In vitro production of Mallory bodies and intracellular hyaline bodies: The central role of sequestosome 1 / p62†
Potential conflict of interest: Nothing to report.
Abstract
Mallory bodies (MBs) and intracellular hyaline bodies (IHBs) are characteristic hepatocellular inclusions. MBs are hallmarks of steatohepatitis, whereas IHBs have first been detected in hepatocellular carcinoma. MBs and IHBs contain ubiquitin and sequestosome 1 / p62 (p62), a stress-inducible adapter protein with affinity to polyubiquitinated proteins. MBs differ from IHBs by their keratin content and morphology. In vitro transfections were undertaken to study under defined conditions MB and IHB formation, their pathogenesis, and relationship. CHO-K1, TIB73, and HeLa cells were transfected with keratin 8, keratin 18, ubiquitin, p62, and p62 lacking the ubiquitin binding domain (p62ΔUBA) and analyzed by immunofluorescence, immunoelectron microscopy, and immunoblotting. Transfection of p62 complementary deoxyribonucleic acid (cDNA) alone led to cytoplasmic aggregates consisting of filaments mostly arranged in parallel arrays resembling amyloid and type 1 MBs. Transfection of p62 and ubiquitin resulted in globular cytoplasmic aggregates with indistinct fibrillar ultrastructure resembling IHBs. Cotransfection of p62, keratin 8, and ubiquitin was necessary to produce in vitro type 2 MBs-like aggregates consisting of randomly oriented 10- to 15-nm filaments. A similar result was obtained when keratin 8 was replaced by keratin 18. After cotransfection of p62ΔUBA, keratin 8, and ubiquitin, keratin formed irregular aggregates with electron-dense granular-amorphous ultrastructure (resembling type 3 MBs), whereas p62ΔUBA remained in diffuse cytoplasmic distribution. Conclusion: Our studies show that in vitro development of classical type 2 MBs requires overexpression of keratin 8 (or keratin 18), ubiquitin, and p62 containing the ubiquitin binding domain, whereas IHBs result from overexpression of p62 together with ubiquitin without keratin involvement. (HEPATOLOGY 2007.)
Mallory bodies (MBs) and intracellular hyaline bodies (IHBs) are hepatocellular inclusions that may occur in a diversity of chronic liver disorders.1, 2 They differ in their light and electron microscopic appearance and chemical composition but share sequestosome 1/p62 (p62) as a major component.1-4 Whereas MBs contain in addition abnormal keratins, ubiquitin, and heat shock proteins, keratins are lacking in classical IHBs.1, 4 P62 is a multifunctional protein5, 6 with several structural motifs that determine its various functions: the SH2 domain binds the tyrosine kinase p56lck in a phosphotyrosine-independent manner, an acidic interaction domain associates with the atypical protein kinase C ζ and mediates the role of p62 as adapter in tumor necrosis factor alpha–initiated, interleukin-1–initiated, and nerve growth factor–initiated signal transduction, and a ZZ-type zinc finger binds the receptor interactive protein involved in tumor necrosis factor–induced apoptosis. Moreover, p62 contains a binding site for the tumor necrosis factor receptor–associated factor 6, which is an E3 ubiquitin ligase, two PEST (regions rich in proline, glutamate, serine, and threonine) sequences, and the ubiquitin-association (UBA) domain.5-7
We have recently described the simultaneous occurrence of MBs and IHBs in neoplastic (hepatocellular carcinoma) and non-neoplastic (idiopathic copper toxicosis) hepatocytes, and of “hybrid” inclusions consisting of both types, suggesting pathogenetic similarities and interactions.4 A disturbance and rarefaction of the keratin intermediate filament cytoskeleton is associated with MB formation in ballooned hepatocytes, whereas IHBs are usually found in small- or normal-sized hepatocytes with an intact cytoskeleton. Because overexpression of p62 and intermediate filament alterations can be the result of a cellular stress, particularly oxidative stress, response, classical MBs may arise if p62 and damaged keratins concur, and possibly hybrid inclusions are formed when overexpression of p62 (together with ubiquitin) prevails over, or precedes, keratin alterations. In contrast, IHBs seem to result from p62 and ubiquitin overexpression and aggregation without accumulation of sufficient amounts of abnormal keratins.4 Expression of abnormal keratins (e.g., due to mutation) alone leads to keratin-containing cytoplasmic inclusions in keratinocytes, as evident in certain blistering skin diseases and tissue culture cells.8-11 The mechanisms underlying inclusion body formation are still unclear, and its significance is controversial. They may be the result of a cellular attempt to segregate and neutralize abnormal proteins that could interfere with normal cell function.12-18 Experimental studies as well as observations in human liver disease showing that p62-positive inclusion bodies, including MBs and IHBs, are associated with living, even activated, cells suggest that p62 is involved in the protection of cells from the toxicity of abnormal (e.g., misfolded) proteins.19 However, in a certain context inclusion bodies also may be cytotoxic.4, 12-24
The aim of our studies was to obtain closer insight into MB and IHB formation by in vitro transfection of their major components, keratins, p62, and ubiquitin, and correlating the ultrastructure of the resulting aggregates with their chemical composition. Keratin 8 was primarily chosen because we and others have shown that keratin 8 is essential for MB formation in vivo.1, 25-27 Because p62 as well as ubiquitin are present in inclusion bodies associated with a variety of chronic neurodegenerative disorders, such as Alzheimer and Parkinson disease,3, 28 results of this study may be relevant in an even greater context.
