Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome†
Potential conflict of interest: Nothing to report.
Abstract
Mortality in patients with alcoholic hepatitis (AH) remains high, and although corticosteroids are widely used for treatment, the results vary considerably. In AH, neutrophils are primed and infiltrate the liver to produce injury, but paradoxically, the main cause of death in such patients is infection. Our prospective study addressed this paradox of primed neutrophils on the one hand and increased risk of infection on the other. We hypothesized that the full activation of neutrophils by a humoral factor such as endotoxin renders them unable to respond to further bacterial challenge. We analyzed neutrophil oxidative burst and phagocytosis in whole blood by fluorescence-activated cell sorting analysis in 63 alcoholic patients with cirrhosis and patients with cirrhosis with superimposed AH (cirrhosis+AH). In 16 patients, ex vivo studies determined whether the removal of endotoxin restored neutrophil function. A resting burst greater than or equal to 5%, indicating neutrophil activation and a reduced phagocytic capacity lower than 42%, was associated with significantly greater risk of infection, organ failure, and mortality. This defective neutrophil function was transmissible through patients' plasma to normal neutrophils, and patients' neutrophil function could be restored by normal plasma. The ex vivo removal of endotoxin from patients' plasma decreased the resting burst and increased the phagocytic function. Conclusions: Our study provides the rationale for a goal-directed approach to the management of patients with cirrhosis and AH, in which the assessment of neutrophil function may be an important biomarker to select patients for immunosuppressive therapy. The neutrophil dysfunction in cirrhosis and AH is reversible, with endotoxin-removal strategies providing new targets for intervention. (HEPATOLOGY 2007.)
In patients with severe alcoholic hepatitis (AH), infection complicates the course of illness and is associated with significant morbidity and mortality.1-4 Neutrophils are an essential component of the innate immune response and key players in the pathogenesis of AH.5 Data on neutrophil function in AH are paradoxical, with some studies suggesting neutrophil priming, indicating a readiness to respond to bacterial challenge (for a review, see Condliffe et al.6), as measured by hydrogen peroxide overproduction, decreased L-selectin expression,7 and high levels of neutrophil elastase.8 In contrast, other studies show decreased neutrophil phagocytic capacity correlating with disease severity.9 Endotoxin is known to be elevated in patients with AH10 and has the ability to both prime6, 11 and activate neutrophils.12, 13
The clarification of this controversy has important clinical implications. Current strategies for treating AH include the administration of immunosuppressive agents such as corticosteroids and, more recently, anti–tumor necrosis factor (TNF)α strategies, which may further potentiate susceptibility to infection. The results of studies using these drugs in patients with AH are conflicting,14, 15 and although there are some data showing benefits from anti-TNFα strategies,16, 17 one trial showed increased risk of infection and mortality, which resulted in early trial termination.18
To reconcile the apparent paradox, we assessed whether neutrophils exist in a primed or fully activated state in patients with different alcoholic liver diseases [alcoholic cirrhosis and AH superimposed on cirrhosis (cirrhosis+AH)] and studied the association of this with the development of infection and clinical outcome. We hypothesized that full activation is associated with impaired neutrophil responses to ongoing bacterial challenge rendering patients more susceptible to infection. Alternatively, if neutrophils are primed but not activated, immunosuppressive therapy may be advantageous. Thus, our prospective study systematically examined neutrophil oxidative burst and phagocytosis in patients with cirrhosis+AH to evaluate whether altered neutrophil function was associated with infection, organ failure, and survival. In ex vivo studies, we investigated whether the defect in neutrophil function was due to a humoral factor and whether endotoxin removal from plasma would restore a patient's neutrophil function.
Abbreviations
AH, alcoholic hepatitis; AUROC, area under the receiver operator curve; CRP, c-reactive protein; CV, coefficient of variation; FACS, fluorescence-activated cell sorting; fMLP, N-formylmethionyl-leucyl-phenylalanine; GMFI, geometric mean of fluorescence intensity; IL, interleukin; LPS, lipopolysaccharide; MDA, malondialdehyde; MELD, model for end-stage liver disease; TNF, tumor necrosis factor.
Patients and Methods
Patient Selection
All patients gave written informed consent, and the study was approved by the local ethics committee. Patients admitted with evidence of alcoholic cirrhosis19 were enrolled into the study at the time of a liver biopsy, which was performed to evaluate the presence or absence of AH. The included patients had been admitted with acute decompensation of alcoholic cirrhosis manifested by increasing jaundice, with no clinical or microbiological evidence (chest radiographs or routine cultures of urine, blood, sputum, and ascites) of infection. Prophylactic antibiotics (cefotaxime) were prescribed following initial cultures if there was a suspicion of infection, and they were stopped if subsequent cultures proved negative. Patients were excluded if they were less than 18 or greater than 75 years and had evidence of organ failure (inotrope requirement, creatinine > 150 μmol/L, hepatic encephalopathy > grade 2, requirement for mechanical ventilation), hyponatremia, and hepatic/extrahepatic malignancy, less than 3 days post gastrointestinal bleeding or if they received any immunomodulatory therapy prior to entry in the study. Blood from 20 age-matched and sex-matched healthy volunteers with no history of liver disease was studied to serve as controls for the comparison of neutrophil function.
