C/EBPα and HNF6 protein complex formation stimulates HNF6-dependent transcription by CBP coactivator recruitment in HepG2 cells†
Potential conflict of interest: Nothing to report.
Abstract
We previously demonstrated that formation of complexes between the DNA-binding domains of hepatocyte nuclear factor 6 (HNF6) and forkhead box a2 (Foxa2) proteins stimulated Foxa2 transcriptional activity. Here, we used HepG2 cell cotransfection assays to demonstrate that HNF6 transcriptional activity was stimulated by CCAAT/enhancer-binding protein α (C/EBPα), but not by the related C/EBPβ or C/EBPδ proteins. Formation of the C/EBPα–HNF6 protein complex required the HNF6 cut domain and the C/EBPα activation domain (AD) 1/AD2 sequences. This C/EBPα–HNF6 transcriptional synergy required both the N-terminal HNF6 polyhistidine and serine/threonine/proline box sequences, as well as the C/EBPα AD1/AD2 sequences, the latter of which are known to recruit the CREB binding protein (CBP) transcriptional coactivator. Consistent with these findings, adenovirus E1A–mediated inhibition of p300/CBP histone acetyltransferase activity abrogated C/EBPα–HNF6 transcriptional synergy in cotransfection assays. Co-immunoprecipitation assays with liver protein extracts demonstrate an association between the HNF6 and C/EBPα transcription factors and the CBP coactivator protein in vivo. Furthermore, chromatin immunoprecipitation assays with hepatoma cells demonstrated that increased levels of both C/EBPα and HNF6 proteins were required to stimulate association of these transcription factors and the CBP coactivator protein with the endogenous mouse Foxa2 promoter region. In conclusion, formation of the C/EBPα–HNF6 protein complex stimulates recruitment of the CBP coactivator protein for expression of Foxa2, a transcription factor critical for regulating expression of hepatic gluconeogenic genes during fasting. (HEPATOLOGY 2006;43:276–286.)
Transfection studies in hepatoma cell lines demonstrated that synergistic transcriptional activation of hepatocyte-specific genes requires simultaneous binding of multiple hepatocyte nuclear factor to their promoter/enhancer regions.1 However, the mechanisms by which these hepatocyte nuclear factor transcription factors functionally interact to elicit transcriptional synergy have yet to be deciphered. The CCAAT/enhancer binding protein α (C/EBPα) transcription factor regulates expression of hepatocyte-specific genes, whose protein products are involved in glucose, lipid, and circulatory homeostasis.2-9 The C/EBPα and C/EBPβ transcription factors are coexpressed in hepatocytes and use a C-terminal basic region leucine zipper bipartite motif to bind to DNA as either homodimers or heterodimers.10 Structure–function studies demonstrate that the C/EBPα protein stimulates transcription through the conserved C/EBPα activation domain (AD) 1 sequences (amino acids 51-136), which recruits CREB binding protein (CBP)/p300 transcriptional coactivators.11
Hepatocyte nuclear factor 6 (HNF6) protein regulates transcription of hepatocyte-specific genes critical for adult liver function and for development of the hepatic biliary tree.1, 12, 13 The HNF6 protein belongs to the ONECUT (OC) family of transcription factors, which use a C-terminal DNA binding motif consisting of a single cut domain and homeodomain.14-18 Formation of HNF6 and Forkhead Box a2 (Foxa2) protein complexes stimulated Foxa2 transcriptional activity by recruiting the CBP coactivator protein through the HNF6 cut–homeodomain sequences.19 Nuclear magnetic resonance structural determination of the HNF6 protein–DNA complex demonstrates that the HNF6 cut–homeodomain sequences that are necessary for recruitment of the CBP coactivator are inaccessible when this HNF6 motif is bound to its DNA recognition sequence.20 These nuclear magnetic resonance structural studies predict that DNA recognition by the HNF6 cut–homeodomain sequences precludes recruitment of the CBP coactivator, suggesting that HNF6 stimulates transcription through its N-terminal serine/threonine/proline (STP) box domain.16 Furthermore, we recently showed that the HNF6 cut domain lysine 339 residue is acetylated by the CBP coactivator protein and that this modification is essential for HNF6 protein stability.21
In the present study, we examined whether HNF6 associates with another liver transcription factor to further stimulate HNF6 activity. We show that formation of the C/EBPα and HNF6 complex stimulated HNF6-dependent transcription through recruitment of the CBP coactivator protein and defined sequences required for this protein association. Co-immunoprecipitation experiments with liver protein extracts and chromatin immunoprecipitation assays with hepatoma cells allowed us to demonstrate a functional association between the HNF6 and C/EBPα transcription factors to stimulate recruitment of the CBP coactivator promoter to the endogenous Foxa2 promoter region.