Abbreviations
cDNA, complementary deoxyribonucleic acid; DDC, 3,4-diethoxycarbonyl-1,4-dihydrocollidine; Ig, immunoglobulin; IHB, intracellular hyaline body; MB, Mallory body; p62, sequestosome 1/p62; UBA, ubiquitin-association domain.
Materials and Methods
Complementary DNA Constructs for Transfection.
Complementary DNA (cDNA) constructs are summarized in Fig. 1. Full-length human p62 cDNA6 was subcloned into the EcoRI site of the multiple cloning site of the pcDNA4/HisMax expression vector (Invitrogen, Groningen, The Netherlands). In addition, a p62 construct lacking the ubiquitin binding (association) domain (p62ΔUBA), that is, the bases 1220 through 1372 of p62 [Acc.no. U46751] or the amino acids 389 through 439, was generated by polymerase chain reaction from the human p62 cDNA construct using the forward primer 5′-CTCGCTATGGCGTCGCTCACCGTG-3′ (corresponding to positions 47–70 of U46751) and the reverse primer 5′-TCACTC TGGCGGGAGATGTGGGTA-3′ (corresponding to positions 1219–1199 plus a stop codon). The polymerase chain reaction product was cloned into the pCR2.1 vector using the TA cloning kit and subcloned into the pcDNA4/HisMax expression vector (Invitrogen). Mouse keratin 8 cDNA (provided by T. M. Magin, Bonn, Germany) and mouse keratin 18 cDNA (provided by A. Alonso, Heidelberg, Germany) were subcloned into the EcoRI site of the pcDNA3 expression vector. The pRBG4-ubiquitin-his-myc construct was provided by R. Kopito (Stanford, CA). All restriction enzymes were from Roche Diagnostics (Roche GmbH, Vienna, Austria).
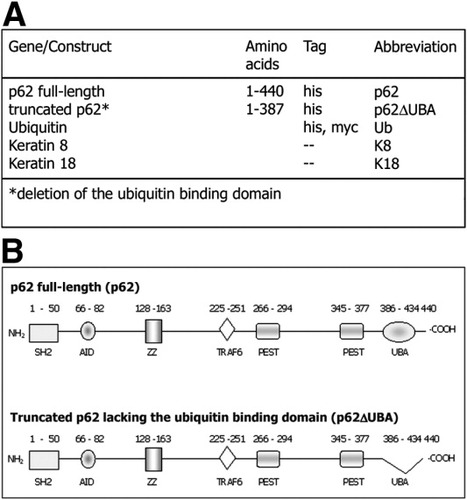
(A) Overview of the gene constructs used for transfection of CHO-K1, TIB73, and HeLa cells. (B) Structural motifs and binding sites of full-length and truncated p62 lacking the ubiquitin binding (association) domain (p62ΔUBA).
Transfection Experiments.
CHO-K1 (chinese hamster ovary), TIB73 (mouse liver), and HeLa cells (all from ATCC, Manassas, VA) were cultured in Ham's F12 medium, Dulbecco's modified medium, and minimum essential medium, respectively, each supplemented with 10% fetal bovine serum. Cells were seeded in 12-well plates onto glass coverslips 18 hours before transfection and transfected with 1.6 μg cDNA and 6 μl Lipofectamine 2000 (Invitrogen) according to the instructions of the supplier. For contransfection, up to 3 constructs were combined; for single transfections and cotransfection of 2 constructs, empty vector was added to keep the total cDNA amount constant. In single transfections as well as in cotransfection experiments, the same cDNA amount of the individual constructs (i.e., one third of the total cDNA amount) was used. In experiments with reduced amounts of keratin 8 or p62 cDNA, keratin 8 or p62 cDNA was reduced from 1 of 3 to 1 of 9 (Table 1) replaced by empty vector. Cells were analyzed 17 to 24 hours after transfection. Transfection efficiency was quantified in 3 independent experiments by counting transfected (protein-expressing) cells in relation to the total number of cells in 20 vision fields at 250× magnification. The transfection efficiency for the double and triple transfectants was estimated based on double positive staining. The transfection efficiency with different constructs is shown in Table 2.
| Transfection regimens | Transfected Constructs | ||||
|---|---|---|---|---|---|
| K8 | Ub | p62 | K18 | empty vector | |
| p62 | — | — | 1/3 | — | 2/3 |
| p62+Ub | — | 1/3 | 1/3 | — | 1/3 |
| K8 | 1/3 | — | — | — | 2/3 |
| K8+Ub | 1/3 | 1/3 | — | — | 1/3 |
| K8+p62 | 1/3 | — | 1/3 | — | 1/3 |
| K8+Ub+p62 | 1/3 | 1/3 | 1/3 | — | — |
| K8↓+Ub+p62 | 1/9 | 1/3 | 1/3 | — | 2/9 |
| K8+Ub+p62↓ | 1/3 | 1/3 | 1/9 | — | 2/9 |
| K18+Ub+p62 | — | 1/3 | 1/3 | 1/3 | — |
| Constructs | Transfection Efficiency (%) |
|---|---|
| p62 | 13.7 ± 3.7 |
| K8 | 5.2 ± 0.4 |
| p62ΔUBA | 12.2 ± 6.3 |
| Ub | 8.4 ± 4.9 |
| K8+Ub | 4.3 ± 3.4 |
| p62+Ub | 14.1 ± 4.4 |
| K8+Ub+p62 | 8.4 ± 1.7 |
Immunofluorescence and Immunoelectron Microscopy.