Patients were classified histologically into those with cirrhosis and superimposed inflammatory AH (cirrhosis+AH) with a histological grading system similar to that of nonalcoholic steatohepatitis,20 taking into account steatosis, neutrophil infiltration, the ballooning of hepatocytes, and the formation of Mallory's hyaline, and compared with cirrhosis alone.
Study Design
Following the correction of any electrolyte disturbances or hypovolemia, peripheral venous blood was aseptically collected into pyrogen-free tubes (BD Vacutainer lithium-heparin, 60 U per tube, BD, Plymouth, UK) and used for routine biochemistry, neutrophil function, cytokine profile, and detection of markers of oxidative stress. For experiments with cells, blood was kept at room temperature (maximum 1 hour); for harvesting plasma, blood was placed immediately on ice. After centrifugation, plasma was aliquoted under pyrogen-free conditions into nonpyrogenic cryotubes (Corning Inc., Corning, NY) and stored at −80°C until further analysis. Whole blood or isolated neutrophils mixed with plasma were used to perform the Phagoburst or Phagotest (Orpegen Pharma GmbH, Heidelberg, Germany). For all experiments, we took strict precautions to avoid endotoxin contamination by working aseptically and using endotoxin-free equipment. Bilirubin, albumin, liver function tests, coagulation parameters, full blood count, and c-reactive protein (CRP) were routinely performed. Maddrey's discriminant function,21 the model for end-stage liver disease (MELD),22 and the Pugh score23 were calculated. The patients were followed prospectively over a period of 90 days. The occurrence of organ dysfunction and mortality was recorded.
Neutrophils
Neutrophils were investigated either in a whole blood assay (as described below) or after isolation by a 1-step gradient centrifugation.
Neutrophil Isolation.
Whole blood (4 mL) was layered over 5 mL of Polymorphprep (Axis-Shield, Oslo, Norway) and spun for 30 minutes at 400g at room temperature. Neutrophils were harvested from the second interface and washed with phosphate-buffered saline (Sigma Aldrich, St. Louis, MO). Neutrophils were counted in a Thoma hemocytometer and resuspended in phosphate-buffered saline at a density of 5 × 105 in 50 μL. Fifty microliters of the cell suspension and 50 μL of plasma were used per assay. The viability was tested by Trypan Blue exclusion and was over 98%.
Neutrophil Activation (Oxidative Burst).
The Phagoburst kit (Orpegen Pharma, Heidelberg, Germany) was used to determine the percentage of neutrophils that produced reactive oxidants with or without stimulation according to the manufacturer's instructions. In brief, 100 μL of heparinized whole blood or 50 μL of isolated neutrophils and 50 μL of plasma (as indicated) were incubated for 20 minutes with 20 μL of opsonized Escherichia coli (2 × 107 bacteria), N-formylmethionyl-leucyl-phenylalanine (fMLP; 5μM), and phorbol-12-myristate-13-acetate (8.1 μM) or without a stimulus at 37°C. The formation of reactive oxidants was monitored by the oxidation of dihydrorhodamine 123 to rhodamine, which produced green fluorescence. To identify neutrophils, cells were stained with anti–CD16-PE antibody (Immuntools, Friesoythe, Germany) and analyzed by fluorescence-activated cell sorting (FACS; Becton Dickinson FACScan, San Jose, CA) with Cellquest software. Neutrophils were gated on forward and side scatter characteristics (Fig. 1A), and subsequently, the percentage of CD16-positive cells producing reactive oxygen metabolites (green fluorescence) was calculated (Fig. 1C,E,G). The samples were analyzed in duplicate or triplicate. The interassay coefficient of variation (CV) for the resting burst was 5.4%, and for the stimulated burst, it was 4.2%. The intra-assay CV for the resting burst was 4.7%, and it was 2.4% for the stimulated burst.
