Glucocorticoid-induced leucine zipper: A key protein in the sensitization of monocytes to lipopolysaccharide in alcoholic hepatitis†
Potential conflict of interest: Nothing to report.
Abstract
Glucocorticoid-induced leucine zipper (GILZ), a recently identified protein induced by glucocorticoids (GCs), inhibits the nuclear factor κB pathway and the activation of monocytes/macrophages by lipopolysaccharides (LPS). This study aimed to elucidate the contribution of GILZ to the pathogenesis of alcoholic hepatitis (AH): we (1) assessed GILZ expression in the livers of patients with AH and (2) treated patients with severe AH with GCs (prednisolone 40 mg/day) and studied the effect of GILZ modulation on circulating monocyte function. We quantified GILZ expression in the livers of 42 consecutive alcoholic patients (21 with and 21 without AH). GILZ messenger RNA (mRNA) levels were lower in the livers of patients with AH versus those without AH (P < 0.05). We collected circulating monocytes from patients with severe AH before and 48 hours after GC treatment to quantify GILZ expression and cytokine secretion. GC treatment induced significantly higher levels of GILZ mRNA than that observed before treatment and impaired LPS-induced tumor necrosis factor-α (TNF-α) and regulated upon activation, normal T cell–expressed secretion (RANTES) by these monocytes. We transfected circulating monocytes with GILZ small interfering RNA (siRNA), specifically blocking GILZ expression, to demonstrate the role of GILZ in mediating GC effect. GILZ siRNA abrogated the effect of GC treatment on LPS-induced TNF-α and RANTES secretion. Conclusion: Low expression of GILZ may contribute to liver inflammation in AH. GCs enhance GILZ expression, abrogating macrophage sensitivity to LPS and proinflammatory cytokine secretion. These findings may explain the beneficial effect of GC treatment in patients with severe AH. (HEPATOLOGY 2007;46:1986–1992.)
Proinflammatory cytokines play a major role in the pathophysiology of alcoholic hepatitis (AH). Cytokines are produced and released by many cells, including monocytes, macrophages, and—particularly relevant to the liver—Kupffer cells.1 The proinflammatory cytokine tumor necrosis factor-α (TNF-α), TNF-α–inducible cytokines, and chemokines are critical mediators of the hepatotoxicity of alcohol. Reactive oxygen species and lipopolysaccharide (LPS),2 a membrane component of gram-negative commensal bacteria present in the digestive tract, are 2 major activators of Kupffer cells in AH. Alcohol ingestion increases intestinal barrier permeability3 and induces the diffusion of bacterial products into the lumen of the portal vein. LPS is hepatotoxic, and plasma endotoxin concentrations increase in experimental and human AH.4
AH is a life-threatening condition. Mortality for patients with the most severe forms of AH is 50%-75%. Although glucocorticoid (GC) treatment continues to be discussed, GCs are the only family of drugs that has shown efficacy in improving short-term survival in patients with severe AH, defined as a Maddrey discriminant function ≥ 32.5 GCs are potent anti-inflammatory and immunosuppressive drugs.6 It is hypothesized that their therapeutic effects in AH are due to their ability to inhibit many macrophage functions. They inhibit the production of cytokines, including TNF-α, interleukin-1, and interleukin-6; the production of chemokines, including regulated upon activation, normal T cell–expressed secretion (RANTES)/CCL57, 8; and the migration of leukocytes to sites of inflammation.6 However, the molecular mechanisms of GC effects are only partly identified, particularly in AH.9
A comparison of complementary DNAs from untreated and dexamethasone-treated murine T cells led to the identification of GC-induced leucine zipper (GILZ), a new member of the leucine zipper family.10 GILZ is produced in healthy subjects by cells of the monocyte/macrophage lineage, which includes monocytes, macrophages, Kupffer cells, and mesangial cells.11 It inhibits the nuclear factor-κB (NF-κB) and activator protein-1 (AP-1) pathways.12, 13 Macrophages, including Kupffer cells, constitutively produce GILZ in normal tissues of humans and mice, and this production is enhanced by GC administration.11 GILZ deactivates monocytes/macrophages by inhibiting their response to LPS, resulting in the production of lower amounts of proinflammatory cytokines and chemokines. In addition, GILZ inhibits the expression of Toll-like receptors, which sense the presence of microbial compounds in the environment.11
GC treatment has a beneficial effect in patients with AH. Thus, we hypothesized that impaired production or function of GILZ contributes to the pathophysiology of AH and that GC treatment counteracts this defect. We adopted a dual approach to test these hypotheses. Firstly, we assessed liver expression of GILZ in patients with alcohol-induced liver disease. Secondly, we treated patients with severe AH with GCs and studied the effects of GILZ modulation on the function of circulating monocytes.