Immunofluorescence and immunoelectron microscopy were performed as previously described.29 Transfected cells grown on coverslips were fixed in methanol at −20°C for 5 minutes and in acetone (−20°C) for 5 seconds. For immunofluorescence microscopy, the following antibodies were used: (1) primary antibodies to keratin 8 (Ks 8.7; Progen), keratin 18 (Ks18.04; Progen), keratin 8/18 (50K160; 4), p62 (Transduction Laboratories, Lexington, KY), myc-tag (Abcam, Cambridge, UK), and ubiquitin (ID Labs Inc., London, ON, Canada); (2) secondary antibodies were: Alexa Fluor 488 nm-conjugated goat anti-mouse immunoglobulin (Ig) (Molecular Probes, Leiden, The Netherlands), TRITC-conjugated swine anti-rabbit Ig (Dako, Glostrup, Denmark), and Rhodamine Red-X–conjugated goat anti-guinea pig Ig (Jackson Immune Research, West Grove, PA). Immunofluorescent specimens were analyzed with a Zeiss LSM 510 laser-scanning confocal microscope (Zeiss, Oberkochen, Germany). As controls, primary antibodies were omitted or replaced by isotype-matched Ig (Dako). For immunoelectron microscopy, the same primary antibodies were used; secondary antibodies were 18 nm gold–conjugated goat anti-mouse Ig and 12 nm gold-conjugated swine anti-rabbit Ig (Jackson). The types of aggregates were classified and quantified based on their ultrastructure (Fig. 7). Approximately 30 cells (with aggregates) from 2 to 3 independent transfection experiments were evaluated.
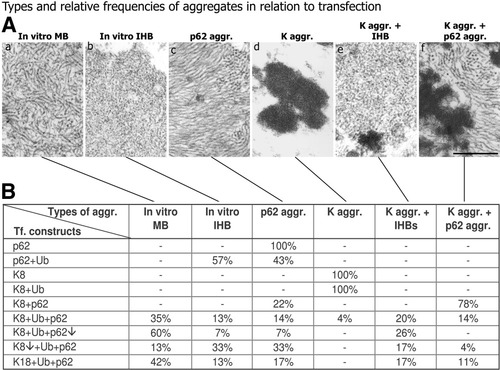
Types of cytoplasmic aggregates as revealed by electron microscopy (A) in relation to transfection of CHO-K1 cells with different cDNA constructs (B). Note that classical (type 2) MBs with randomly oriented filaments only arise if keratin 8 or 18 is cotransfected with ubiquitin and p62 (K8 + Ub + p62). In addition to type 2 MBs, IHBs, p62 (p62 aggr.), and keratin aggregates (K aggr.) and combinations (K aggr. + IHB, K aggr. + p62 aggr.) arise. Lowering keratin 8 expression (K8↓) decreases, whereas lowering p62 expression (p62↓) increases type 2 MB formation. Under cell culture conditions, keratin 18 is able to induce type 2 MBs in combination with ubiquitin and p62 (K18 + Ub + p62). Bar, 250 nm.
Analysis of Ubiquitinated Keratin.
CHO-K1 cells were cotransfected with keratin 8 and his-tagged ubiquitin as described. Ubiquitinated proteins (keratins) were isolated using the Talon Metal Affinity Resins System (Clontech, Vienna, Austria) following the instructions of the supplier. In brief, transfected cells were lysed with nondenaturing (detergent-free) and denaturing (containing 8 M urea) buffer, respectively. Ubiquitinated proteins were isolated from 12,000 g supernatants by affinity precipitation via the his-tag. Precipitates, supernatants, and pellet fractions were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and analyzed by immunoblotting using keratin 8 antibodies (Troma I; Developmental Studies Hybridoma Bank, Iowa City, IA) and enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ) for visualization.
Western Blotting.
Western blotting was performed as described.2 Equal amounts of total lysates of transfected CHO-K1 cells were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis followed by protein transfer onto polyvinylidine fluoride membranes. The following antibodies were used: (1) primary antibodies to keratin 8 (Troma I) to detect transfected keratin 8, p62 (Transduction Laboratories) to detect transfected p62, p62CT2 to detect endogenous and transfected p62, myc-tag (Abcam) to detect transfected ubiquitin, and β-actin (Sigma-Aldrich, Vienna, Austria) to detect endogenous β-actin; (2) as secondary antibodies, horseradish peroxidase–conjugated rabbit anti-mouse Ig, swine anti-rabbit Ig, and rabbit anti-guinea pig Ig (Dako) were used.