Abbreviations
HNF6, hepatocyte nuclear factor 6; Foxa2, forkhead box a2; C/EBPα, CCAAT/enhancer-binding protein α; AD, activation domain; CBP, CREB binding protein; STP, serine/threonine/proline; CMV, cytomegalovirus; cDNA, complementary DNA; GFP, green fluorescent protein; HA, hemagglutinin; GST, glutathione-S-transferase; Ad, adenovirus; CMV-TetO, 7 copies of the tetracycline operator sequence linked to the minimal CMV promoter; AdCMV-TA, adenovirus expressing tetracycline transcriptional activator; ChIP, chromatin immunoprecipitation; PCR, polymerase chain reaction; WT, wild-type; PH, polyhistidine; Co-IP, co-immunoprecipitation.
Materials and Methods
Expression and Reporter Plasmids and Cotransfection Assays.
The cytomegalovirus (CMV) promoter expression vectors containing the HNF6 complementary DNA (cDNA) alone or tagged with green fluorescent protein (GFP) or V5 and the C/EBPα, C/EBPβ, or C/EBPδ expression vectors were previously described.17, 21, 22 The SV40 promoter pECE expression vectors containing mutant HNF6 ΔSTP box (Δ98-123) or HNF6 Δpolyhistidine cDNAs were a generous gift from Frédéric Lemaigre (Brussels, Belgium) and have been previously described.16 The CMV pCDNA3.1 expression vectors containing the hemagglutinin (HA)-tagged C/EBPα or HA-C/EBPα deletion mutants lacking either the transcriptional AD2 (amino acids Δ116-254) or AD1 sequences (119-360) and the C/EBPα K300E DNA binding mutant have been previously described.23, 24 The pGEX4-T-1 plasmid was used to express the glutathione-S-transferase (GST)-C/EBPα fusion proteins consisting of either AD1 (1-119), both AD1 and AD2 (1-226 or 1-286), the basic leucine zipper DNA binding domain (280-336) or the basic domain (313-336) and has been previously described.23 To generate the GST-C/EBPα AD2 (92-271) fusion protein, we blunted the BssHII fragment from the human C/EBPα cDNA into the SmaI site of the pGEX4T-1 expression plasmid. The pGEM1 HNF6 expression plasmids were used to synthesize radioactively labeled full-length or truncated HNF6 proteins and have been previously described.19 The HNF6-dependent reporter gene (6X HNF6 TATA luciferase plasmid) has been previously described.17, 19
Human hepatoma HepG2 cells, mouse hepatoma Hepa1-6 cells (ATCC #CRL-1830), or human osteosarcoma U20S cells were maintained in monolayer cultures and grown as previously described18, 25 and cotransfected using Fugene 6 reagent (Roche, Mannheim, Germany) according to the manufacturer's protocol. HepG2 or U2OS cells were transfected with 1.6 μg of the 6X HNF6 TATA luciferase plasmid and 200 ng of CMV C/EBPα (wild-type or mutant) or CMV C/EBPβ expression plasmid with or without 200 ng of the CMV HNF6 expression vector. We also performed cotransfection assays with 1.6 μg of the 6X HNF6 TATA luciferase reporter plasmid and 200 ng of CMV HNF6 and CMV C/EBPα with or without 200 ng of the CMV adenovirus E1A expression vector.