Abbreviations
AH, alcoholic hepatitis; AP-1, activator protein-1; AU, arbitrary unit; GC, glucocorticoid; GILZ, glucocorticoid-induced leucine zipper; LPS, lipopolysaccharide; mRNA, messenger RNA; NF-κB, nuclear factor-κB; RANTES, regulated upon activation, normal T cell–expressed and secreted; RT-PCR, real-time polymerase chain reaction; siRNA, small interfering RNA; TNF-α, tumor necrosis factor α.
Patients and Methods
Patients.
All patients were admitted to the hepatogastroenterology department of Antoine Beclere Hospital, Clamart, France, for alcoholism and abnormal liver function tests. Patients were considered for inclusion if they had drunk at least 50 g of alcohol per day over the previous year, were negative for the presence of hepatitis B surface antigen and antibodies to hepatitis C virus in the serum, and had given their informed consent. Exclusion criteria were gastrointestinal bleeding, bacterial infection, hepatocellular carcinoma or other carcinoma, acute pancreatitis, severe associated disease, presence of anti-HIV antibodies, presence of dyslipidemia, and diabetes mellitus.
We used a specific questionnaire to collect information about alcohol consumption.14 The patients' families were also interviewed, when possible. Patients were questioned about the total duration of alcoholism and their daily alcohol intake over the 5 years preceding their first hospitalization in our department due to alcoholism or alcoholic liver disease. This was done because it has been reported that patients might have difficulty remembering a longer history of dietary intake and alcohol consumption. The questionnaire included the following items concerning alcohol consumption: duration of alcohol abuse; daily consumption of beer, wine, before-dinner drinks (aperitifs), and spirits (strong liquors); and the types of aperitifs ingested (aniseed, whisky, or others). The daily intake of each beverage was expressed in grams of pure ethanol, and the total daily consumption of ethanol was obtained by totaling the amounts consumed for each type of beverage. A total of 65 patients with alcoholic liver disease were included.
Patients with severe AH (discriminant function >32) (n = 23) underwent transjugular biopsy and were treated with GCs. Due to the limited size of the biopsy, GILZ production in the liver could not be studied in these patients. The effect of GC treatment on monocyte functions was tested in their circulating monocytes. Transparietal liver biopsies from the other 42 patients with alcoholic liver disease were used to compare GILZ production in patients with and without AH. This study was approved by the local ethics committee.
Liver Biopsies.
Patients underwent liver biopsy 5.6 ± 0.5 days after admission. One sample was used for histological assessment. When possible, another sample, weighing 5-10 mg, was immediately frozen in liquid nitrogen and stored at −80°C until used for RNA extractions. The staining procedure included hematoxylin-eosin-saffron, Masson's trichrome, and picrosirius stains. All biopsy samples were evaluated independently by 2 pathologists who were blinded to the clinical and biological data of the patients and to the amounts and durations of their alcohol intake. Internationally accepted morphological criteria15 were used for this evaluation. AH was defined according to the extent of liver cell damage, typically evident as ballooning degeneration with areas of necrosis and infiltration of polymorphonuclear leukocytes. A scoring system of alcoholic steatohepatitis combined the detailed alcoholic features: necrosis, polymorphonuclear leukocytes, Mallory bodies, and ballooning. Each feature was scored from 0-,2 with a total score ranging from 0-8. Steatosis was assessed semiquantitatively in 5 grades: none = 0; mild = 1 (1%-5% of hepatocytes); moderate = 2 (6%-32%); marked = 3 (33%-66%); and severe = 4 (67%-100%). Fibrosis was semiquantified by determining a modified METAVIR score16: 0 = no fibrosis; 1 = pericentral and/or periportal fibrosis without fibrous septa; 2 = pericentral and/or periportal fibrosis with few fibrous septa; 3 = many fibrous septa without cirrhosis; and 4 = cirrhosis. Liver biopsies were placed into 2 categories according to the presence or absence of AH.