Results
Transfection of Individual MB and IHB Components.
For transfection we preferentially used CHO-K1 cells, which lack keratins and thus provide an appropriate system to study MB and IHB formation in vitro. Transfection of CHO-K1 cells with p62 cDNA led to irregularly outlined variably sized p62 aggregates with filamentous ultrastructure (Fig. 2A,B). Filaments, 10 to 15 nm in diameter, were mostly arranged in parallel arrays (Fig. 2B). Immunofluorescence and immunoelectron microscopy did not reveal ubiquitination by endogenous ubiquitin (not shown). The same immunofluorescence and immunoelectron microscopic images were obtained, when p62 was transfected into cells containing an intrinsic keratin intermediate filament cytoskeleton (TIB73, HeLa), indicating that p62 did not interact with keratins assembled as intermediate filaments (Fig. 2C,D). Transfection of CHO-K1 cells with keratin 8 alone led to irregular cytoplasmic aggregates with electron-dense granular-amorphous ultrastructure (Fig. 2E,F). An identical result was achieved by transfection with keratin 18 (Fig. 4A,B). Transfection of CHO-K1 cells with keratin 8 and keratin 18 resulted in the development of a network of intermediate filament bundles as typically seen in epithelial cells.3 After transfection of p62ΔUBA, a diffuse p62 distribution without aggregate and filament formation ensued (Fig. 2G,H), and a similar diffuse cytoplasmic staining was obtained after transfection of ubiquitin (Fig. 2I,J).
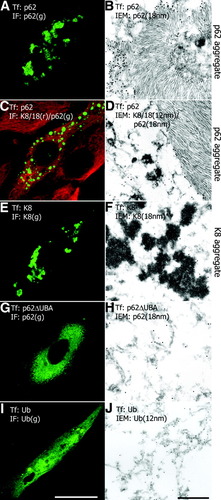
Immunofluorescence and immunoelectron microscopy of transfected tissue culture cells (CHO-K1: A, B, E, F, G, H, I, J; TIB73: C, D; Tf: transfected cDNA; IF: antibodies used in immunofluorescence microscopy; g, green; r, red; IEM, immunoelectron microscopy). (A) Irregularly sized p62-positive cytoplasmic aggregates in p62-transfected CHO-K1 cells; they consist of filaments in parallel arrays decorated by p62 antibodies [(B) 18 nm gold particles]. (C) Transfection of TIB73 cells with p62 leads to p62 aggregates without association with the intrinsic keratin cytoskeleton (keratin, red; p62, green). The ultrastructure of the p62 aggregates resembles that shown in B [(D) p62 antibodies: 18-nm gold particles] keratin antibodies decorate intermediate filaments [(D) 12-nm gold particles]. (E) Transfection of keratin 8 (K8) leads to irregular aggregates with electron-dense granular-amorphous ultrastructure [(F) keratin antibodies: 18-nm gold particles]. (G) Aggregate formation does not occur after transfection of p62ΔUBA as revealed by diffuse p62 staining. Transfected p62ΔUBA exists as indistinct filamentous or granular structures [(H) p62 antibodies: 18-nm gold particles]. (I) Transfection of ubiquitin (Ub) yields diffuse cytoplasmic distribution. Electron microscopy confirms this distribution without distinct ultrastructure or association with specific organelles (J). Bar (A, C, E, G, I), 10 μm; Bar (B, D, F, H, J), 500 nm.
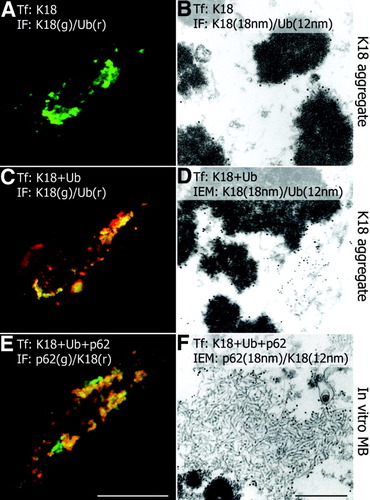
Immunofluorescence and immunoelectron microscopy of transfected and cotransfected CHO-K1 cells. (A) Transfection of keratin 18 (K18) leads to irregular aggregates consisting exclusively of keratin 18 (green immunofluorescence with keratin 18 antibodies; no reactivity with ubiquitin antibodies) and showing electron-dense granular-amorphous ultrastructure (B; keratin antibodies: 18-nm gold particles; ubiquitin antibodies: 12-nm gold particles). (C) Cotransfection of keratin 18 and ubiquitin (K18+Ub) results in irregular aggregates (K18 aggregates) consisting of keratin and ubiquitin (yellow in C) with electron-dense granular-amorphous ultrastructure (D; keratin antibodies: 18-nm gold particles; ubiquitin antibodies: 12-nm gold particles). (E) Cotransfection of keratin 18, ubiquitin, and p62 (K18 + Ub + p62) shows aggregates containing p62, keratin 18, and ubiquitin (yellow in E; ubiquitin reactivity not shown), in addition to p62-positive (green in E) and keratin-positive (red in E) aggregates. Inclusions containing p62, ubiquitin, and keratin 18 consist of randomly oriented filaments resembling type 2 MBs (F; p62 antibodies: 18-nm gold particles; keratin antibodies: 12 nm). Bar (A, C, E), 10 μm; Bar (B, D, F), 500 nm.