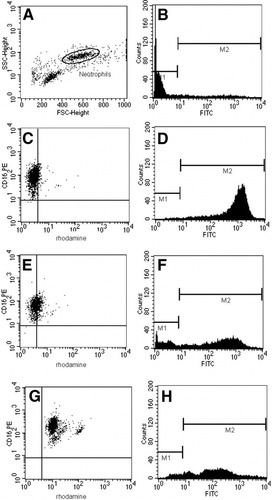
Representative FACS analysis plots for Phagotest and Bursttest are shown. (A) Neutrophils are gated according to their forward and side scatter characteristics. (B) Analysis of phagocytosis: on a sample without bacteria, markers are set so that more than 99% of the gated neutrophils are within the first maker. (C) Representative FACS plot from a healthy control subject (resting oxidative burst = 8.3%). The percentage of double positive cells (shown in the right upper quadrant) is measured. (D) Corresponding FACS plot of a healthy control with a normal phagocytic capacity (100%). (E) Representative FACS plot from a patient with a low resting oxidative burst (39.2%). (F) Corresponding FACS plot of a patient with a relative GMFI of 50%. (G) Representative FACS plot from a patient with a high resting oxidative burst (99.9%). (H) Corresponding FACS plot of the same patient in part C with a relative GMFI of 17%.
Neutrophil Phagocytic Capacity.
The Phagotest (Orpegen Pharma) was used to measure phagocytosis with fluorescein isothiocyanate–labeled opsonized E. coli bacteria. Whole blood (100 μL) or 50 μL of isolated neutrophils and 50 μL of plasma (as indicated) were incubated with 20 μL of bacteria (2 × 107) at 37°C for 20 minutes, whereas a negative control sample remained on ice. Neutrophils were identified and gated as described previously, and subsequently, the geometric mean of fluorescence intensity (GMFI), corresponding to the number of bacteria engulfed by a single cell, was analyzed (Fig. 1B,D,F,H). To avoid misinterpretation of the results due to batch-to-batch variability of the bacteria, the results were normalized to the mean of at least 3 healthy control samples for each new batch of bacteria used. The samples were analyzed in duplicate or triplicate. The interassay CV was 10.1%, and the intra-assay CV was 1.6%.
Incubation with Endotoxin
Endotoxin (E. coli 0111:B4 lot 085K4068, Sigma, Poole, United Kingdom) was prepared as a stock solution of 1 mg/mL and diluted with phosphate-buffered saline (Sigma) to required concentrations at the time of experimentation. Whole blood was incubated for 1 hour with the respective endotoxin concentration in a water bath at 37°C before the Phagotest or Bursttest was performed.
Endotoxin Removal from Patient Plasma
Using Polymixin B.
Detoxi-Gel affinity-pack prepacked columns (Pierce Biotechnology, Rockford, IL) containing immobilized polymixin B, which binds the lipid A portion of bacterial lipopolysaccharide (LPS), were used to remove endotoxin from plasma samples (diluted 1:1 with phosphate-buffered saline). The endotoxin-free, diluted plasma (100 μL) was incubated with 50 μL of a cell suspension, and the Bursttest or Phagotest was applied.
Using Anti-CD14 Antibody.
Plasma (50 μL) and 50 μL of isolated neutrophils were incubated for 60 minutes with 5 μL of an anti–human anti-CD14 antibody (Clone 11D18, Immuntools, Friesoythe, Germany) known to neutralize LPS before the Phagotest or Bursttest was performed.
Cytokines
TNFα, interleukin-6 (IL-6), and interleukin-8 (IL-8) were determined by an enzyme-linked immunosorbent assay from ethylenediaminetetraacetate anticoagulated plasma samples with commercially available antibody sets (BioSource International, Nivelles, Belgium) in accordance with the manufacturer's instructions as described.17 The lower limit for the detection of the cytokines was 3 pg/mL. The intra-assay CV was 5.4%–6.4%. IL-6 and IL-8 were undetectable in the controls.
Malondialdehyde (MDA) and Prostaglandin F2α
MDA was determined with a modified thiobarbituric acid–reactive substance assay described by Lapenna et al.24 in which the major interfering/oxidizable component in the plasma is inhibited by the addition of sodium sulfate.
Free 8-isoprostane F2α was assayed with a commercial enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions.
Statistics
For a comparison of 2 groups, a chi-square test, t test, or Mann-Whitney test was used (whatever was appropriate), whereas an analysis of variance test with Tukey's (equal variances) or Dunnett C (nonequal variances) post hoc analysis was used for the comparison of more than 2 data sets. To assess the diagnostic accuracy, the areas under the receiver operator curves (AUROCs) were calculated. Differences in survival were analyzed by the log-rank test. Pearson's correlation coefficient was used to assess relationships between variables. The results are presented as means ± SEM, with P < 0.05 considered significant.