Quantification of GILZ Gene Expression in the Liver.
Liver was homogenized in QIAzol lysis buffer with a Polytron. Total RNA was extracted using the RNeasy Lipid kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The deoxyribonuclease step was performed using an ribonuclease-free deoxyribonuclease kit (Qiagen) to eliminate residual DNA. The amount of RNA was determined at 260 nm with a spectrophotometer. RNA was amplified using the Message Amp II aRNA kit (Ambion, Austin, TX). M-MuLV RT (Invitrogen, Carslbad, CA) was used to reverse-transcribe 5 μg with random hexamers (Roche). Amplification was performed for the GILZ gene, and results were normalized according to actin expression [expressed as GILZ messenger RNA (mRNA) arbitrary units (AU)/103 actin mRNA (AU)] as described previously.17
Blood Collection.
Blood was collected prospectively from 23 consecutive patients with severe AH (discriminant function >32). Inclusion and exclusion criteria were the same as described above. A transjugular liver biopsy was performed for all of these patients and used for liver pathology to confirm AH diagnosis. Prednisolone, a member of the GC class of hormones, was given in a single dose of 40 mg each morning for 28 days. Heparinized blood samples (20 mL each) were collected at 8 AM from fasting patients before GC treatment and and 48 hours after the start of treatment.
Cell Purification.
Peripheral blood mononuclear cells were purified on a ficoll-hypaque density gradient, and monocyte-enriched antigen-presenting cells17 were isolated from peripheral blood mononuclear cells with a negative selection Dynal kit (Dynal, Compiègne, France). Purity of monocytes after selection was 92.3% ± 4.1%, as assessed by the presence of CD14.
Monocyte Stimulation, Quantification of GILZ Expression, and Cytokine Production.
A fraction of purified monocytes was used for quantification of GILZ gene expression via quantitative real-time polymerase chain reaction (RT-PCR) (light cycler, Roche) as described.17 Results were normalized according to actin expression (expressed as GILZ mRNA (AU)/103 actin mRNA (AU)) as described previously.17
Another monocyte fraction was cultured in polypropylene tubes at a density of 1 × 106 cells/mL in RPMI supplemented with 10% human serum albumin and 1% antibiotics. Cells were stimulated with 1 μg/ml Escherichia coli–derived LPS (Sigma, St. Quentin Fallavier, France) for 48 hours. Supernatants were then harvested, and the concentrations of CCL5/RANTES and TNF-α were assessed via enzyme-linked immunosorbent assay (Diaclone, Besançon, France; detection range >10 pg/mL).
Inhibition of GILZ Expression.
GILZ expression was inhibited by transfecting monocytes with small interfering RNAs (siRNAs) specific for the GILZ gene, as described previously.17 The sequence of the sense strand of the GILZ siRNA (siGILZ) was 5′-AAC AGC UUC ACC UGA CAA CGAdTdT-3′. The sense strand of the control siRNA was synthesized with random nucleotides and had no known specificity: 5′-CAU AAC GAG CGG AAG AAC GdTdT-3′ (MWG Biotech, Ebersberg, Germany).
To assess the efficacy of GILZ knock-down by siRNA transfection, monocytes were purified from buffy coats (healthy donors) and transfected with GILZ-specific or control siRNA. Dexamethasone (10−7 M) was added 6 hours after transfection; cells were collected 14 hours later. GILZ gene expression was determined via PCR as described previously18, 19 and immunoprecipitation followed by Western blotting. GILZ was immunoprecipitated with a polyclonal anti-GILZ antibody, and the immune complex was separated with protein G (Sigma). Detection of GILZ via Western blotting and sodium dodecyl sulfate–polyacrylamide gel electrophoresis was performed as described.18
Immunohistochemistry.