In Vitro Reproduction of MBs and IHBs by Cotransfections.
Cotransfection of p62 and ubiquitin resulted in globular cytoplasmic aggregates resembling IHBs,2, 4 with an indistinct fibrillar to granular ultrastructure and colocalization of p62 and ubiquitin (Fig. 3A,B). Cotransfection of p62, keratin 8, and ubiquitin led to irregular aggregates of different sizes; they resembled type 2 MBs with respect to both shape and characteristic ultrastructure consisting of randomly oriented filaments of intermediate (10–15 nm) dimensions (Fig. 3C,D). In addition, small aggregates containing keratin 8 alone as well as p62-positive but keratin 8-negative inclusions were found (Fig. 3C). Transfection of keratin 8 together with ubiquitin resulted in disordered aggregates consisting of keratin and ubiquitin with electron-dense granular-amorphous ultrastructure (Fig. 3E,F).
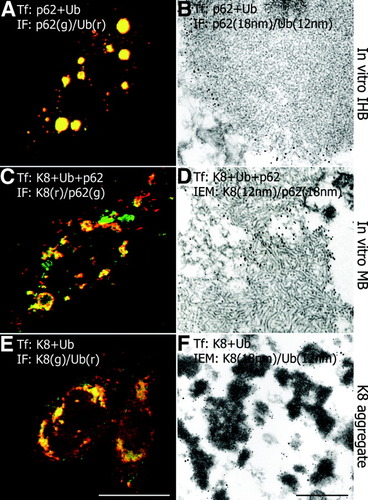
Immunofluorescence and immunoelectron microscopy of cotransfected CHO-K1 cells. (A) Cotransfection of p62 and ubiquitin (p62+Ub) yields globular cytoplasmic inclusions with colocalization of p62 and ubiquitin (indicated by the yellow color in A) and indistinct fibrillar to granular ultrastructure resembling IHBs (B; p62 antibodies: 18-nm gold particles; ubiquitin antibodies: 12-nm gold particles). (C) Cotransfection of keratin 8, ubiquitin, and p62 (K8 + Ub + p62) shows aggregates with colocalization of p62, keratin 8, and ubiquitin (yellow in C), in addition to only p62-positive (green in C) and only keratin-positive (red in C) aggregates. Inclusions containing p62, ubiquitin, and keratin 8 consist of randomly oriented filaments resembling type 2 MBs (D; p62 antibodies: 18-nm gold particles; keratin antibodies: 12-nm gold particles). (E) Cotransfection of keratin 8 and ubiquitin (K8+Ub) results in irregular aggregates (K8 aggregates) consisting of keratin and ubiquitin (yellow in E) with electron-dense granular-amorphous ultrastructure (F; keratin antibodies: 18 nm gold particles; ubiquitin antibodies: 12 nm gold particles). Bar (A, C, E), 10 μm; Bar (B, D, F), 500 nm.
Animal experiments with modification of keratin 8 and 18 and with keratin 8 and keratin 18 knockout mice revealed the essential role of keratin 8 for MB formation,25-27 although the analysis of in vivo developed MBs disclosed the presence of keratin 18 in addition to keratin 8.30 We therefore tested the capability of transfected keratin 18 to produce MBs in vitro. Cotransfection of keratin 18 and ubiquitin led to granular-amorphous aggregates identical to that obtained with keratin 8 (Fig. 4C,D). When keratin 18 was cotransfected with ubiquitin and p62 type 2 MBs were produced (Fig. 4E,F). This suggests that the inability of keratin 8 knockout mice expressing only keratin 18 to produce MBs25 does not rest on the type of keratin protein per se but on other factors, such as decreased stability of keratin 18 in vivo.
Interaction of p62, Keratin 8, and Ubiquitin.
P62 binds ubiquitinated proteins through its UBA domain. We therefore determined the ubiquitination state of keratin 8 after cotransfection with his-tagged ubiquitin into CHO-K1 cells. To assess the solubility of (ubiquitinated) keratin 8, transfected CHO-K1 cells were lysed with nondenaturing and denaturing buffer, respectively, and supernatant and pellet fractions after centrifugation were evaluated by immunoblotting (Fig. 5A). Most of the keratin 8 was insoluble in nondenaturing buffer and remained in the pellet fraction (Fig. 5A, lane 3) but was soluble in denaturing buffer and then present in the supernatant (Fig. 5A, lane 2). In addition to keratin 8 with a molecular weight of approximately 55,000, higher molecular weight bands were detected (Fig. 5A, lane 2). The latter resembled ubiquitinated keratin as demonstrated by affinity precipitation of his-tagged ubiquitin from the supernatants and immunoblotting with keratin 8 antibody (Fig. 5A, lane 6). These results support previous observations by Ku and Omary31 and our group3 showing keratin 8 ubiquitination in cultured cells and in MBs, respectively.
