or 4 × 109 pfu AdLacZ (n = 5 for each group). *P < 0.05 versus mice HP-injected with PBS.
HP Injection of Adenovirus Vector–Induced Liver Injury Depends on the Presence of Hepatic NK Cells.
AdLacZ HP injection preferentially led a substantial increase of NK cells in infected liver (day 1) (Fig. 2A, B). Mice were pretreated with either anti-NK1.1 (depletion of both NK and NKT cells) or anti-AsGM1 (depletion of NK cells) and then infected with virus. As shown in Fig. 2C, depletion of NK/NKT cells or NK cells prevented mice from adenovirus vector–induced liver injury. Because the depletion of NK/NKT cells yielded the same result as that of NK cells, we believe the adenovirus vector–induced injury was mainly dependent on the presence of NK cells. To further examine this possiblity, SCID mice and αβT cell–depleted mice were HP-injected with AdLacZ virus and assayed for serum ALT values. Figure 2D shows that SCID mice or αβT cell–depleted mice responded to virus infection significantly, and this response could be completely suppressed by injection of anti-AsGM1 antibody. Therefore, neither T cells, B cells, nor NKT cells are required for NK-mediated liver injury. It has been reported that NK cells are major source of liver IFN-γ at early times after virus infection.15 Consistent with this, we also found that IFN-γ transcripts and serum IFN-γ levels were strongly suppressed in NK cell–depleted mice, but not significantly in SCID mice or αβT cell–depleted mice in our model of adenovirus infection (Fig. 2E,F).
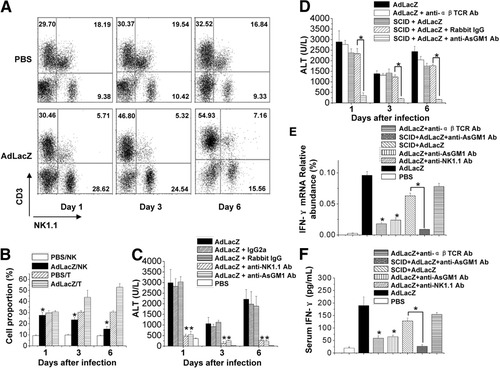
NK cell dependence in HP injection of adenovirus-induced liver injury. B6 mice were treated with 4 × 109 pfu AdLacZ virus via HP injection. After various time points, the hepatic lymphocyte populations were analyzed via FACS. (A) Representative example of 3 independent experiments. (B) Means ± SEM of hepatic NK and T cell proportions at indicated time points after HP injection. *P < 0.05 versus mice HP-injected with PBS. (C) Depletion of NK cells in adenovirus-induced liver injury. Mice were injected intravenously with anti-NK1.1 (PK136), anti-AsGM1, or control antibodies as indicated daily from days −1 to 5, and 4 × 109 pfu AdLacZ virus was HP-injected on day 0. Sera were collected as indicated and assayed for ALT. Values from groups of 6 mice are expressed as the means ± SEM. *P < 0.05. (D) B6 mice were injected intravenously with anti-αβTCR mAb every other day from days −1 to 5 and SCID mice were injected intravenously with anti-NK1.1, anti-AsGM1 or control antibodies as indicated daily from days −1 to 5. AdLacZ virus (4 × 109 pfu) was HP-injected on day 0. Sera were collected as indicated and assayed for ALT. Values from groups of 6 mice are expressed as the means ± SEM. *P < 0.05. Normal, anti-NK1.1, anti-AsGM1, or anti-αβTCR mAb-treated B6 mice and SCID mice treated with anti-AsGM1 mAb were HP-injected with 4 × 109 pfu AdLacZ virus on day 0. Twenty-four hours after injection, infected livers and sera were harvested. Total RNA from the infected livers was extracted and assayed via FQ-PCR for IFN-γ transcripts (E). Data from 3 independent experiments are expressed as relative to GAPDH expression (means ± SEM). *P < 0.05. Serum levels of IFN-γ were measured via ELISA (F). Values are expressed as the means ± SEM (n = 5). *P < 0.05..
CX3CL1 and CX3CR1 Are Up-regulated During Viral Infection and Are Responsible for Accumulation of Hepatic NK Cells.