Sections (7 μm thick) of frozen liver tissue were subjected to immunohistochemistry. The sections were fixed in 4% paraformaldehyde at 4°C and treated with citrate buffer (pH 6) (Dako, Trappes, France) in a microwave for antigen retrieval. Endogenous peroxidase and biotin activity were quenched by incubating the sections with peroxidase-blocking solution (Dako) and then with Biotin-Blocking System reagents (Dako). Sections were incubated with 10% normal rabbit serum for 30 minutes at room temperature to block nonspecific antibody binding sites. The sections were then incubated overnight at 4°C with 2 μg/mL polyclonal goat GILZ antibody (sc-26518; Santa Cruz Biotechnology, Santa Cruz, CA) and then probed with a biotinylated rabbit anti-goat antibody (Vectastain Elite Avidin-Biotin-Complex kit, Vector, Burlingame, CA). The signal was amplified with avidin-biotin-complex reagent (Vector) and visualized with the chromogen AEC (3-amino-9-ethylcarbozole) (Dako). Slides were counterstained with Mayer's hematoxylin solution. Controls included omission of the primary antibody and replacement of the primary antibody with normal goat immunoglobulin G (sc-2028, Santa Cruz Biotechnology).
Statistical Analysis.
All values are expressed as the mean ± standard error of the mean. A nonparametric Mann-Whitney test was used for comparisons between alcoholic patients with and without AH. A nonparametric-paired Wilcoxon signed-rank test was used to compare results before and after treatment with GCs.
Results
Decreased GILZ Expression in Livers of Patients with AH.
To determine whether decreased production of GILZ by Kupffer cells contributes to alcohol-induced liver inflammation, we investigated GILZ gene expression in the livers of 42 consecutive alcoholic patients. All 42 patients had histological abnormalities in the liver characterizing alcohol-induced liver disease. We classified these patients according to the absence or presence of AH. There was no significant difference between patients with and without AH for age, sex ratio, body mass index, and alcohol consumption over the past 5 years (data not shown).
We quantified GILZ gene expression in the liver via RT-PCR. Its level was lower in patients with AH than without AH (17,753 ± 3839 and 44,683 ± 10,242 GILZ mRNA (AU)/103 actin mRNA (AU), respectively; P < 0.05) (Fig. 1). This finding indicates that low liver expression of the GILZ gene in alcoholic liver disease patients is associated with AH.
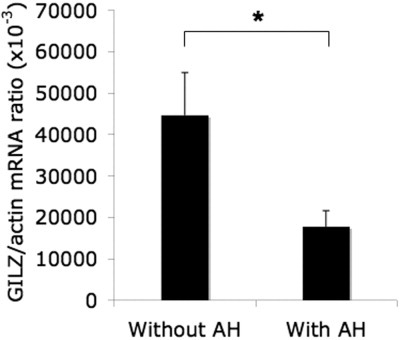
Decreased GILZ expression in the liver of patients with AH. GILZ expression was quantified via RT-PCR in livers from alcoholic patients with and without AH. Results are expressed as the mean ± standard error of the mean (n = 21 patients per group). *P < 0.05 (Mann-Whitney test).
GILZ Is Produced by Kupffer Cells in AH.
We have shown previously that normal human liver Kupffer cells express the GILZ gene, as ascertained by in situ hybridization experiments.11 We confirmed this finding via immunohistochemical analysis of GILZ production in normal human livers. Kupffer cells were identified as the dominant source of GILZ in normal human liver (Fig. 2A). We then asked whether Kupffer cells were also the main source of GILZ in the liver of alcoholic patients. Immunohistochemical analysis showed that GILZ-producing cells in alcoholic patients were Kupffer cells, based on location, morphologic findings, and phenotype (i.e., CD68-positive). GILZ staining was lower in alcoholic patients than in normal human liver, in accordance with GILZ mRNA quantification results. There was no staining with the control antibody (Fig. 2B). These results indicate that the presence of AH does not affect the cellular source of GILZ in the liver.