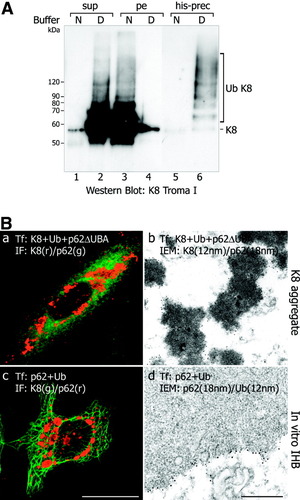
(A) Western blot analysis of ubiquitinated keratin 8. Lysis of transfected CHO-K1 cells with denaturing buffer (D) releases keratin 8 (K8; molecular weight approximately 55,000) and higher molecular weight keratins resembling ubiquitinated species (Ub K8) into the supernatant (sup) as revealed by immunoblotting with keratin 8 antibodies (K8 Troma I; compare lanes 2 and 4), in contrast to lysis with nondenaturing (N, “native”) buffer where keratins largely remain in the pellet (pe; compare lanes 1 and 3). After affinity precipitation of the his-tag (his-prec), higher molecular weight supernatant proteins are identified as ubiquitinated keratin 8 (Ub K8) by immunoblotting with keratin 8 antibodies (lane 6; note lack of ubiquitinated keratins in the supernatant of cells lysed with nondenaturing buffer, lane 5). (B) Cotransfection of keratin 8, ubiquitin, and p62ΔUBA (K8 + Ub + p62ΔUBA) results in irregular aggregates in CHO-K1 cells that are positive for keratin (a, red) and diffuse p62 immunostaining (a, green). Immunoelectron microscopy discloses the keratin nature of the granular-amorphous aggregates (b; keratin 8 antibodies: 12 nm; p62: 18-nm gold particles). c. Cotransfection of p62 and ubiquitin (p62 + Ub) into HeLa cells with intact keratin cytoskeleton leads to globular aggregates (red in c) without association with the intrinsic keratin cytoskeleton (green in c). Electron microscopy shows the IHB-like ultrastructure (see also Fig. 3B) and decoration by p62 (18-nm gold particles) and ubiquitin (12-nm gold particles) antibodies (d). Bar (a, c), 10 μm; bar (b, d), 500 nm.
To further investigate the interaction of ubiquitinated keratin 8 with p62 and the role of the UBA-domain of p62, we cotransfected keratin 8, ubiquitin, and p62ΔUBA. This experiment revealed that p62ΔUBA abrogated type 2 MB formation. In the transfected cells, keratin 8 aggregates without p62 were formed, whereas p62ΔUBA remained dispersed in the cytoplasm. The aggregates consisted of electron-dense granular-amorphous material (Fig. 5Ba, Bb) identical to the granular-amorphous keratin aggregates formed when keratin 8 was transfected alone or together with ubiquitin. This shows that the UBA domain of p62 acting as the binding site for ubiquitinated keratin 8 is essential for MB formation.
Because in diseased liver MBs and IHBs occur in hepatocytes containing a keratin intermediate filament network, we cotransfected p62 and ubiquitin into HeLa and TIB73 cells. Immunofluorescence and immunoelectron microscopy showed that under these conditions IHB-like aggregates, but not MBs, arose (Fig. 5Bc, Bd). Transfected ubiquitin colocalized with p62 while the keratin network remained unaffected (Fig. 5Bc, Bd). Thus, MBs are not formed when keratin 8 is properly assembled with its partner keratin 18 as intermediate filament.
Transfection Efficiencies and Morphology of the Resulting Inclusions in Relation to Protein Expression.