It has been reported that CX3CL1 plays an important role in NK cell migration10 and activation11, 12 as well as IFN-γ production.13 In the present study, we found that expression of CD69 (PBS injection, 10.3% ± 2.2%; AdLacZ injection, 78.4% ± 6.4%) and CX3CR1 (PBS injection, 16.8% ± 1.8%; AdLacZ injection, 73.3% ± 2.8%) on hepatic NK cells was elevated 24 hours after injection (Fig. 3A). The up-regulation of CD69 indicated that NK cells were rapidly activated after infection. Real-time fluorescent quantitative PCR (FQ-PCR) analysis revealed that CX3CL1 and IFN-γ mRNAs were also significantly increased in the liver (Fig. 3B,C). It was also observed that there was a substantial increase of hepatic NK1.1+ cells with the colocalized expression of CX3CR1 protein in the infected liver (confocal microscopic data being consistent with FACS data, but not shown). Because NKT cells do not express CX3CR1 receptor, we can conclude that the preferentially recruited NK1.1+CX3CR1+ cells were NK cells.
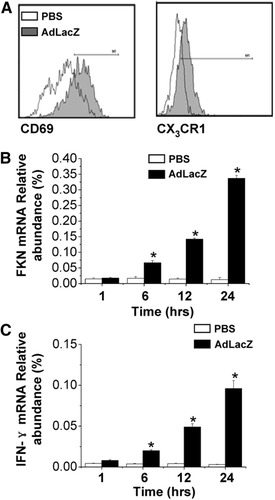
Involvement of FKN/CX3CR1 interaction in NK cell recruitment and activation in adenovirus vector–induced liver injury. (A) Expression of CD69 and FKN on hepatic NK cells in the form of histogram 24 hours after AdLacZ infection (4 × 109 pfu). A representative example of 3 independent experiments is shown. Quantification of (B) FKN or (C) IFN-γ transcript expression in livers from PBS or AdLacZ HP-injected mice via FQ-PCR. Data from 3 independent experiments are expressed as relative to GAPDH expression (means ± SEM). *P < 0.05 versus mice HP-injected with PBS.
Overexpression of CX3CL1 (FKN) Aggravates Liver Injury.
Western blot analysis of HP injection with AdFKN revealed much greater FKN protein in the liver (Fig. 4A). Overexpression of FKN after AdKFN infection induced more lymphocyte infiltration (Fig. 4B) and tissue injury (Fig. 4B) and higher ALT serum levels (Fig. 4C). Furthermore, the percentage of NK cells in AdFKN-infected livers was greater than AdLacZ-infected liver (AdLacZ, 28.6% ± 1%; AdFKN, 36.4% ± 1.3%) (Fig. 5A), and CX3CR1 was up-regulated in the infiltrating NK cells of AdFKN-infected livers (PBS, 16.8% ± 1.8%; AdLacZ, 73.3% ± 2.8%; AdFKN, 85.7 ± 1.6%) (Fig. 5B). Meanwhile, there was a significant increase of serum IFN-γ levels in AdFKN-infected mice compared with AdLacZ-treated mice (Fig. 5C). These observations confirmed that overexpression of FKN recruited more CX3CR1+ NK cells into the liver.
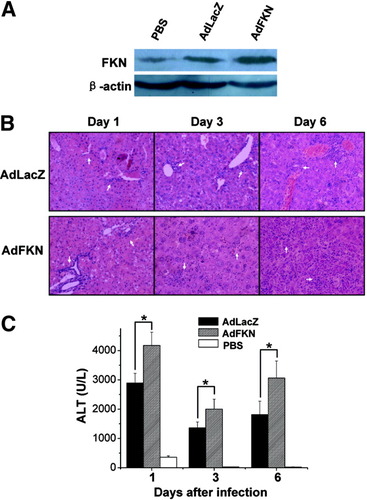
Overexpression of FKN aggravates hepatic inflammation. (A) B6 mice were HP-injected with AdLacZ (4 × 109 pfu), AdFKN (4 × 109 pfu), or PBS. Twenty-four hours later, liver samples were harvested and expression of CX3CL1 protein was determined via western blotting. (B) Liver samples of 4 × 109 pfu AdLacZ or 4 × 109 pfu AdFKN HP-injected mice were collected for hematoxylin-eosin staining (n = 3) (original magnification ×100). The arrows indicate areas of necrosis. (C) Sera of 4 × 109 pfu AdLacZ, 4 × 109 pfu AdFKN, or PBS HP-injected mice were collected as indicated and assayed for ALT. Values are expressed as the means ± SEM of 6 mice at each time point. *P < 0.05.
