Race, insulin resistance and hepatic steatosis in chronic hepatitis C†
Potential conflict of interest: Dr. Zacks is on the speakers' bureau of Roche. He also received grants from Salix Pharmaceuticals.
Abstract
Hepatic steatosis is common in chronic hepatitis C and has been linked to concurrent obesity, insulin resistance, diabetes, disease severity, and poor response to therapy. Racial differences in rates of obesity and diabetes may contribute to racial differences in hepatic steatosis and treatment response. The aim of the present study was to compare hepatic steatosis and its associations between African American (AA) and Caucasian American (CA) patients with chronic hepatitis C, genotype 1, participating in a prospective study of peginterferon and ribavirin therapy. Liver biopsy results were available from 194 AA patients and 205 CA patients. The 2 groups were compared for anthropometric, clinical, and biochemical features and insulin resistance estimated by the homeostasis model assessment index (HOMA-IR). Sixty-one percent of the AA patients and 65% of the CA patients had hepatic steatosis (P = 0.38). In univariable analysis, steatosis was associated with HOMA-IR, body mass index, waist circumference, serum triglycerides, aminotransferase level, and histological scores for inflammation and fibrosis. After adjusting for these features, AA patients had a lower risk of steatosis than did CA patients (OR 0.54, 95% CI 0.32-0.91, P = 0.02). Insulin resistance but not steatosis was associated with a lower rate of sustained virological response when adjusted for known factors that predict response (relative risk 0.87, 95% CI 0.77-0.99, P = 0.028). Conclusion: After adjusting for the higher prevalence of features associated with hepatic steatosis, AA patients had a lower prevalence of hepatic steatosis than did CA patients with chronic hepatitis C, genotype 1. Insulin resistance but not steatosis was independently associated with lower sustained virological response. (HEPATOLOGY 2006;45:80–87.)
Hepatic steatosis is a common histological finding in chronic hepatitis C virus (HCV) infection, found in 40%-70% of patients.1-3 In the largest series reported, the prevalence of steatosis was 65%, and its presence was associated with more advanced disease, especially in patients with genotype 1 infection.4 The mechanisms of hepatic steatosis in HCV are thought to be multifactorial.5-14 Several possibilities have been proposed including the presence of insulin resistance. Insulin resistance is a key factor in the development of steatosis in nonalcoholic fatty liver disease (NAFLD) and has been observed in individuals with HCV, especially in HCV, genotype 1 infection (referred to as metabolic steatosis).15
Compared to other racial groups, African Americans (AA) appear to have a lower prevalence of fatty liver,16, 17 despite a higher prevalence of risk factors for NAFLD. Clinical series have also found an unexplained low representation of African Americans among patients with nonalcoholic steatohepatitis (NASH), the most severe form of NAFLD.18 There have been no large studies of African Americans and Caucasian Americans (CA) designed to compare risk factors for and the presence of NAFLD determined by histopathology. Among patients with HCV, the relationship between race and the presence or severity of steatosis is unknown in genotype 1 infection, which is the predominant type found among both AA and CA. The aim of this study was to compare the prevalence and severity of hepatic steatosis according to anthropometry and other measures of insulin resistance among a large cohort of AA and CA with chronic hepatitis C, genotype 1, enrolled in a trial of antiviral therapy. We also wanted to assess whether the presence and severity of hepatic steatosis and/or insulin resistance were important factors to predict virological response in this population.
Patients and Methods
Patient Population.
The Virahep-C study was a multicenter study of combination peginterferon and ribavirin therapy of chronic hepatitis C designed to assess the rates and predictors of response among AA and CA with genotype 1 infection and to identify reasons for nonresponse to therapy. The design and primary outcomes of the Virahep-C trial have been reported.19 Eight clinical centers across the United States participated. Adult patients 18 years and older who were treatment naive, infected with genotype 1, had detectable HCV RNA, and had histologic evidence of chronic HCV were eligible to participate. Patients with a history of alcohol consumption of more than 2 drinks or the equivalent (>20 g) per day or evidence of alcohol abuse in the preceding 6 months were excluded. All patients had undergone liver biopsy within 18 months of enrollment. The study was designed to enroll at least 400 patients, equally divided between AA and CA. Patients were classified by race as either African American or Caucasian race and by ethnicity as either Hispanic or non-Hispanic based on self-report. All participants were required to have been born in the United States. Between July 2002 and December 2003, a total of 401 patients were enrolled and started on therapy.
Study Design.