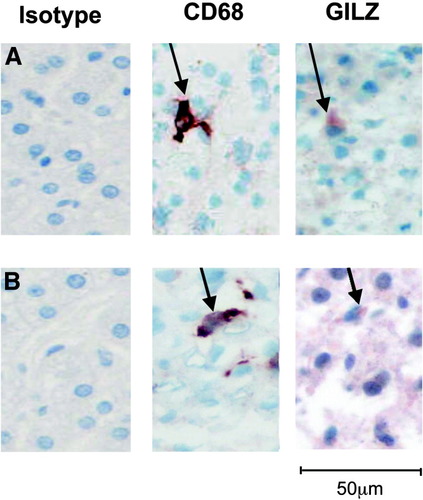
GILZ is expressed by Kupffer cells in livers of alcoholic and nonalcoholic patients. GILZ expression was determined via immunohistochemistry with anti-GILZ and anti-CD68 antibodies in serial sections of the same liver sample. Kupffer cells, identified as CD68-positive cells, are GILZ-positive in both (A) control and (B) AH livers.
GC Treatment of Patients with Severe AH Increases GILZ Expression in Circulating Monocytes.
GILZ is involved in the anti-inflammatory action of GCs, especially in cells belonging to the monocyte/macrophage lineage. Thus, we investigated whether GILZ contributes to the anti-inflammatory effect of GCs in AH.
We tested whether GC treatment stimulates GILZ expression in circulating monocytes of patients with severe AH. We used RT-PCR to quantify the level of GILZ mRNA in monocytes purified from patients with severe AH before and 48 hours after the start of GC treatment. GILZ gene expression levels were significantly higher after GC treatment than before treatment (3093 ± 812 and 1700 ± 198 of GILZ mRNA (AU)/103 actin mRNA (AU), respectively; P < 0.01) (Fig. 3).
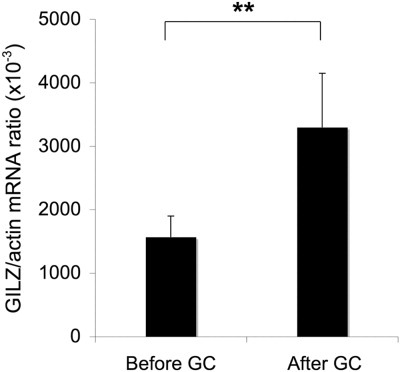
Glucocorticoid treatment of patients with severe AH increases GILZ expression in circulating monocytes. GILZ expression was quantified via RT-PCR in circulating monocytes from patients with severe AH before and 48 hours after the start of GC treatment. Results are expressed as the mean ± standard error of the mean (n = 12 patients per group). **P < 0.01 (Wilcoxon signed-rank test).
GC Treatment of Patients with Severe AH Abrogates Production of Proinflammatory Cytokines by LPS-Stimulated Monocytes.
The beneficial effect of GC treatment in severe AH may depend on its ability to inhibit the production of inflammatory mediators in response to bacterial products. To support this hypothesis, we analyzed proinflammatory cytokine and chemokine production by monocytes of patients treated with GCs. We cultured blood monocytes purified from patients with severe AH before and 48 hours after the start of GC treatment. We measured the concentration of TNF-α and RANTES produced in response to LPS challenge of these monocytes. In patients with severe AH, GC treatment significantly decreased production of TNF-α (from 1520 ± 480 pg/mL before treatment to 650 ± 193 pg/mL after treatment; P < 0.05) and RANTES (from 1080 ± 270 pg/mL before treatment to 144 ± 36 pg/mL after treatment, P < 0.01) (Fig. 4A–B). These results show that treatment with GC for two days substantially impairs the ability of circulating monocytes from AH patients to produce proinflammatory cytokines and chemokines in response to LPS.