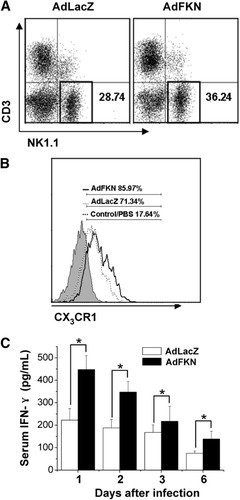
CX3CR1+ NK cell accumulation in FKN-overexpressing liver. (A) Liver MNCs of B6 mice were prepared 24 hours after HP injection with 4 × 109 pfu AdLacZ or 4 × 109 pfu AdFKN and then subjected to flow cytometry analysis. (B) Expression of CX3CR1 on the surface of hepatic NK cells from mice HP-injected with 4 × 109 pfu AdLacZ, 4 × 109 pfu AdFKN, or PBS for 24 hours was determined via flow cytometry. (C) Serum samples were collected at various times after viral infection, and serum levels of IFN-γ were measured via ELISA. Values are expressed as the means ± SEM (n = 5). *P < 0.05.
Therapeutic RNA Silencing of CX3CL1 Gene Prevents Mice from Adenovirus Vector–Induced NK Cell–Mediated Acute Liver Injury.
In order to elucidate the role of FKN in adenovirus vector–induced NK cell–mediated liver injury, we constructed an adenoviral vector inserted with siRNA-FKN (AdsiFKN) to silence FKN expression in vivo. FKN expression was successfully inhibited in AdsiFKN-treated livers on day 1 in both mRNA and protein levels, respectively (Fig. 6A,B). AdsiFKN elicited much lower serum ALT levels compared with control AdsiNeg (Fig. 6C). Meanwhile, recruitment of hepatic NK cells 24 hours after infection was significantly decreased when infected with AdsiFKN virus compared with that of AdsiNeg-infected livers (AdsiNeg, 27.8% ± 0.4%; AdsiFKN, 14.9% ± 0.8%) (Fig. 6D). FKN expression was significantly higher in AdsiNeg-infected livers than in AdsiFKN-treated livers (Fig. 6E). Immunostaining of CX3CR1 also indicated that there were much fewer CX3CR1+ lymphocytes in AdsiFKN-infected livers (Fig. 6E).
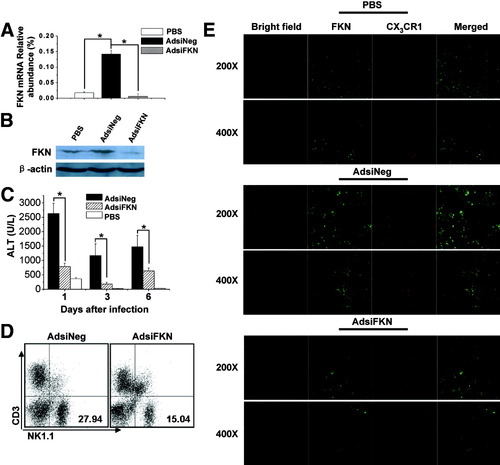
Therapeutic RNA silencing of FKN protects mice from adenovirus vector–induced liver injury after injection of AdsiFKN virus. (A) Detection of FKN transcript expression via real-time FQ-PCR in the liver tissues of B6 mice 24 hours after HP injection of AdsiNeg (4 × 109 pfu), AdsiFKN (4 × 109 pfu), or PBS. Data from 3 independent experiments are expressed as relative to GAPDH expression (means ± SEM). *P < 0.05. (B) FKN expression of protein levels was determined via western blotting. (C) ALT values in B6 mice treated with 4 × 109 pfu AdsiNeg, 4 × 109 pfu AdsiFKN, or PBS. Values are expressed as the means ± SEM of 6 mice at each time point. *P < 0.05. (D) Liver MNCs of B6 mice injected with 4 × 109 pfu AdsiNeg or 4 × 109 pfu AdsiFKN for 24 hours were prepared and analyzed via flow cytometry. (E) Expression of FKN and CX3CR1 in the livers of mice HP-injected with PBS, 4 × 109 pfu AdsiNeg, or 4 × 109 pfu AdsiFKN for 24 hours was detected via confocal microscope analysis (n = 3) (original magnification ×200 and ×400).
To confirm the role of FKN-CX3CR1 signaling in vivo, virus-infected mice were blocked by specific neutralizing antibodies against FKN or CX3CR1. The serum ALT levels of mice pretreated with the specific anti-FKN antibody or anti-CX3CR1 antibody were significantly down-regulated (Fig. 7A). Furthermore, the amount of lymphocytes in livers of FKN antibody–treated mice was significantly decreased (Fig. 7B). AdLacZ infection induced high levels of IFN-γ, but when mice were treated with FKN antibody, the serum levels of IFN-γ were significantly down-regulated, consistent with the decrease of NK cells (Fig. 7C).