Baseline anthropometric measurements, including height and weight to calculate body mass index (BMI) and waist circumference and waist-hip ratio to assess truncal obesity, were recorded at screening. Fasting glucose and insulin were obtained at baseline, and insulin resistance (IR) was assessed by the homeostasis model assessment index (HOMA): {fasting insulin [μU/ml] × (fasting glucose [mg/dl]/18)}/22.5.20, 21 Other baseline blood tests included serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and fasting triglycerides. All patients were questioned about history of diabetes, hypertension, hyperlipidemia, and medication use for these conditions.
All patients were required to have had liver biopsies performed within 18 months of screening, which were read by a central pathologist for whom all clinical information including race was masked. All biopsies were assessed for severity of hepatitis C by grading the inflammation and staging the fibrosis using the modified histologic activity index (HAI) scoring system described by Ishak et al.22 In addition, 2 other histological features, steatosis and features of steatohepatitis, were scored. Steatosis was graded on a scale from 0 to 4 according to the percentage of cells with fat, with 0 = none, 0.5 (trace) = <5%, 1 = 5% to <25%, 2 = 25% to <50%, 3 = 50% to <75%, and 4 = 75% to 100%. Because few patients had marked steatosis, those with steatosis grades 2, 3, and 4 were combined for analysis. Three specific features of steatohepatitis were recorded as either present or absent: zone 3 ballooning degeneration, zone 3 perisinusoidal fibrosis, and Mallory bodies. A biopsy was considered to show steatohepatitis if 2 of the 3 features were present in addition to a steatosis grade of 1 or above. HOMA is used routinely to assess longitudinal changes including assessment of the effects of treatment.20, 21 In general a HOMA index value of more than 1.5 is considered abnormal based on repeat testing measurements performed by both HOMA assessment and by euglycemic clamp technique and is considered representative of decreased insulin sensitivity. Although insulin secretion is pulsatile, the correlation between HOMA computed from repeat sampling (using a mean of 3 samples taken at 5-minute intervals to compute HOMA) and the value obtained from a single basal sample to determine insulin sensitivity has been shown to be near perfect, even in patients with type 2 diabetes (r = 0.99, P < 0.0001).21 In this study we decided to use a HOMA index value of more than 2.0 as the criterion to represent insulin resistance.
Patients received peginterferon alfa-2a (Pegasys, Roche Pharmaceuticals, Nutley, NJ) in a dose of 180 μg weekly and ribavirin (Copegus, Roche Pharmaceuticals, Nutley, NJ) in a dose of 1000-1200 mg daily for at least 24 weeks. The study was designed such that patients who became HCV RNA negative by week 24 continued treatment for a total of 48 weeks, whereas those who remained HCV RNA positive stopped treatment. The primary endpoint of the trial was a sustained virological response, defined as the absence of detectable HCV RNA for at least 24 weeks after stopping therapy. HCV RNA testing was done at a central laboratory (SeraCare BioServices, Gaithersburg, MD) using the Cobas Amplicor Assay (sensitivity 50 IU/ml: Roche Molecular Diagnostics, Alameda, CA). Selected samples were tested for HCV RNA levels by Cobas Amplicor Monitor Assay and for HCV RNA genotype by Versant HCV Genotype Assay (Bayer, Tarrytown, NY).
The design and details of this study were approved by the institutional review boards of the participating institutions and by a central Data Safety and Monitoring Board assembled by the National Institute of Diabetes and Digestive and Kidney Diseases to oversee and monitor the trial. All patients gave written informed consent for participation.
Statistical Analysis.
Prevalence and severity of steatosis and steatohepatitis were investigated in relation to patient characteristics including race, age, BMI, waist circumference in inches, waist-hip ratio, HOMA, baseline fasting triglyceride level, total HAI score, HAI inflammation score, Ishak fibrosis score, ALT and AST levels, alcohol consumption (number of drinks/week), use of lipid-lowering agents (yes/no), diagnosis of diabetes (yes/no), baseline HCV RNA level, and HCV subtype. Some of these variables were log-transformed (natural log, unless stated otherwise) to reduce the skew in the distribution and minimize the presence of outliers.
To compare the distributions of demographic and clinical features among steatosis grades, the Kruskal-Wallis test was used for continuous and semicontinuous variables, and the Jonckheere-Terpstra test was used for categorical variables. Odds ratios from simple logistic regression of steatosis (steatohepatitis) on individual continuous and semicontinuous variables were calculated to assess the strength and direction of the relationship between steatosis (steatohepatitis) and independent variables. Chi-square tests were used to assess differences in proportions among categorical variables. When the expected frequencies were small, exact tests were used.