Results
Patient Characteristics
Sixty-three patients, all of whom fulfilled the study criteria, were enrolled. Patients classified histologically as having cirrhosis+AH (n = 23, all with moderate to severe steatohepatitis) were more severely ill, as evidenced by higher MELD and Pugh scores (P < 0.001) in comparison with patients with only cirrhosis (n = 40). Patients with cirrhosis+AH also had significantly higher CRP (P < 0.005) and white cell counts (P < 0.001). All patients had higher levels of TNFα, IL-6, IL-8, MDA, and isoprostane F2α than healthy controls. As anticipated, patients with cirrhosis+AH had significantly higher levels of IL-6 and IL-8 than those with cirrhosis alone, but the measures of oxidative stress did not differ significantly between these patient subsets, nor did they correlate with liver disease severity. Furthermore, there was no correlation between levels of cytokines and markers of oxidative stress and neutrophil function. Table 1 outlines baseline characteristics for all patients and distinguishes between the groups of cirrhosis+AH and cirrhosis alone. For ex vivo experiments, whole blood, isolated neutrophils, or plasma from 16 of the 63 patients was used (6 had cirrhosis+AH and 10 had cirrhosis alone), and the baseline clinical characteristics for these 16 patients were not significantly different from those of the whole cohort.
| Normal Range | All Patients (n = 63) | Cirrhosis (n = 40) | Cirrhosis+AH (n = 23) | Patients Selected for Ex Vivo Studies (n = 16) | |
|---|---|---|---|---|---|
| Age (years) | 50.3 ± 1.3 | 52.2 ± 1.7 | 47.1 ± 1.9 | 51.8 ± 2.7 | |
| Biochemistry/liver function | |||||
| Bilirubin (μmol/L) | 3–17 | 151.2 ± 20.9 | 94.0 ± 17.4 | 249.2 ± 39.9* | 145.8 ± 47.7 |
| Prothrombin time (seconds) | 10–12 | 15.3 ± 0.6 | 13.6 ± 0.5 | 17.2 ± 1.0* | 13.3 ± 0.6 |
| Albumin (g/L) | 35–53 | 29.8 ± 1.1 | 32.7 ± 1.2 | 27.6 ± 1.4* | 33.9 ± 1.3 |
| White blood cell count (109/L) | 3–10 | 10.1 ± 0.9 | 7.9 ± 0.9 | 14.1 ± 1.5* | 7.9 ± 1.2 |
| CRP (mg/L) | 0–5 | 30.9 ± 4.5 | 21.4 ± 5.2 | 45.7 ± 7.0* | 22.6 ± 7.1 |
| ALT (U/L) | 8–63 | 62.8 ± 10.8 | 73.9 ± 15.8 | 40.8 ± 5.7 | 82.7 ± 34.7 |
| Maddrey's discriminant function | — | — | 43.3 ± 6.2 | — | |
| Pugh score | 9.3 ± 0.4 | 8.0 ± 0.3 | 11.1 ± 0.4* | 8.7 ± 0.6 | |
| MELD | 15.6 ± 1.8 | 10.7 ± 1.1 | 23.5 ± 4.1* | 15.1 ± 2.8 | |
| Cytokine/inflammation | |||||
| TNFα (pg/mL) | 0–5 | 19.6 ± 6.5† | 18.3 ± 6.9† | 22.3 ± 14.6† | 39.8 ± 14.6 |
| IL-6 (pg/mL) | 0–5 | 49.4 ± 14.9† | 21.9 ± 7.9† | 106.1 ± 39.6*† | 43.2 ± 17 |
| IL-8 (pg/mL) | 0–5 | 180.5 ± 56.9† | 101.8 ± 55.8† | 337.9 ± 122.8*† | 39.7 ± 10.1 |
| Oxidative stress | |||||
| MDA (μmol/L) | 1.2–2.5 | 3.2 ± 0.5† | 2.7 ± 0.5† | 4.3 ± 0.8† | 2.3 ± 0.5 |
| Isoprostane F2α (pg/mL) | 40–100 | 346.8 ± 49.6† | 296.9 ± 43.8† | 394.7 ± 81.2† | 343.9 ± 46.3 |
| Outcome | |||||
| Death n (%) | 14 (22) | 3 (8) | 11 (48) | 3 (19) | |
| Organ failure n (%) | 17 (27) | 3 (8) | 14 (61) | 4 (25) | |
- * P < 0.001 versus cirrhosis.
- † P < 0.05 versus control.
Oxidative Burst and Phagocytosis in Patients with Alcoholic Liver Disease
Patients with cirrhosis+AH had a significantly higher resting oxidative burst than patients with cirrhosis alone (P < 0.001) or controls (P < 0.001, Fig. 2). Patients with cirrhosis alone also had a higher resting oxidative burst than controls (P < 0.01, Fig. 2). Stimulation with fMLP, a synthetic peptide that triggers minimal oxidative burst in unstimulated neutrophils but a significantly higher response in primed neutrophils, resulted in a much greater oxidative burst in patients with cirrhosis (P = 0.01) and cirrhosis+AH (P = 0.001) in comparison with controls, suggesting prior priming of these patients' neutrophils. There was no difference in response to E. coli or phorbol-12-myristate-13-acetate as a high stimulus between the patient subgroups. There was no difference between the resting burst and response to fMLP in patients with cirrhosis+AH anymore, whereas in patients with cirrhosis alone, the fMLP response was 22% higher than the resting burst (P < 0.05). Furthermore, upon stimulation with E. coli, the relative increase in the oxidative burst from resting levels was significantly reduced in patients with cirrhosis+AH in comparison with patients with cirrhosis alone (P = 0.001) or controls (P < 0.001, Fig. 2). Furthermore, patients with cirrhosis+AH engulfed significantly less bacteria than controls (P < 0.05, Fig. 2).