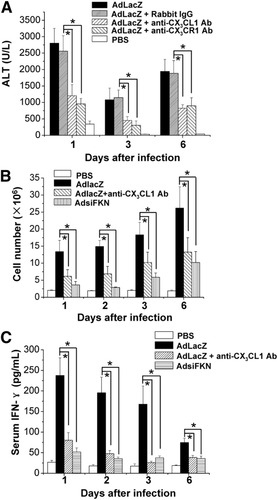
Blockade of interaction between FKN and CX3CR1 alleviates adenovirus vector–induced liver injury. (A) Mice were injected intravenously with anti-CX3CL1, anti-CX3CR1, or control antibodies every other day from days −1 to 5, and 4 × 109 pfu AdLacZ virus was HP-injected on day 0. Sera were collected as indicated and assayed for ALT. Values are expressed as the means ± SEM of 6 mice at each time point. *P < 0.05. (B) B6 mice were HP-injected with 4 × 109 pfu AdsiFKN or PBS or pretreated with anti-CX3CL1 antibody followed by HP injection of AdLacZ. After various time points, total hepatic MNCs were counted (n = 5). *P < 0.05. (C) Serum levels of IFN-γ were measured via ELISA. Values are expressed as the means ± SEM (n = 5). *P < 0.05.
Discussion
Adenovirus vectors are highly efficient in the transfer of genes to a wide variety of target cells. Furthermore, they are capable of expressing exogenous genes at a high level. In fact, these adenoviruses have substantial tropism for the respiratory tract as well as the liver when administered intravenously at high titer with a hydrodynamics procedure; over 80% of hepatocytes can be transferred with adenoviruses 48 hours after injection (data not shown). Therefore, adenovirus-based vectors offer a great opportunity to target gene expression in the liver via systemic delivery. However, previous studies have demonstrated that adenoviral vectors may activate innate inflammatory response and stimulate the synthesis of tumor necrosis factor-α.3-9 This increased tumor necrosis factor-α production not only reduces the duration and magnitude of transgene expression,3 it also induces an acute inflammatory condition that contributes to liver injury. Therefore, the acute inflammatory response elicited by adenovirus vectors in the liver is now believed to be the major limiting factor in the use of adenovirus vectors in gene therapy. Until now, few investigations have sought approaches to preventing adenovirus vector–induced liver injury. At present, few studies have reported that the adenoviral expression of the anti-inflammatory cytokines interleukin-10 or cytoprotective interleukin-6 could be successfully used for the treatment of necrotizing pancreatitis16 or inflammatory acute hepatitis,17 respectively. However, these gene transfer approaches planned to improve the inflammatory injury induced by chemicals through adenovirus vector delivery, but did not observe the injury induced by adenovirus vectors themselves, and they did not explore the immune effector cells in the process of inflammation. In the present study, after exploring the cellular and molecular factors leading to liver injury by adenovirus vector injection, we found that recruitment and activation of hepatic NK cells and NK cell–dependent IFN-γ are critical in adenovirus vector–induced liver injury. Based on these findings, we constructed an adenovirus vector by inserting FKN-siRNA to silence FKN mRNA; administration of this FKN-siRNA–containing adenovirus vector prevented adenovirus-induced acute severe liver injury, suggesting a strategy to prevent or alleviate adenovirus vector–induced acute liver injury by blocking FKN-CX3CR1 interaction in adenovirus vector–based gene therapy.
Recombinant replication-deficient adenoviruses are a useful tool for investigating how virus vectors induce liver injury and how effector cells are involved in this injury process. Unfortunately, conventional injection of adenovirus vectors—in which transfer efficiency into hepatocytes was very low by fast blood flow—only caused mild liver injury, because the inflammatory infiltration was not significant15, 18, 19 (Fig. 1). We employed a hydrodynamics-based procedure, an efficient method of transfection, to deliver adenoviral vectors to murine livers. Adenoviruses delivered in this way were present around hepatocytes immediately after injection and successfully infected several hepatocytes, causing high levels of gene expression and acute liver injury. Based on the principle of the hydrodynamics-based delivery, a high-volume and high-speed intravenous injection of naked plasmid DNA or siRNA could be applicable to a liver-restricted delivery method and has been used frequently as a simple and convenient in vivo transfection method.14, 20-22 In our study, consistent with the enhanced transfecting efficiency and expression of target genes, liver injury became much heavier after HP injection versus conventional injection (Fig. 1B), the model of which greatly improved our study.