The relationship between steatosis and the independent variables was studied in multiple logistic regression models. Significant variables (P < 0.05) from unadjusted analysis, with other clinically important variables including race, sex, HCV RNA level, and HCV genotype were chosen as candidate explanatory variables. Independent variables whose distributions were skewed, such as ALT, AST, HOMA, and triglyceride level were log-transformed to obtain approximate symmetry, except for BMI, for which a reciprocal transformation was applied. Stepwise analysis was used to eliminate variables possibly unrelated to steatosis. In addition, race-stratified models were constructed to determine whether significant predictors of steatosis differed within each race. The Hosmer-Lemeshow statistic was used to test the goodness of fit of each logistic regression model.23 The Hosmer-Lemeshow test is a modified chi-square goodness-of-fit test specifically designed for logistic regression analysis. It compares the observed and expected frequencies in different groups. Contrary to the Pearson chi-square test of goodness of fit, where the groups are formed by covariate values, the groups in the Hosmer-Lemeshow test are formed on the basis of the percentiles of the predicted probabilities. Similar methods were used to investigate the association between steatohepatitis and independent variables. However, race-stratified analyses were not possible because of the small number of patients with steatohepatitis in the cohort.
The proportions of AA and CA with sustained virological response (SVR) were compared using the chi-square test of association with continuity correction. A recent analysis from the same group (VIRAHEP-C Study) using the modified Poisson regression approach19 showed SVR to be significantly associated with race, sex, baseline viral level, ISHAK score, and proportion of maximum doses taken. Following the same methodology, we investigated whether inclusion of steatosis and insulin resistance in addition to the above covariates in the model explained the racial difference in SVR. The association between SVR and other covariates adjusting for potential confounders was assessed through Poisson regression models to calculate the relative risk.24 Relative risk estimates the relative change in the rate of outcome (SVR) per unit increase in the explanatory variable. Uncertainty in the relative risk estimates were estimated by the sandwich estimator of the variance. Statistical analyses were performed using SAS 8.02 (Statistical Analysis Software, SAS Institute, Cary, NC.25
Results
Frequency of Steatosis and Correlations with Clinical Factors.
Liver biopsy samples of 399 of the 401 patients enrolled in the Virahep-C trial (194 AAs and 205 CA) were adequate for reading. Hepatic steatosis was present in 253 patients (63%), with prevalence similar among AA (61%) and CA (65%) patients (P = 0.38). The severity of steatosis was generally mild, with 30% of patients having trace fat (0.5: <5%), 27% having mild af (1: 5%-25%), and only 6.5% having moderate to severe fat (2: >25%). There was a trend toward a less severe degree of steatosis among AA patients (Table 1). Patients with any degree of steatosis had higher BMI, larger waist circumference, larger waist-to-hip ratio, higher HOMA index value, and higher fasting triglyceride level and were more likely to have diabetes and hypertension than those without steatosis. These differences were present even when patients without steatosis were compared to those with trace steatosis. Thus, the presence of even minor amounts of hepatic steatosis correlated with metabolic and anthropometric factors associated with overweight and obesity.