Our transfection methodology using CHO-K1 cells resulted in transfection efficiencies as shown in Table 2. The lower transfection efficiency of keratin 8 may be attributable to the well-known instability of individual keratin proteins not assembled as intermediate filaments.32, 33 The size of aggregates varied in individual transfected cells, probably depending on cDNA uptake, expression time, and protein stability. Western blotting (Fig. 6) and electron microscopy (Fig. 7A) showed a correlation between protein expression and frequency and types of resulting aggregates (Fig. 7B): (1) Transfection of p62 alone resulted exclusively in the formation of p62 aggregates consisting of filaments in parallel arrays. Endogeneous p62 (and ubiquitin), probably because of its low concentration (Fig. 6, lane 1), did not interfere with aggregate formation in transfected CHO-K1 cells. (2) Transfection of p62 and ubiquitin resulted in aggregates consisting of less distinct filaments and beads resembling IHBs. (3) Transfection of keratin 8 (or keratin 18) with or without ubiquitin produced exclusively granular-amorphous keratin aggregates. (4) After triple transfection (keratin 8 or 18/ubiquitin/p62), different types of inclusions were observed in different proportions: classical type 2 MBs consisting of randomly oriented filaments prevailed, but, in addition, aggregates with the ultrastructure of IHBs, p62 aggregates, granular-amorphous keratin aggregates and combinations with IHBs, and p62 aggregates were also observed. Low p62 expression improved (type 2) MB formation, indicating that excess of p62 because of its high affinity to ubiquitin and competition with keratin 8 for ubiquitin counteracts type 2 MB formation. Conversely, low keratin 8 expression shifted the balance to IHBs and p62 aggregates. Thus, the in vitro situation closely resembled the coexistence of type 2 and type 3 MBs with IHBs in diseased liver as reported previously.4
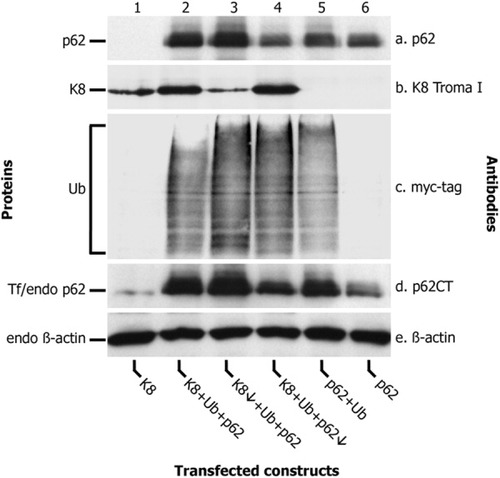
Western blot analysis of total cell lysates of transfected CHO-K1 cells using antibodies to p62 to detect transfected p62 (a), keratin 8 (b), myc tag for detection of transfected ubiquitin (c), p62CT to detect endogenous and transfected p62 (d), and β-actin to detect endogenous β-actin (e). Lanes 1 through 6 show the effects of the different transfection regimens on protein expression. Transfection of decreased amounts of keratin 8 cDNA (K8↓ + Ub + p62; lane 3) and of p62 cDNA (K8 + Ub + p62↓; lane 4) resulted in a clearcut lower expression of the respective protein. Note the presence of only small amounts of endogenous p62 (lane 1) in cells not transfected with the respective construct.
Discussion
Our in vitro studies demonstrate that MBs and IHBs can be induced in tissue culture cells by transfection with keratin 8 or 18/p62/ubiquitin and p62/ubiquitin, respectively. MBs and IHBs associated with diseased liver differ with respect to chemical composition, light microscopy, and ultrastructure.1, 2, 4, 34, 35 In IHBs, the indistinct fibrillar and granular ultrastructure prevails.2 The ultrastructure of MBs is variable.1, 4, 34, 35 Yokoo et al.34 distinguished 3 types: (1) type 1 is rare and consists of bundles of filaments with a mean diameter of approximately 14 nm arranged in parallel arrays, closely resembling amyloid fibrils; (2) type 2, which is the most common and classical type, displays closely packed, randomly oriented filaments with a mean diameter of 15 nm and fuzzy surface coat; (3) type 3 shows increased electron density and loss of fibrillarity, leading to granular-amorphous appearance.
We show in the current study that the ultrastructure of the aggregates resulting from transfections depends on their chemical composition (Fig. 8): classical type 2 MBs arise when keratin 8 (or keratin 18), ubiquitin, and p62 are cotransfected and overexpressed. Transfection of keratin 8 (or keratin 18) alone or in combination with ubiquitin leads to keratin aggregates resembling type 3 MBs. IHBs result from overexpression of p62 together with ubiquitin, whereas aggregates of pure p62 resemble the amyloid-like type 1 MBs. Cotransfection of p62 together with ubiquitin prevented the formation of amyloid-like filaments, suggesting that in this situation ubiquitin interacted with p62 by noncovalent binding to its ubiquitin binding site, thus hindering its ordered filamentous aggregation. Seibenhener et al.21 demonstrated the importance of the UBA domain of p62 for formation of p62 aggregates. Our results extend these observations by proving that the UBA domain of p62 is essential for the development of MBs and IHBs. Thus, p62 not only has the capacity of self-aggregation37 but also promotes aggregation of ubiquitinated proteins through interaction with ubiquitin.38
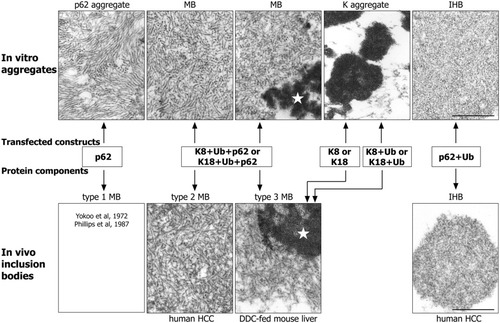
Comparison of the ultrastructure of MBs types 1 through 3 and of IHB associated with human and experimental liver disease (hepatocellular carcinoma, HCC; DDC-fed mouse liver; lower panel) with that of aggregates produced by transfection in CHO-K1 cells (upper panel). Transfection of keratin 8, p62, and ubiquitin (K8 + p62 + Ub) results in aggregates resembling type 2 MBs, which may contain areas with granular-amorphous ultrastructure resembling type 3 MBs (asterisks). Cotransfection of p62 and ubiquitin (p62 + Ub) leads to aggregates identical to IHBs present in HCC. Transfection of keratin 8 (but also of keratin 18) with (K8 + Ub or K18 + Ub) or without (K8 or K18) ubiquitin exclusively leads to granular-amorphous keratin aggregates (K aggregate), which ultrastructurally resemble type 3 MBs. Transfection of p62 results in aggregates consisting of filaments in regular parallel arrays, which resemble type 1 MBs as published by Yokoo et al.34 and Phillips et al.35 They are not shown in this figure because we could not find type 1 MBs in our biopsy or experimental material. Bar, 250 nm.