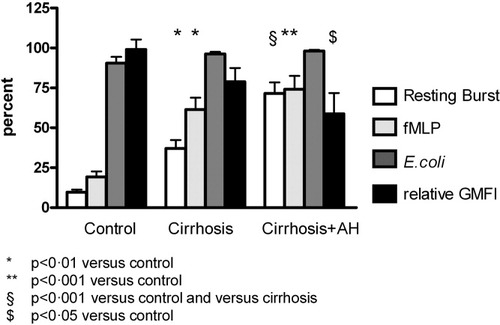
The column bars represent the percentage of cells undergoing a resting oxidative burst in untreated neutrophils (white) and after stimulation with fMLP (light gray) or E. coli (dark gray) in samples from controls (n = 13), patients with cirrhosis (n = 40), and patients with cirrhosis+AH (n = 23). The last column bar (black) depicts the phagocytic capacity (relative GMFI) in the study groups.
Association of Resting Oxidative Burst and Phagocytosis with Organ Failure and Survival
Seventeen patients (27%) developed organ failure, and 14 (22%) died within 90 days (30-day mortality 13%). Renal failure occurred in 15 (88%) and was associated with ventilatory and circulatory failure in 4. The resting oxidative burst was found to be predictive of 90-day mortality (AUROC 0.77, P < 0.005, Fig. 3A,B) and organ failure (AUROC 0.76, P < 0.001). A resting burst of greater than 55% had a sensitivity of 77% and a specificity of 69% for predicting death. The phagocytic function was also predictive of death (AUROC 0.80, P < 0.05, Fig. 3C,D) and organ failure (AUROC 0.91, P < 0.0001). A phagocytic capacity less than 42% of normal among the studied patients had a sensitivity of 86% and a specificity of 70% to predict mortality.
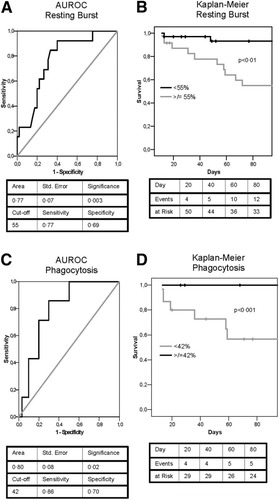
(A) AUROC shows the predictive utility of the measurement of the oxidative burst in determining survival. (B) A resting burst of greater than 55% had the best sensitivity and specificity on AUROC analysis to group the patients and was used to construct a Kaplan-Meier survival curve; the log-rank analysis shows a significantly higher mortality (P < 0.01) in the group of patients with a high burst (≥55%). (C) AUROC analysis also reveals that the phagocytic capacity is predictive in determining survival. (D) A phagocytic capacity of less than 42% had the best sensitivity and specificity on AUROC analysis and was used to construct a Kaplan-Meier survival curve; the log-rank analysis shows a significantly higher mortality (P < 0.001) in the group of patients with decreased phagocytic capacity (<42%).
Association of the Resting Oxidative Burst and Phagocytosis with the Development of Infection
Although none of the included patients had a proven infection at the time of assay for neutrophil function, in 38 (60%) patients, infection was clinically suspected during the course of the hospital admission. In 19 of 38 patients (50%), a culture-positive infection was verified within 30 days of admission, albeit our management protocol necessitates the use of broad-spectrum antibiotics as soon as an infection is suspected. In 8, more than 1 organism was found. The following species were cultured: Enterococcus (n = 9), coagulase-negative Staphylococcus (n = 8), Candida sp. (n = 3), E. coli (n = 2), methicillin-resistant Staphylococcus aureus (n = 2), S. aureus (n = 2), and Propionibacterium sp. (n = 1). Patients with cirrhosis+AH were more likely to develop culture-positive infections (57% versus 15%, P = 0.001). Furthermore, when patients were stratified for resting burst, those with a high resting burst (≥55%) were more likely to develop culture-positive infections than patients with a low resting burst (48% versus 15%, P < 0.005, Fig. 4A). Moreover, only patients with a high burst developed infections with more than 1 organism, and they also developed these infections earlier during their hospital stay in comparison with patients with a low burst (7 versus 16 days, P = 0.005). Additionally, 53% of the patients with a phagocytic capacity lower than 42% developed a culture-positive infection versus 13% of the patients with a phagocytic capacity greater than or equal to 42% (P < 0.05). Those patients who developed culture-positive infections were more likely to develop organ failure (P = 0.001) and to die (P < 0.0001, Fig. 4B).