| Variable | No Steatosis (mean ± SD) | Steatosis < 5% (mean ± SD) | Steatosis 5%–25% (mean ± SD) | Steatosis 25% or Above (mean ± SD) | P Value |
|---|---|---|---|---|---|
| N = 146 | N = 120 | N = 107 | N = 26 | ||
| Race | |||||
| AA | 75 (51%) | 63 (52%) | 46 (43%) | 10 (38%) | 0.06 |
| CA | 71 (49%) | 57 (48%) | 61 (57%) | 16 (62%) | 0.65 |
| Female (n, %) | 50 (34%) | 40 (33%) | 42 (39%) | 8 (31%) | |
| Age (years) | 46.8 ± 8.3 | 48.5 ± 8.0 | 48.3 ± 7.5 | 46.6 ± 6.2 | 0.57 |
| Body mass index (kg/m2) | 27.4 ± 5.3 | 29.1 ± 5.4 | 32.3 ± 6.3 | 31.8 ± 6.0 | < 0.0001 |
| Female | 27.0 ± 6.1 | 27.9 ± 5.7 | 33.8 ± 6.6 | 36.7 ± 7.1 | < 0.0001 |
| Male | 27.6 ± 4.9 | 29.6 ± 5.2 | 31.4 ± 6.0 | 29.9 ± 4.5 | < 0.0001 |
| Waist circumference (inches) | 36.4 ± 5.3 | 39.0 ± 5.7 | 40.8 ± 5.8 | 41.2 ± 4.6 | < 0.0001 |
| Female | 34.4 ± 5.6 | 36.2 ± 6.2 | 40.1 ± 6.4 | 41.5 ± 3.9 | < 0.0001 |
| Male | 37.5 ± 4.8 | 40.3 ± 5.0 | 41.2 ± 5.3 | 41.1 ± 4.9 | < 0.0001 |
| Waist-hip ratio | 0.89 ± 0.11 | 0.93 ± 0.11 | 0.92 ± 0.09 | 0.95 ± .07 | < 0.0001 |
| Female | 0.85 ± 0.13 | 0.86 ± 0.11 | 0.88 ± 0.09 | 0.93 ± .04 | 0.005 |
| Male | 0.91 ± 0.09 | 0.96 ± 0.10 | 0.94 ± 0.07 | 0.96 ± .08 | 0.001 |
| Fasting glucose (mg/dl) | 89.7 ± 17.8 | 98.0 ± 27.2 | 106.4 ± 37.1 | 124.5 ± 54.5 | < 0.0001 |
| Fasting insulin (units/dl) | 14.5 ± 18.0 | 19.7 ± 16.2 | 22.2 ± 29.4 | 22.4 ± 17.1 | < 0.0001 |
| HOMA index | 3.5 ± 5.0 | 4.3 ± 5.5 | 6.1 ± 9.0 | 6.8 ± 6.0 | < 0.0001 |
| Fasting triglycerides (g/dl) | 102.4 ± 48.5 | 127.5 ± 102.8 | 141.4 ± 90.6 | 161.7 ± 75.7 | < 0.0001 |
| ALT | 79.1 ± 71.4 | 89.3 ± 75.1 | 97.7 ± 76.0 | 121.1 ± 77.9 | 0.001 |
| AST | 52.4 ± 34.3 | 68.6 ± 57.0 | 77.3 ± 58.5 | 88.4 ± 62.2 | < 0.0001 |
| Log HCV RNA level | 6.3 ± 0.8 | 6.3 ± 0.7 | 6.20 ± 0.7 | 6.3 ± .6 | 0.62 |
| History of DM (n, %) | 8 (5.5%) | 15 (12.5%) | 11 (10%) | 5 (19%) | 0.04 |
| History of LLA use (n, %) | 3 (2.1%) | 0 (0.0%) | 1 (0.9%) | 1 (3.9%) | 0.77 |
| History of HTN (n, %) | 36 (25%) | 37 (31%) | 43 (40%) | 11 (42%) | 0.01 |
| Alcohol use (drinks/week) | |||||
| <1 | 117 (81%) | 83 (74%) | 84 (80%) | 17 (65%) | |
| 1–<7 | 15 (10%) | 15 (13%) | 6 (6%) | 5 (19%) | 0.20 |
| 7–<14 | 7 (5%) | 9 (8%) | 7 (7%) | 1 (4%) | |
| >14 | 5 (4%) | 6 (5%) | 8 (8%) | 3 (12%) | |
| HCV subtype (n, %) | |||||
| 1a | 76 (52%) | 62 (52%) | 57 (53%) | 13 (50%) | 0.66 |
| 1b | 50 (34%) | 46 (38%) | 42 (39%) | 10 (38%) | |
| Other (a/b, unspecified) | 20 (14%) | 12 (10%) | 08 (08%) | 03 (12%) | |
| Histology: | |||||
| Total HAI score | 9.1 ± 3.2 | 10.2 ± 3.4 | 11.1 ± 3.4 | 12.1 ± 2.9 | < 0.0001 |
| HAI inflammation score | 7.8 ± 2.6 | 8.5 ± 2.5 | 9.1 ± 2.6 | 9.9 ± 2.3 | < 0.0001 |
| Ishak fibrosis score | 1.6 ± 1.1 | 2.3 ± 1.7 | 2.6 ± 1.4 | 3.0 ± 1.6 | < 0.0001 |
| Bridging fibrosis/cirrhosis (n, %) | 33 (23%) | 42 (35%) | 56 (52%) | 15 (58%) | < 0.0001 |
| SVR (n, %) | 67 (46%) | 45 (38%) | 41 (38%) | 8 (31%) | 0.10 |
- NOTE. P values are from the Kruskal-Wallis test for continuous/semicontinuous variables and from the Jonckheere-Terpstra trend test for categorical variables.