The following prerequisites are required for MB formation in vivo as well as in vitro1, 25, 26: (1) imbalance of the keratin 8 to keratin 18 ratio; (2) ubiquitination of keratin; (3) p62 with intact UBA domain for binding ubiquitinated keratin; and (4) overexpression of these components. The importance of p62 for MB formation is also emphasized by the studies of Nan et al.,36 using primary cultures of hepatocytes from drug-primed mice. These authors found that blocking p62 expression inhibited, whereas p62 overexpression increased, MB formation.
Keratin 18 in combination with p62 and ubiquitin is also able to form classical type 2 MBs in vitro. This agrees with the observation that transfected keratin 18 is even more prone to aggregation than keratin 8.39 Thus, differences exist between the in vivo and the in vitro situation because MB development is inhibited in 3,4-diethoxycarbonyl-1,4-dihydrocollidine (DDC)-intoxicated keratin 8 knockout mice expressing only keratin 18. It could be related to rapid degradation of keratin 18 under these conditions.25, 31
Protein aggregation is a complex process that involves several intermediate stages (i.e., soluble oligomers, prefibrillar and profibrillar aggregates), finally resulting in filamentous or amorphous aggregates.12, 13, 15, 16, 40, 41 The earliest aggregates are relatively disorganized structures, whereas aggregates resembling amyloid fibrils are highly organized and stable. With respect to the biological consequences of the aggregation process, filamentous aggregates seem to behave relatively benignly, whereas oligomers and profibrillar aggregates can be damaging because the exposure of hydrophobic residues may lead to aberrant interaction with membranes or other cellular components. Based on ultrastructural appearance, IHB and MB development in human liver resembles the fate of unfolded or intermediately folded proteins entering the so-called off-folding pathway of protein aggregation with 2 possible routes and endpoints, namely, amorphous aggregation and fibril formation.12, 13, 40, 41 Aggregation of keratin resembles the amorphous route; that of p62, the amyloid route. The formation of regular amyloid-type filaments by p62 is abrogated by the presence of additional components, such as keratin and ubiquitin, in support of the fact that mixed or composite amyloids do not exist.40 The ultrastructure of MBs1, 34, 35 suggests that both routes of the off-folding pathway may occur simultaneously in vivo.
The question arises as to why MB formation is not more ubiquitous in liver diseases. Human as well as experimental liver diseases associated with MBs have in common their chronic nature resulting from long-term intoxication or metabolic disturbance (e.g., metabolic syndrome) and their association with oxidative stress.1, 9, 33 As revealed by numerous experimental studies using the DDC-MB model and keratin 8 or keratin 18 knockout or overexpressing mice, overexpression and increase in the keratin 8 to keratin 18 ratio are key factors for MB formation, but additional stimuli are required to ultimately trigger MB formation.1, 25, 26, 27 The imbalance in the keratin 8 to keratin 18 ratio may be the result of transcriptional or posttranscriptional events or differences in keratin protein turnover (e.g., inhibitory effect of keratin 8 hyperphosphorylation on keratin degradation; increased keratin 8 stability due to transglutaminase action26, 42). Accordingly, one can postulate that MBs are more likely to arise in diseases that result in a keratin 8 > keratin 18 protein imbalance with accumulation of keratin 8. Moreover, the final formation of inclusion bodies is a complex process, being the result of evasion of cellular rescue mechanisms against abnormal (unfolded or misfolded) and potentially toxic proteins, and requires additional factors, such as impairment of chaperone-dependent processes for refolding and of protein degradation systems.1, 13, 18, 20, 26, 27 The role of additional stimuli for MB formation is clearly demonstrated by the results obtained with keratin 18 knockout mice: The “widowed” keratin 8 expressed by these animals is normally rapidly degraded and does not accumulate as inclusions. However, these mice respond much more rapidly than wild-type animals to DDC intoxication by MB formation (Stumptner et al., unpublished observation) and spontaneously develop MB at an older age.43
Studies on MB formation in vivo and in vitro may also be informative in the context of other human diseases associated with inclusion bodies. Ubiquitin-containing and p62-containing cellular protein aggregates are characteristic morphological features of a wide range of chronic degenerative diseases, including Alzheimer disease, Parkinson disease, amyotrophic lateral sclerosis, and Huntington disease,3, 28 and protein misfolding seems to play an essential role in these disorders. Misfolded proteins may result from mutation or as a consequence of conditions, such as oxidative stress, that favor protein unfolding.13, 14, 15, 18, 20 Oxidative stress is also a classical inducer of p626, 19, 24 and therefore seems to be the central player in these disease processes. Moreover, keratin mutations prime hepatocytes to oxidative injury and may thus predispose patients to liver cirrhosis.44