In Vitro GST-C/EBPα Pull-Down Assay, Western and Co-immunoprecipitation With Transfected HepG2 Nuclear Extracts.
Production and isolation of GST fusion proteins from BL21 Escherichia coli was performed as previously described.26 Binding buffer and wash buffer for the in vitro GST pull-down assay and co-immunoprecipitation assays with nuclear extracts were performed as previously described.19, 22 The immunoprecipitates were eluted by the addition of SDS sample buffer containing 1% β-mercaptoethanol and boiled for 5 minutes. Eluted proteins were resolved via SDS-PAGE and transferred to nitrocellulose membranes for Western blot analysis with either monoclonal antibody specific to GFP antibody (Clontech, Palo Alto, CA, for GFP-HNF6), HA epitope tag (Invitrogen, Carlsbad, CA, for HA-C/EBPα), V5 epitope tag (Invitrogen, for V5-HNF6), or C/EBPβ (sc-150 [C-19]; Santa Cruz Biotechnology, Santa Cruz, CA) and detected as previously described.19, 21, 27 For co-immunoprecipitation assays, 750 μg of total protein extract from quiescent mouse liver (0h) or regenerating mouse liver was immunoprecipitated with HNF627 or rabbit serum (Vector Laboratories, Burlingame, CA) and then subjected to Western analysis with rabbit anti-C/EBPα antibody (sc-61; Santa Cruz Biotechnology), C/EBPβ antibody (sc-150 [C-19]; Santa Cruz Biotechnology) or rabbit anti–CBP C-terminal antibody (Upstate, Lake Placid, NY). Peroxidase-conjugated ImmunoPure Recombinant Protein A/G (Pierce, Rockford, IL) was used to identify antibody–antigen bands and was detected via enhanced chemiluminescence (ECL-+ Amersham Pharmacia Biotech, Piscataway, NJ) followed by autoradiography.
Construction of Adenovirus With Inducible C/EBPα Expression and Adenovirus Purification.
The adenovirus (Ad)-tetracycline responsive element–HA-C/EBPα adenoviral construct was produced using the Adeno-X Tet-Off expression system by inserting the HA-tagged human C/EBPα cDNA (1-360) into the tetracycline responsive element shuttle vector following the manufacturer's instructions (BD Biosciences, Palo Alto, CA). The adenovirus vector used 7 copies of the tetracycline operator sequence linked to the minimal CMV promoter (CMV-TetO) to drive expression of the HA-C/EBPα protein. Induced expression was accomplished by coinfection with a second adenovirus containing the CMV promoter driving expression of the tetracycline transcriptional activator (AdCMV-TA), which is able to transcriptionally activate in the absence of doxycycline (Adeno-X Tet-Off system). Expression of HA-C/EBPα from this inducible adenovirus delivery system was detected via Western blot analysis with either the C/EBPα antibody or HA-tag antibody (Upstate). Recombinant adenoviruses were used to infect HEK 293A cells, and cell lysates were harvested at 72 hours postinfection. Generation and infection with the adenovirus containing the CMV-HNF6 cDNA expression cassette (AdHnf6) has been previously described,28 and adenovirus CMV GFP was purchased from BD Biosciences. The large-scale production of recombinant adenovirus particles was performed via infection of QBO-293 cells and subsequent purification following the protocol of Quantum Biotechnologies (Montreal, Canada). The number of infectious particle units was determined using the Adeno-X Rapid Titer Kit (BD Biosciences) according to the manufacturer's instructions.
Infection of Hepatoma Hepa1-6 Cells With Recombinant Adenoviruses and Chromatin Immunoprecipitation Assays.