- Abbreviations: AA, African American; CA, Caucasian American; HOMA, homeostasis model assessment; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DM, diabetes mellitus; LLA, lipid-lowering agent; HTN hypertension; HAI, histological activity index; SVR, sustained virological response.
Patients with steatosis were also more likely to have more severe liver disease than patients without steatosis, having higher average ALT and AST values as well as higher liver biopsy scores for necroinflammation and fibrosis (Table 1). Bridging fibrosis or cirrhosis was twice as prevalent in patients with steatosis as in those without (45% vs. 23%, P < 0.0001), and all except 1 patient with cirrhosis had fat on liver biopsy (28/29, 97%). Patients with trace hepatic steatosis had higher mean ALT, AST, and HAI scores than patients with no steatosis. Thus, the presence of even minor amounts of fat on liver biopsy correlated with serum biochemical and histological features of disease severity.
Factors that did not correlate with the presence or severity of hepatic steatosis included age, estimated duration of infection, current alcohol consumption, HCV RNA level, and subtype of genotype 1 (1a vs. 1b). Most patients in this cohort drank little if any alcohol, with approximately 90% reporting alcohol intake of less than 1 drink per day.
Steatohepatitis defined by strict criteria was identified in 28 patients, and the rates were similar among AA (6%) and CA (8%). In univariate analysis, factors that correlated with the presence of steatohepatitis were similar to those associated with the presence of steatosis (at any level) and included BMI, waist circumference, HOMA index, triglyceride level, ALT level, AST level, history of diabetes, and hypertension, as well as necroinflammatory and fibrosis scores on liver biopsy (data not shown).
Factors Associated with Steatosis in Multivariable Analysis.
Whereas the crude prevalence of steatosis did not differ between the two racial groups, AA patients had higher rates of many of the metabolic risk factors for steatosis. In multivariate analysis, after controlling for metabolic factors and fibrosis, AA patients had a substantially lower risk of steatosis than CA (OR 0.54, 95% CI 0.32-0.91, P = 0.02; Table 2). Other variables that were statistically significantly associated with steatosis in multivariable analysis were HOMA index, BMI, and Ishak fibrosis score. Using a greater degree of fat (1 or more: >5%) to define steatosis yielded similar predictive factors in both univariate and multivariable analyses (data not shown).
| Characteristics | Steatosis | ||
|---|---|---|---|
| Odds Ratio | 95% CI | P Value | |
| Race (African American) | 0.54 | 0.32-0.91 | 0.02 |
| HOMA index* | 2.19 | 1.57-3.05 | 0.001 |
| BMI** | 1.62 | 1.07-2.44 | 0.023 |
| Ishak fibrosis score | 1.45 | 1.19-1.76 | 0.0002 |
- Abbreviation: HOMA, homeostasis model assessment; BMI, body mass index.
- * Variable entered into the model in logarithmic (natural log) scale.
- ** Variable entered into the model as reciprocal transformation 100 (1-1/BMI).
Because metabolic factors were strongly associated with hepatic steatosis and were more frequent among AA patients than among CA patients, further analysis was done that compared the frequency of hepatic steatosis of any degree for different levels of BMI, HOMA, and fibrosis score by the two racial groups (Table 3). These analyses showed that AA patients were less likely to have hepatic steatosis than CA patients with a given level of metabolic abnormality such as HOMA (P = 0.03) or BMI (P = 0.091), but were similarly likely to have steatosis with a given degree of hepatic fibrosis (P = 0.60). Similar findings were found (lower rates of steatosis among AA than CA) when rates of steatosis by other metabolic factors such as waist circumferences and triglyceride level were compared (data not shown). Similarly, when analyzed by different features of disease severity, rates of steatosis were similar for AA and CA, such as by ALT and AST elevations and HAI score (data not shown).
| AA Patients | CA Patients | AA/CA Odds Ratio (95% CI)* | |||
|---|---|---|---|---|---|
| N | % with Steatosis | N | % with Steatosis | ||
| BMI | 0.69 (0.44, 1.06), | ||||
| 18–<25 kg/m2 | 32 | 41 | 57 | 40 | P = 0.091 |
| 25–<30 kg/m2 | 75 | 59 | 77 | 65 | |
| 30–56 kg/m2 | 84 | 71 | 69 | 86 | |
| HOMA index quartile | 0.58 (0.35, 0.94), | ||||
| 0.24–<1.75 | 30 | 33 | 55 | 40 | P = 0.03 |
| 1.75–<2.99 | 44 | 57 | 42 | 67 | |
| 2.99–<5.39 | 40 | 70 | 44 | 89 | |
| 5.39-74.71 | 52 | 75 | 33 | 82 | |
| Ishak fibrosis score | 0.90 (0.59, 1.36), | ||||
| 0 | 20 | 40 | 22 | 55 | P = 0.60 |
| 1-2 | 107 | 59 | 104 | 55 | |
| 3-4 | 57 | 68 | 60 | 77 | |
| 5-6 | 10 | 90 | 19 | 100 | |
- Abbreviations: AA, African American; CA, Caucasian American; BMI, body mass index; HOMA, homeostasis model assessment.