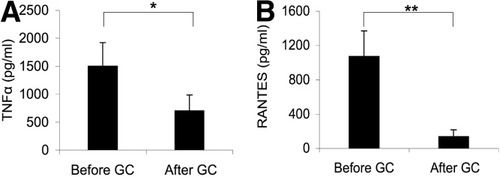
GC treatment of patients with severe AH abrogates production of TNF-α and RANTES by LPS-stimulated monocytes. Circulating monocytes were isolated from patients with severe AH before and 48 hours after the start of GC treatment. Cells were stimulated with LPS for 48 hours and TNF-α, and RANTES in supernatants were quantified via enzyme-linked immunosorbent assay. (A) TNF-α. (B) RANTES. Results are expressed as the mean ± standard error of the mean (n = 14). *P < 0.05; **P < 0.01 (Wilcoxon signed-rank test).
GILZ Expression Desensitizes Monocytes to LPS.
GCs stimulate GILZ production by monocytes and macrophages, possibly contributing to their anti-inflammatory effect in patients with AH. To test this hypothesis, we analyzed whether inhibiting GILZ expression in monocytes prevented the effect of GC treatment on the production of inflammatory mediators. For this purpose, we specifically blocked GILZ expression by transfecting monocytes with GILZ siRNA. We first confirmed that this approach inhibited dexamethasone-induced GILZ expression in monocytes from healthy individuals, either at the mRNA or protein level (Fig. 5A–B). We then studied the effect of GILZ expression inhibition on cytokine and chemokine production by monocytes, isolated before and 48 hours after start of GC treatment in patients with AH. Monocytes isolated before GC administration produced similar levels of TNF-α and RANTES following stimulation with LPS, regardless of whether they were transfected with control or GILZ siRNA (Fig. 6A–B). Monocytes isolated after GC treatment and then transfected with control siRNA produced lower levels of TNF-α and RANTES than those isolated before treatment. This finding confirms the anti-inflammatory effect of GCs. Inhibition of GILZ gene expression by transfection with GILZ siRNA abrogates the effect of GCs on TNF-α and RANTES secretion, demonstrating that induction of GILZ expression by GCs is key to the anti-inflammatory effect in AH patients. Therefore, administration of GCs to patients with severe AH stimulates GILZ expression by circulating monocytes, resulting in decreased sensitivity of monocytes to LPS and decreased synthesis of proinflammatory mediators.
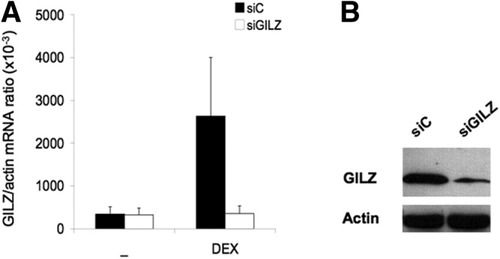
siRNA blocks dexamethasone induction of GILZ gene expression at the mRNA and protein level. Monocytes from healthy controls were transfected with control siRNA or GILZ siRNA and treated or not with dexamethasone (DEX) (10−7 M). GILZ production was assessed via RT-PCR or immunoprecipitation followed by Western blotting. (A) GILZ mRNA expression. (B) GILZ protein expression.
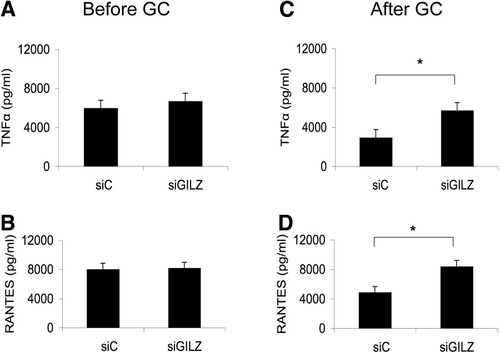
GILZ expression desensitizes monocytes to LPS. Circulating monocytes were isolated from patients with severe AH before and 48 hours after the onset of GC treatment. They were transfected either with GILZ siRNA or control siRNA. Cells were then stimulated with LPS for 48 hours. TNF-α and RANTES in supernatants were quantified via enzyme-linked immunosorbent assay. Inhibition of GILZ expression has no effect on (A) TNF-α or (B) RANTES secretion in the absence of GC treatment. Inhibition of GILZ expression abrogates GC effects on (C) TNF-α and (D) RANTES secretion. Results are expressed as the mean ± standard error of the mean (n = 6 patients per group); *P < 0.05 (Wilcoxon signed-rank test).