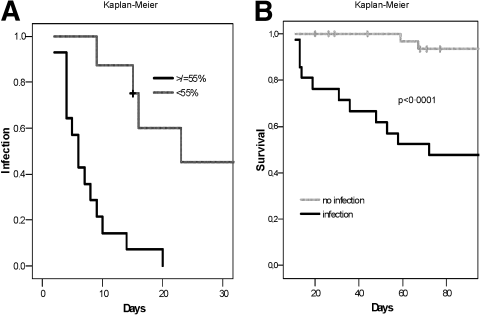
(A) An analysis of the time to infection (Kaplan-Meier and log-rank) shows that patients with a high resting burst (≥55%) have a significantly higher risk (P < 0.005) of developing culture-positive infections within 30 days. (B) According to Kaplan-Meier and log-rank analyses used to determine differences in survival between patients with and without culture-positive infections, those with infections have a significantly higher 90-day mortality (P < 0.001).
Effect of Patients' Plasma and Normal Plasma on the Neutrophil Oxidative Burst
Plasma from patients with a high resting burst (≥55%; n = 6) induced a high resting burst in normal neutrophils (P = 0.005), whereas plasma from patients with a low resting burst (<55%; n = 6) failed to do so (Fig. 5A). This result suggests the presence of a transmissible factor in patients' plasma promoting neutrophil activation.
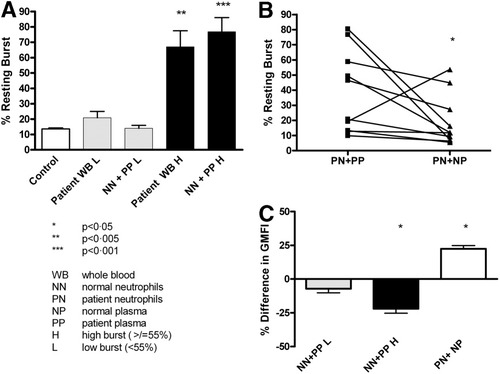
(A) The bars show the resting oxidative burst in the whole blood of controls, in the whole blood of patients, and in normal neutrophils incubated with patients' plasma. Plasma from patients with a high resting burst induced a high burst in normal neutrophils, whereas plasma from patients with a low resting burst failed to do so. (B) The elevated resting oxidative burst in patients' neutrophils incubated with their own plasma is reversed after incubation with normal plasma. (C) The incubation of normal neutrophils with plasma from patients with a low resting burst does not change phagocytosis; plasma from patients with a high burst decreases phagocytosis. The incubation of patients' neutrophils with normal plasma restores phagocytic function.
When isolated neutrophils from patients with a known high resting burst were incubated with normal plasma, the resting burst decreased significantly in comparison with isolated neutrophils incubated with the patients' own plasma (P = 0.02; Fig. 5B). This may suggest the removal of a factor present in patients' plasma by the transfer of their neutrophils into a more healthy environment.
Effect of Patients' Plasma and Normal Plasma on Phagocytosis
Neutrophils from healthy controls incubated with plasma from patients with a low resting burst did not differ from those of controls, whereas neutrophils from healthy controls incubated with plasma from patients with a high resting burst showed a 22% decrease in the phagocytic capacity (P < 0.05, n = 6). Conversely, incubating patients' neutrophils for 60 minutes with plasma from healthy controls showed a 22% increase (P < 0.05, n = 6) in phagocytosis in comparison with patients' neutrophils incubated with their own plasma (Fig. 5C). These results concur with derangements in oxidative burst and indicate that impaired phagocytic function may be due to a transmissible and reversible serum factor.
Effect of Endotoxin on Oxidative Burst and Phagocytosis
Incubating neutrophils from healthy volunteers (n = 5) with increasing endotoxin concentrations resulted in a concentration-dependent increase in the resting burst (P < 0.0001, Fig. 6A). Furthermore, incubating patients' neutrophils with endotoxin reduced the phagocytic capacity by 20% (n = 8, P < 0.05, Fig. 6B). These results suggest that endotoxin modulates the neutrophil function in a manner comparable to that seen after exposure to patient plasma.