- * Adjusted for risk factor strata.
Hepatic Steatosis and Virological Response to Combination Therapy.
The relationship of hepatic steatosis with SVR to combination therapy with peginterferon and ribavirin was analyzed on the basis of any degree and different degrees of steatosis. Among patients with any steatosis at baseline, 37% achieved SVR compared to 46% of those without steatosis (P = 0.09). In addition, there was only a statistically nonsignificant trend toward lower SVR rate with increasing grade of steatosis (Table 1).
Because hepatic steatosis is linked to BMI, HOMA, and hepatic fibrosis, these related variables were analyzed for association with SVR. The SVR rate did not appear to be significantly affected by BMI (P = 0.15), with overall rates of 46% in patients of normal weight (BMI 20-25), 42% in overweight patients (BMI 25-30), and 35% in obese patients (BMI > 30) patients (P = 0.18). In contrast, the SVR rate was significantly associated with HOMA index value, with overall SVR rates of 49% in patients with HOMA index values of less than or equal to 2 and 36% in those with HOMA values greater than 2. Furthermore, there was a stepwise decrease in SVR rates by quartiles of HOMA values: 49%, 45%, 40%, and 25% (P < 0.001). Finally, as previously reported,19 the degree of fibrosis was strongly associated with SVR (P = 0.01): 62% for patients without fibrosis, 40% for patients with mild fibrosis (Ishak score of 1-2), 38% for patients with moderate fibrosis (Ishak score of 3-4), and 24% for patients with severe fibrosis and cirrhosis (Ishak score of 5-6).
In previously reported multivariable regression analyses from this study, race, sex, baseline viral level (and its interaction with race), fibrosis score, and proportion of peginterferon taken (over the first 24 weeks of treatment) were independently associated with SVR.19 In that model, the relative risk (RR) of SVR among CA compared to AA was 1.89 (95% CI 1.46-2.46). Furthermore, in the final multivariable regression model, BMI and steatosis were not independently associated with SVR. In the current analysis, HOMA index was added as a factor in the model and was found to be independently associated with SVR (RR = 0.87, 95% CI 0.77-0.99: P = 0.03; Table 4). The addition of HOMA to the model resulted in loss of sex as an independent variable but affected other factors minimally: the RR for Caucasian race decreased from 1.97 (1.48-2.62, P < 0.001) to 1.85 (1.3-2.53, P = 0.0001). Thus, SVR rates were higher with lower HOMA index values in both AA and CA patients, and at all levels response rates were lower in AA patients. These relationships are shown graphically in Fig. 1, which displays the likelihood of SVR by HOMA index level, controlling for other predictive factors separately for AA and CA patients. Thus, insulin resistance was associated with lower SVR rate, and despite greater insulin resistance among AA patients, this factor did not account for the differences in response rate of the two racial groups.
| Variable | Relative Risk | 95% Confidence Limits | P Value | |
|---|---|---|---|---|
| CA race | 1.85 | 1.35 | 2.53 | 0.0001 |
| Baseline viral level (log10 IU/ml) | 0.57 | 0.44 | 0.74 | < 0.0001 |
| Ishak fibrosis score | 0.90 | 0.83 | 0.98 | 0.015 |
| Proportion of maximum peginterferon dose taken for first 24 weeks (per 0.1 increase) | 1.37 | 1.17 | 1.60 | < 0.0001 |
| Interaction of baseline viral level (log10 IU/ml) and CA race | 1.53 | 1.15 | 2.04 | 0.004 |
| HOMA index° | 0.87 | 0.77 | 0.99 | 0.028 |
- ° Variable entered into the model in logarithmic (natural log) scale.