Discussion
Our findings show that GILZ is key in the desensitization of monocytes to LPS-induced proinflammatory cytokine secretion in AH, possibly contributing to the pathophysiology of AH and to the beneficial effect of GC treatment. We show that the level of GILZ expression is lower in the liver of patients with AH than in alcoholic patients without AH. This quantitative change is not associated with a change in the source of GILZ, because Kupffer cells are the prominent source of GILZ in livers of patients with AH and in normal human liver controls. We also show that GC treatment of patients with severe AH increases GILZ gene expression in circulating monocytes and abrogates production of proinflammatory cytokines after LPS challenge. siRNA transfection experiments demonstrate that impaired GILZ production in monocytes prevented the anti-inflammatory effects of GCs. Therefore, GC-induced GILZ production desensitizes monocytes/macrophages to LPS stimulation. This finding may also extend to Kupffer cells. Stimulation of GILZ production in patients with AH may then contribute to the beneficial effect of GCs (i.e., the resolution of liver inflammation).
In livers of healthy individuals, constitutive production of cytokines is low or absent. Chronic consumption of alcohol enhances the secretion of TNF-α by monocytes/macrophages.19 Activation of the innate immune system by LPS from the digestive tract has emerged as a key factor in the triggering of AH.3, 4, 19-24 Alcohol abuse impairs the function of the intestinal barrier, possibly enhancing the translocation of bacterial toxins.3 Ethanol intake in rats substantially increases translocation of LPS from the gut lumen into the portal blood.4 Patients with alcoholic cirrhosis have higher endotoxin levels than heavy drinkers without cirrhosis and healthy controls.21 Kupffer cells are located at the first site of exposure to gut-derived endotoxin. Their stimulation with microbial products leads to production of proinflammatory mediators.22, 23 In rats exposed to ethanol by gastric infusion, antibiotic treatment decreases TNF-α expression and liver injury.22 Mice lacking functional Toll-like receptor 4, the LPS receptor, do not respond to endotoxin25 and are resistant to alcohol-induced liver injury.24 GILZ induction by GCs abrogates the LPS-induced secretion of proinflammatory chemokines by the THP-1 monocyte cell line,11 and by monocytes from patients with severe AH, as shown in this study.
Activation of NF-κB is a key abnormality in the mediation of cytokine disturbance in AH.9 Activation of the NF-κB pathway and the stimulation of NF-κB–dependent gene transcription are common to activation of Toll-like receptors 4, 2, and 626, 27 and are essential to the initiation of an inflammatory response. NF-κB is activated in monocytes in patients with AH.28 NF-κB binding activity is stronger in the livers of rats chronically exposed to ethanol by gastric infusion than in controls, a response that is thought to occur in Kupffer cells.29 Gut-derived LPS activation of Kupffer cells leads to NF-κBκdependent synthesis of cytokines and chemokines. Ethanol also activates the transcription factor AP-1.30 AP-1 proteins bind to genes regulating cell proliferation and death.30 AP-1 is also important in regulating transcription of CD14, the LPS coreceptor.31 GILZ, which is expressed by Kupffer cells, binds to the cytoplasmic p65 subunit of NF-κB, preventing its nuclear translocation and activation.11 GILZ also directly interferes with AP-1.13 GC treatment of patients with AH probably inhibits both NF-κB and AP-1 by inducing GILZ expression, conferring resistance to LPS.
In conclusion, our findings demonstrate that GILZ is a critical player in the desensitization of monocytes/macrophages to LPS in AH. Low GILZ expression in the liver is associated with AH. Induced GILZ expression leads to the anti-inflammatory effects of GC in circulating monocytes of patients with AH. Through induction of GILZ expression, GCs abrogate LPS sensitivity of macrophages and subsequent proinflammatory cytokine secretion, making it a beneficial treatment in patients with severe AH.