There were many applications of RNA interference targeting at specific genes in vitro, either when transfected directly as siRNA or when generated from DNA vectors (short hairpin RNA). However, there were fewer RNA interference applications practiced in vivo due to lack of proper delivery with high efficiency. Two groups of researchers silenced Fas expression in vivo via HP injection of synthesized siRNAs specific for Fas to protect mice from renal ischemia–reperfusion injury and fulminant hepatitis, respectively.23, 24 Chu et al.25 and Wooddell et al.26 used the hydrodynamic technique to codeliver a target reporter gene with siRNA expression constructs to perform RNA interference in vivo. Adenoviral vectors can generate high levels of gene or siRNA expression, and by using this property of adenoviruses, some researchers have taken advantage of recombinant adenoviruses to successfully knock down specific gene expression in vitro.27-29 We combined a hydrodynamics technique with FKN-siRNA–expressing adenoviruses to knock down FKN expression in the murine liver. Our findings revealed that siFKN generated by AdsiFKN markedly inhibited FKN expression in infected livers and that RNA interference targeted at FKN expression in vivo attenuated adenovirus-induced liver injury.
NK cell recruitment and activation in a particular tissue or organ are considered a critical step in viral infection,30, 31 and more interestingly, NK cells constitute a major cell type responsible for early injury in the virus-infected liver in adenoviral infection models of immunocompetent animals. Although previous studies have suggested that FKN acts through its receptor, CX3CR1, to influence NK cell functions in vitro,12, 13 the contribution of FKN-CX3CR1 in regulating NK cell functions in vivo in the intact animal is not clear. FKN-CX3CR1 interactions are important in several systems and may play a role in various disease states.32-34 The up-regulation or induction of FKN expression in cultured endothelial or smooth muscle cells in response to inflammatory stimuli has been reported.35-38 In human hepatitis, FKN/CX3CL1 was up-regulated in areas of necrosis and inflammation and regenerating epithelial cells within bile duct–like structures, whereas its receptor, CX3CR1, is expressed in the majority of NK cells and monocytes and in some T cells (CD4+ and CD8+ T cells).10, 39 In our study, we noted that after injection of adenoviruses into mice, FKN, which functions as an adhesion molecule inducing the capture and firm adhesion of CX3CR1-expressing cells, was rapidly induced in areas of inflammation in the liver. Furthermore, most NK cells that significantly infiltrated the infected livers had high levels of CX3CR1 on their surface. Based on these findings, we speculated that distinct increased levels of hepatic FKN and CX3CR1 expression in this acute viral hepatitis and the FKN/CX3CR1 interaction led to the potent immunoactivation of NK cells.
The purpose of the present study was to assess the contribution of FKN-CX3CR1 to the pathogenesis of NK cell–mediated hepatitis induced by adenoviral vector administration in mice. We induced acute liver injury in mice via HP injection of adenovirus vectors, which have been used to obtain the highest transfer efficiency,20-22 and found that the severity of liver injury was dependent on the presence of NK cells (Fig. 2C) and NK-dependent IFN-γ (Fig. 2E,F), which was also observed in other viral infections.40-43 Because FKN/CX3CR1 interaction has been reported to play an important role in NK cell recruitment and activation in the central nervous system of experimental autoimmune encephalomyelitis44 and possibly in hepatic bile ducts,45 we introduced a series of experiments in order to more clearly verify the actual function of FKN in adenovirus vector–induced NK cell–mediated liver injury. As reported in our study, the recruiting hepatic NK cells expressed high levels of surface CX3CR1 (Fig. 3A) after adenovirus vector HP injection, which may be the result of enhanced FKN production by the liver (Fig. 3B). After exogenous transgenic overproduction of FKN protein by CX3CL1-containing adenovirus vectors, the severity of liver injury increased (Fig. 4), with much more CX3CR1+ NK cell recruitment into the liver (Fig. 5). Moreover, when FKN mRNA was silenced by FKN-siRNA (Figs. 6, 7) or FKN/CX3CR1 interaction was blocked by FKN-specific antibody (Fig. 7), the adenovirus-induced acute severe liver injury was almost prevented, with much lower hepatic NK cell infiltration (Figs. 6D, 7B) and a significant reduction in IFN-γ serum levels (Fig. 7C).
In conclusion, our findings suggest that a blockade of FKN-CX3CR1 interaction in recruitment and activation of hepatic NK cells may prevent or alleviate adenovirus vector–induced acute liver injury to benefit adenovirus vector–based gene therapy.