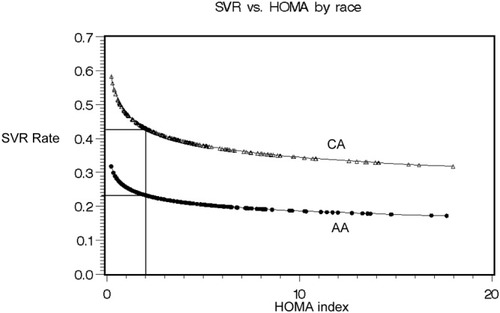
Relationship between HOMA index and adjusted predicted percentage with sustained virological response (SVR) by race showing the predicted probability of an SVR by baseline HOMA level separately for African Americans (AA) and Caucasian Americans (CA) using the example of a patient with a baseline viral level of 1.05 × 107 IU/ml and an Ishak fibrosis score of 2 on pretreatment liver biopsy who took 80% of the maximum peginterferon dose during the first 24 weeks of treatment.
Discussion
This large study of approximately equal numbers of AA and CA patients with chronic hepatitis C, genotype 1, allowed for a detailed analysis of racial differences in the clinical manifestations of disease and the outcomes of treatment. The current analysis focused on factors that correlated with hepatic steatosis and how these affected the outcome of therapy for hepatitis C. Hepatic steatosis is common in chronic hepatitis C; in this study, 63% of patients had some degree of fat on liver biopsy, a rate similar to what has been reported from other large cohorts.4 Although steatosis was common, the degree of fatty infiltration was usually mild. Most patients (30%) had trace fat (<5%), and only a few patients (6.5%) had more than a mild amount (>25% of cells with steatosis). Overall, there was no difference between AA and CA patients in either the frequency (62% vs. 65%) or the severity of steatosis.
Importantly, analysis of factors associated with steatosis showed racial differences in the frequency that a patient had hepatic fat. Steatosis was associated with many clinical factors, most strikingly with higher body weight, higher BMI, higher waist circumference, larger waist-hip ratio, and the presence of insulin resistance as assessed by fasting serum insulin and glucose and quantified by the HOMA index, a validated means of assessing insulin resistance. These metabolic factors were generally worse in AA than in CA, so that with controlling for the effects of these factors, AA were less likely to have hepatic steatosis than CA. The odds ratio for having hepatic steatosis was 0.54 for AA (P = 0.02). Thus, for a given degree of overweight and obesity or insulin resistance, AA were approximately half as likely to have hepatic steatosis as CA. Thirty-nine of the 401 patients (9.7%) enrolled were diabetic, and 24 of these (6%) were on oral antidiabetic agents, the details about which are unavailable. Although the use of thiazolidinediones can affect hepatic steatosis and could possibly have confounded the results, we believe that this is unlikely to have occurred, given that only 6% of the patients were on oral agents that included agents other than thiazolidinediones. In addition, although only a minority of patients in the study had diabetes, and despite that it has been shown that the correlation between HOMA computed from repeat sampling with that computed from a single basal sample is very good in determining insulin sensitivity in patients with type 2 diabetes,21 there is still concern that HOMA may underestimate the degree of insulin resistance in overtly diabetic patients. This possibly could be a limitation to the interpretation of the results of our study.
Analysis of factors associated with hepatic steatosis also showed that severity of liver disease correlated with fat on liver biopsy. Thus, steatosis was present in 48% of the patients with no fibrosis, 57% of the patients with mild fibrosis (Ishak fibrosis score of 1-2), but almost all the patients with cirrhosis (97%). In comparison to CA patients, AA patients appeared to be less likely to have hepatic fat in response to the metabolic effects of obesity and insulin resistance, but they were similarly likely to have steatosis in response to advanced fibrosis or other indices of disease severity. Thus, unlike the relationship of BMI and HOMA to steatosis, the relationship of fibrosis to steatosis was similar in the two races.
Multivariable analysis demonstrated 3 factors that correlated independently with steatosis on liver biopsy: BMI, HOMA, and hepatic fibrosis. Although obesity and insulin resistance were related, they appeared to have independent effects on fat accumulation in the liver. Thus, the more overweight and the greater the obesity, the greater was the likelihood of hepatic fat, which was further increased by the development of insulin resistance. The effect of both overweight/obesity and insulin resistance on hepatic steatosis appeared to be modulated by AA race with AA patients less likely to have hepatic steatosis at any weight or with any level of insulin resistance.