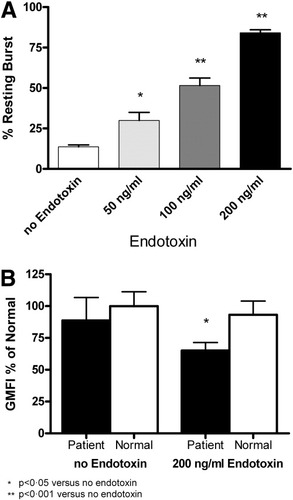
(A) Dose-dependent increase in the neutrophil resting burst following incubation with endotoxin. (B) Incubation with endotoxin does not change phagocytosis in normal neutrophils but decreases phagocytosis further in patients' neutrophils.
Effect of Removing Endotoxin from a Patient's Plasma
Using Polymixin B Columns.
When patient plasma evoking a high resting burst in the whole blood assay was passed over polymixin B columns, the resting burst was reduced by 32% (P < 0.001, n = 9). Plasma from patients with a low resting burst (n = 4) and normal plasma (n = 3) did not change the resting burst of isolated healthy donor neutrophils (Fig. 7A). Endotoxin removal from the plasma of patients with a high resting burst (n = 11) also increased the phagocytic capacity by 31% (P < 0.05) in comparison with cells incubated with untreated plasma but had no significant effect on the plasma of patients with a low burst (n = 8) or normal plasma (n = 5; Fig. 7B). This set of experiments suggests that endotoxin removal by polymixin B reverses the burst-inducing and phagocytosis-decreasing effect of patients' plasma.
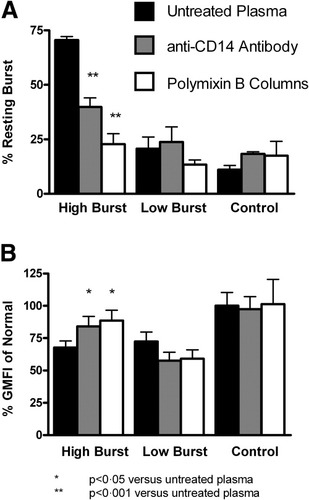
(A) A high resting oxidative burst is reversible by the passage of plasma over an endotoxin-removal column or following incubation with anti-CD14 antibodies. Plasma from patients with a low burst or control plasma passed over the column or incubated with anti-CD14 antibodies does not influence the resting burst. (B) Impaired phagocytosis is reversible by the passage of plasma from patients with a high burst over an endotoxin-removal column or incubation with anti-CD14 antibodies. Plasma from patients with a low burst or from controls passed over the column or incubated with anti-CD14 antibodies does not influence phagocytosis.
Using LPS-Neutralizing Antibodies.
Incubation with an LPS-neutralizing anti–human anti-CD14 antibody prevented the induction of increased burst in normal neutrophils by the plasma of patients with a high burst (P < 0.001, n = 7) but had no effect on neutrophils incubated with the plasma of patients with a low burst (n = 8) or healthy control plasma (n = 3, Fig. 7A). The incubation of plasma of patients with a high burst with the anti-CD14 antibody increased the phagocytic capacity by 20% (P = 0.04, n = 11), whereas this antibody had no effect on the phagocytic capacity when plasma from patients with a low burst (n = 8) or healthy controls (n = 3) was used on normal neutrophils (Fig. 7B). This further supports the concept that endotoxin may be responsible for the observed induction of high resting burst in neutrophils from patients with AH.
Discussion
The results of this study suggest a severe functional failure of neutrophils in a proportion of patients with cirrhosis+AH and that these defects are associated with increased risk of infection, organ failure, and mortality. The ex vivo studies support the notion that this neutrophil dysfunction is contributed by endotoxin and is reversible if the plasma is treated with endotoxin-removal strategies such as passing the plasma over a polymixin B column or using LPS-neutralizing anti-CD14 antibodies. Our results provide important clues to explain the apparent paradox in neutrophil function in the context of inflammation on the one hand and increased susceptibility to infection on the other.
AH is a proinflammatory condition in which TNFα is thought to promote a cascade of proinflammatory signals that perpetuate liver injury.25-27 Evidence for an enhanced proinflammatory cytokine environment was demonstrated in the cirrhosis+AH group in our study. Moreover, circulating neutrophils have been shown to be primed in AH,7, 8 resulting in enhanced responses to a microbial challenge.6 Our data confirm that neutrophils in patients with alcoholic cirrhosis and cirrhosis+AH are primed as shown by increased oxidative burst upon stimulation with fMLP in comparison with controls. The presence of primed neutrophils would suggest good bactericidal function but with the potential for promoting local damage if further activated.