Mouse hepatoma Hepa1-6 cells (1 × 107 cells per 150-mm dish) were infected at a multiplicity of infection of 10 infectious particle units per cell with AdHNF6 or AdC/EBPα (AdCMV-TetO-HA-C/EBPα and AdCMV-TA) either separately or together or with AdGFP control virus alone, as previously described.19, 29 We also used the Nucleofector II apparatus and buffers recommended by the manufacturer (Amaxa, Gaithersburg, MD) to transfect Hepa1-6 cells with CMV expression vectors containing either mouse HNF6 cDNA or rat C/EBPβ cDNA separately or together or with the CMV-GFP expression plasmid alone. The Nucleofector II system transfected approximately 60% of the Hepa1-6 cells as determined by electroporation of CMV GFP expression vector and counting the number of cells positive for GFP fluorescence (data not shown). At 24 hours after infection or electroporation, the cells were processed for chromatin immunoprecipitation (ChIP) assays using published methods.30, 31 The following antibodies were used for ChIP assays as previously described30: Hnf6 rabbit antibody,27 CBP rabbit antibody (sc-369 [A-22]; Santa Cruz Biotechnology), C/EBPα or C/EBPβ rabbit antibody (Santa Cruz Biotechnology; see above), or control rabbit serum (Vector Laboratories). Crosslinks were reversed on all chromatin samples via RNase A and proteinase K digestion, and DNA was then purified using polymerase chain reaction (PCR) purification columns according to the manufacturer's instructions (Qiagen, Valencia, CA) as previously described.30 The total input sample was diluted 1:10, and 2.5 μL was used for PCR (10% total input). To amplify the mouse Foxa2 promoter in ChIP assays, we used the −166 bp sense primer (ctcctgaagtcatcccacaagg) and −67 bp anti-sense primer (ggtgcccaaagcatttcgtaac) with 54°C annealing temperature. The following reaction mixture was used for all PCR samples: 1X of IQ SybrGreen Supermix (Biorad, Carlsbad, CA), 100 nmol/L of each primer, and 2.5 μL of each purified ChIP extract in a 25 μL total volume. Reactions were amplified and analyzed in triplicate using the MyiQ single-color real-time PCR detection system (Biorad). Normalization was performed using the ΔΔCT method as previously described.30, 31 The levels of either AdGFP-infected or CMV-GFP–transfected control samples were set at one. The experimental ChIP binding levels were expressed as a fold induction with respect to these control samples (relative promoter binding) ± SD.
ChIP Assays With Wild-Type Mouse Liver.
Liver tissue was isolated from 3 separate wild-type (WT) mice and washed in phosphate-buffered saline; the weight of each mouse liver was then recorded. The liver tissue was diced with a razor blade, homogenized in phosphate-buffered saline for 2 seconds with a PowerGen 125 Polytron (Fisher Scientific, Pittsburgh, PA), and fixed in 10 mL 1% formaldehyde (diluted in phosphate-buffered saline) for 15 minutes at room temperature with rotation. Glycine was added to 0.125 mol/L and suspension was incubated with rotation for an additional 5 minutes at room temperature to neutralize the formaldehyde. Fixed liver tissue was then pelleted 1,500-2,500 rpm at 4°C for 10 minutes. The cell pellet was homogenized for 1 minute in a Medimachine (Becton-Dickinson, Franklin Lakes, NJ) by resuspending a tissue pellet in 1 mL of cold phosphate-buffered saline containing protease inhibitor cocktail (Roche), then transferring it to a 50-μm medicon using an 18-gauge needle and syringe. Nuclei were recovered through the port in the medicon using a syringe and needle, and the homogenate was reapplied to the medicon 2 more times to purify liver nuclei. The liver nuclei were then pelleted and frozen at −80°C overnight. At this point, the chromatin was sonicated, and the ChIP assay was performed and analyzed as described above for cultured cells.
Results
HNF6 Transcriptional Synergy Requires the C/EBPα AD1/AD2 Sequences and Is Abrogated by E1A-Mediated Inhibition of CBP Activity.