Patients with hepatic steatosis were slightly less likely to have a sustained response to peginterferon-ribavirin combination therapy than were patients without steatosis (30% vs. 46%) although this was not statistically significant (P = 0.09). This weak association may have been a result of the confounding association of hepatic fibrosis and steatosis. The degree of fibrosis was strongly associated with SVR rate, an association confirmed by multivariable analysis. BMI, in contrast, was not clearly associated with response rate, with an SVR occurring in 35% of obese patients, 42% of overweight patients, and 46% of normal-weight patients and an association not found in multivariable analysis. In contrast to steatosis and BMI, the pretreatment HOMA index value correlated significantly with SVR rate and, in multivariable analysis, was independently associated with response rate. These findings indicate that among factors that determine virological response to peginterferon and ribavirin therapy, insulin resistance and fibrosis are important and obesity and steatosis may be less or not as important. The mechanisms by which insulin resistance might affect response to interferon-based therapy for hepatitis C are not obvious from these analyses, but a reasonable hypothesis is that there are important interactions and “cross talk” between intracellular insulin- and interferon-signaling pathways. The importance of these findings is that insulin resistance is a potentially modifiable factor, so that responses to antiviral therapy for hepatitis C may be improved by modulation of insulin signaling and improvements in insulin resistance and glucose control. These possibilities deserve prospective evaluation.
Acknowledgements
Members of Virahep-C who contributed to the study were: Nezam Afdhal (principal investigator) and Tiffany Geahigan (research coordinator) from Beth Israel Deaconess Medical Center, Boston, MA; Robert S. Brown, Jr. (principal investigator), Lorna Dove (co-investigator), Shana Stovel (study coordinator), and Maria Martin (study coordinator) from New York-Presbyterian Medical Center, New York, NY; Norah Terrault (principal investigator), Stephanie Straley, Eliana Agudelo, Melissa Hinds (clinical research coordinator), and Jake Heberlein (clinical research coordinator) from the University of California, San Francisco, San Francisco, CA; Thelma E. Wiley (principal investigator) and Monique Williams (study coordinator) from Rush University, Chicago, IL; Charles D. Howell (principal investigator), Kelly Gibson (project coordinator), Karen Callison (study coordinator), and Jane Lewis (study coordinator) from the University of Maryland, Baltimore, MD; Lennox J. Jeffers (principal investigator), Shvawn McPherson Baker (co-investigator), Maria DeMedina (project manager), and Carol Hermitt (project coordinator) from the University of Miami, Miami, FL; Hari S. Conjeevaram, (principal investigator), Robert J. Fontana (co-investigator), and Donna Harsh (study coordinator) from the University of Michigan, Ann Arbor, MI; Michael W. Fried (principal investigator [K24 DK066144]), Scott R. Smith (co-investigator), Dickens Theodore (co-investigator), Steven Zacks (co-investigator [K23 DK064762]), Roshan Shrestha (co-investigator), Karen Dougherty (co-investigator), Paris Davis (study coordinator), and Shirley Brown (study coordinator) from the University of North Carolina, Chapel Hill, NC; John E. Tavis (principal investigator), Adrian Di Bisceglie (co-investigator), Ermei Yao (co-investigator), Maureen Donlin (co-investigator), Nathan Cannon (graduate student), and Ping Wang (lab technician) from St. Louis University, St. Louis, MO; Huiying Yang (principal investigator), George Tang (project scientist), and Dai Wang (project scientist) from Cedars-Sinai Medical Center, Los Angeles, CA; Hugo R. Rosen (principal investigator), James R. Burton (co-investigator), and Jared Klarquist (lab technician) from the University of Colorado Health Sciences Center, Denver, CO; Scott Weston (lab technician) from the Veterans' Administration, Portland, OR; Milton W. Taylor (principal investigator), Corneliu Sanda (postdoctoral associate), Takuma Tsukahara (statistician), and Mary Ferris (lab assistant) from Indiana University, Bloomington, IN; Steven H. Belle (principal investigator), Richard A. Bilonick (statistician), Geoffrey Block (co-investigator), Jennifer Cline (data manager), Marika Haritos, MS (statistician), KyungAh Im (statistician), Stephanie Kelley (data manager), Sherry Kelsey (co-investigator), Laurie Koozer (project coordinator), Sharon Lawlor (data coordinator), Stephen B. Thomas (co-investigator), Abdus Wahed (statistician), Yuling Wei (project coordinator), Leland J. Yee (consultant), and Song Zhang (statistician) from the Data Coordinating Center, Graduate School of Public Health at the University of Pittsburgh, Pittsburgh, PA; Patricia Robuck (project scientist), James Everhart (scientific adviser), Jay H. Hoofnagle (scientific adviser), Edward Doo (scientific adviser), T. Jake Liang (scientific adviser), and Leonard B. Seeff (scientific adviser) from the National Institute of Diabetes and Digestive and Kidney Diseases; and David E. Kleiner (central pathologist) from the National Cancer Institute.