Paradoxically, despite data suggesting neutrophil priming, it is also established that patients with alcoholic cirrhosis, especially those with AH, are prone to infections,28 with a higher prevalence of sepsis,29 and are more likely to die from sepsis-related complications.14 This clinical finding is supported by several studies showing defects in phagocytosis, bactericidal capacity, chemotaxis, and neutrophil locomotion.9 It is therefore important to distinguish between primed (but not activated) neutrophils and those that are fully activated. Activated neutrophils were defined in this study as neutrophils that have a high resting burst above and beyond the primed state (increased oxidative burst following fMLP stimulation). Additionally, the presentation of a bacterial challenge to activated neutrophils was associated with an inability of these cells to generate a further oxidative burst in comparison with primed cells. The neutrophils in our study population of cirrhosis+AH showed both neutrophil activation and impairment of phagocytosis. We can only speculate on the mechanism by which an increased resting oxidative burst might be related to a decreased phagocytic capacity. Because both oxidative burst and phagocytosis are energy-dependent processes, the induction of oxidative burst may lead to energy depletion and reduction in phagocytosis. This is supported by data showing that the activation of adenosine monophosphate kinases in a state of low cellular energy contributes to a reduction in host defense mechanisms.30
The clinical importance of these neutrophil abnormalities, which are identified by a high resting oxidative burst greater than or equal to 55% and a reduced phagocytic capacity (relative GMFI < 42%), are highlighted by the observation of increased risk of infection and the association with organ failure and mortality in these patients. It is notable that this group of patients in our study had infections with multiple organisms despite antibiotic therapy within a relatively short time frame. Although we attempted to exclude all patients with preexisting infections at the baseline, we cannot fully exclude the possibility of underlying subclinical infections that were not detected by current routine microbiology techniques, which might precipitate a high resting oxidative burst in some patients. It has been shown that 32% of patients with cirrhosis with culture-negative ascites have detectable bacterial DNA in the blood, which may indicate systemic bacterial seeding.31 An important observation made through ex vivo experiments in this study was the demonstration that the neutrophil functional defect could be transmitted to normal neutrophils incubated with patients' serum. We also showed that the neutrophil dysfunction in the patients was reversible following incubation with normal serum. As endotoxin is known to be elevated in patients with alcoholic liver disease, we explored whether this transmissible factor in the patients' plasma imparting the functional disturbance in neutrophils was endotoxin. Endotoxin not only is a priming agent6 but has been reported to fully activate neutrophils12, 13 by up-regulating the NADPH oxidase assembly.32 The highest levels of endotoxin reported in patients with liver disease are found in portal venous blood,33, 34 highlighting the importance of the bowel and altered gut permeability as a possible source of endotoxin.35 However, the difficulties with the measurement of endotoxin are reflected in the wide range of values in the literature and make this an unreliable indicator on which to base further management.33, 34 Our data do support the notion that endotoxin may be the putative humoral factor in patients' plasma responsible for the activation of neutrophils by showing that endotoxin increased the resting burst in healthy neutrophils while reducing phagocytosis in neutrophils from patients with alcoholic cirrhosis.
Having established a potential role for endotoxin, we determined whether different strategies to remove endotoxin from a patient's plasma ex vivo would result in improved neutrophil function. We chose to use 2 approaches, a gel containing bound polymixin B and an anti-CD14 antibody, both of which have been validated in their ability to bind endotoxin. The removal of endotoxin by both these methods resulted in a effective reduction in the high resting oxidative burst and also improved the phagocytic capacity. This novel observation, showing the potential of endotoxin removal to affect the functional status of circulating neutrophils, may have important therapeutic implications. Endotoxin removal has been tested in patients with sepsis and multiorgan failure, with improvements in clinical and biochemical parameters noted.36 However, a definitive survival benefit has not been shown to date, most likely because of the heterogeneity of the conditions studied.
Our results may also have important implications in the selection of patients for immunosuppressive therapy for AH. Although controversy exists around the use of corticosteroids and anti-TNF strategies for the routine treatment of AH, there is little doubt of their effectiveness in selected patients. Our data provide insight into the conflicting results of clinical trials and may suggest a rationale to select an appropriate therapy tailored to the patient. The use of oxidative burst measurements to categorize patients in whom the neutrophils are primed but not activated (indicated by an increased fMLP response but a resting burst lower than 55%) would identify those patients with AH that are likely to respond to anti-inflammatory strategies but have a low risk of precipitated infection. However, patients in whom the neutrophils are activated (indicated by a high resting burst greater than or equal to 55%) are likely to have a high risk of infection, and strategies to remove endotoxin may be more beneficial. This hypothesis will need to be tested in appropriately powered clinical trials.
In conclusion, our study data provide an explanation for the apparent paradox of increased inflammation and simultaneous heightened risk of infection and suggest an important role for endotoxin as the humoral factor that triggers neutrophil activation. We also show the reversibility of neutrophil dysfunction following endotoxin removal and suggest that neutrophil activation renders them hyporesponsive to further bacterial challenge, accounting for the increased rate of infection and mortality. The mechanism for phagocytic dysfunction in fully activated neutrophils requires further study, but our data provide a rationale for improved selection of patients for current therapies and suggest new therapeutic approaches for the management of AH.