In this study, we examined whether HNF6 associates with another liver transcription factor to stimulate HNF6 activity. We cotransfected human hepatoma HepG2 cells with the HNF6-dependent reporter plasmid (6× HNF6 TATA luciferase) and the CMV HNF6 expression plasmid with or without expression vectors containing either HNF4α, fetoprotein transcription factor, C/EBPα, C/EBPβ, or C/EBPδ cDNAs. These cotransfection experiments revealed that only the C/EBPα expression vector stimulated HNF6 transcriptional activity (data not shown). Cotransfection assays with C/EBPα and HNF6 expression vectors and 6× HNF6 TATA luciferase plasmid demonstrated that C/EBPα stimulated HNF6-dependent transcription in either human hepatoma HepG2 or osteosarcoma U2OS cells (Fig. 1A -B). Furthermore, a DNA binding–deficient C/EBPα (K300E) mutant was able to stimulate HNF6-dependent transcription in cotransfection assays (Fig. 1A), suggesting that this transcriptional activation was independent of C/EBPα DNA binding activity. This HNF6 transcriptional activation was specific to the C/EBPα isoform because neither C/EBPβ nor C/EBPδ expression vectors provided significant stimulation of HNF6 transcriptional activity in HepG2 or U2OS cotransfection assays (Fig. 1A-B and data not shown).
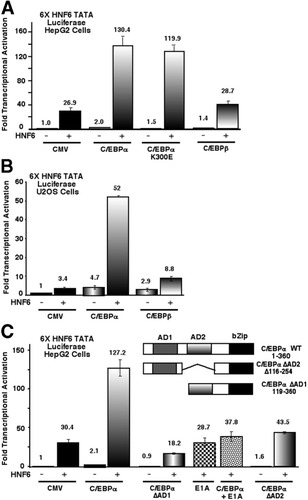
C/EBPα synergistically stimulates HNF6 transcriptional activity in cotransfection assays. (A-B) HNF6-dependent transcription is stimulated by C/EBPα, but not C/EBPβ, in cotransfection assays with either HepG2 or U2OS cells. Human hepatoma HepG2 cells (A) or osteosarcoma U2OS cells (B) were cotransfected with 6× HNF6 TATA luciferase reporter construct and CMV expression constructs containing no insert (CMV), C/EBPα, DNA binding–deficient C/EBPα mutant protein (C/EBPα K300E), or C/EBPβ with or without the CMV HNF6 expression vector. The CMV renilla-luciferase reporter construct was included in all of the transfections and was used as a normalization control. Protein extracts were prepared from transfected HepG2 or U2OS cells at 24 hours following DNA transfection, and a dual luciferase assay was performed to measure luciferase enzyme activity as previously described.19 (C) C/EBPα-HNF6 transcriptional synergy requires the C/EBPα AD1 and AD2 sequences and is inhibited by adenovirus E1A. Schematically shown is the location of the C/EBPα transcriptional AD1 and AD2 sequence and the basic leucine zipper DNA binding domain. HNF6 cotransfection assays were performed with deletion of the C/EBPα AD1 (amino acids Δ1-118) or the AD2 (amino acids Δ116-254) sequences. HepG2 cells were also cotransfected with the 6× HNF6 TATA-luciferase construct together with the CMV HNF6, CMV C/EBPα, and CMV adenovirus E1A (an inhibitor of p300/CBP histone acetyltransferase activity) plasmids or with HNF6 and E1A expression vectors. All of the transfection results are presented as the mean fold induction of promoter activity ± SD from 2 separate experiments in triplicate. Transcriptional activity of the CMV empty is set at 1.0. HNF6, hepatocyte nuclear factor 6; CMV, cytomegalovirus; C/EBP, CCAAT/enhancer-binding protein; AD, activation domain; bZip, basic leucine zipper; WT, wild-type.
Cotransfection studies with expression vectors containing C/EBPα deletion mutants demonstrated that both AD1 and AD2 sequences were essential for C/EBPα-HNF6 transcriptional synergy (Fig. 1C; C/EBPα ΔAD1, or ΔAD2). Because the AD1 sequences were shown to mediate transcriptional activation by the p300/CBP coactivators,11 we performed cotransfection assays with the CMV vector expressing the adenovirus E1A protein, which inhibits histone acetyltransferase activity of the p300/CBP coactivator proteins.32 Cotransfection assays showed that the E1A protein suppressed the ability of C/EBPα to stimulate HNF6 transcriptional activity (Fig. 1C), a finding that supports the hypothesis that C/EBPα stimulates HNF6 transcriptional activity by recruiting the CBP coactivator proteins. In contrast, cotransfection assays with the E1A and HNF6 expression vectors and the 6X HNF6 TATA Luciferase plasmid was unable to inhibit the basal transcriptional activity of HNF6, a finding consistent with previous studies.19 Taken together, these structure–function studies indicated that both the C/EBPα AD1 and AD2 sequences are essential for C/EBPα-HNF6 transcriptional synergy.
The HNF6 Polyhistidine and STP Box Sequences Are Essential for C/EBPα Transcriptional Synergy in Cotransfection Assays.
We examined whether deletion of either the N-terminal HNF6 transcriptional activation STP box domain (HNF6ΔSTP; Δ98-123) or the polyhistidine (HNF6ΔPH; Δ122-139) sequences were necessary for transcriptional synergy with the C/EBPα protein (Fig. 2A ). We performed HepG2 cotransfection studies with the 6X HNF6 TATA luciferase plasmid and expression vectors containing WT HNF6, HNF6ΔSTP, or HNF6ΔPH mutant proteins with or without the CMV C/EBPα expression construct. Consistent with published studies without C/EBPα,16 the HNF6ΔPH mutant displayed higher transcriptional activity than the WT HNF6 protein, while the HNF6ΔSTP mutant exhibited a 30% reduction in transcriptional activity (Fig. 2B). However, both the HNF6ΔSTP and HNF6ΔPH mutant proteins showed a reduction in HNF6-dependent transcriptional activity when combined with the CMV C/EBPα expression vector (Fig. 2B). These results demonstrated that retention of both the HNF6 N-terminal STP box and PH sequences was required for HNF6-C/EBPα transcriptional synergy.
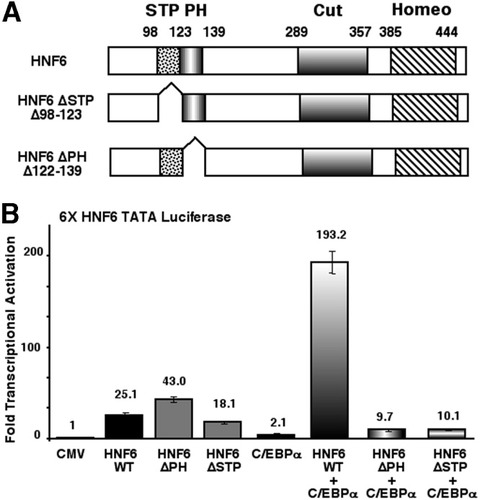
The N-terminal activation domain of HNF6 is required for the transcriptional synergy with C/EBPα. (A) Schematic representation of HNF6 WT protein with N-terminal transcriptional activation STP box domain and the PH region and the C-terminal cut–homeodomain DNA binding motif.16 Also shown are the HNF6 deletion mutant proteins HNF6 ΔSTP (Δ98-123) or HNF6 ΔPH (Δ122-139). (B) The HNF6 STP box and PH sequences are required for C/EBPα-HNF6 transcriptional synergy. HepG2 cells were cotransfected with the 6× HNF6 TATA luciferase reporter and expression vectors containing either the HNF6 WT, HNF6 ΔPH, or HNF6 ΔSTP box with or without the CMV C/EBPα expression vector. Transfections and analysis of transcriptional activity using dual luciferase assay are described in Fig. 1. STP, serine/threonine/proline; PH, polyhistidine; Cut, cut domain; Homeo, homeodomain; HNF6, hepatocyte nuclear factor 6; CMV, cytomegalovirus; C/EBPα, CCAAT/enhancer-binding protein α; WT, wild-type.
HNF6 Associates With the C/EBPα Protein, and HNF6 Cut Domain and C/EBPα AD 1/2 Sequences Are Required for the Interaction.
To define sequences required for association between HNF6 and C/EBPα proteins, we performed pull-down experiments with GST C/EBPα fusion proteins and radioactively labeled in vitro transcription and translation HNF6 proteins as shown in Fig. 3A -B. The HNF6 expression plasmids was used to synthesize 35S-methionine–labeled HNF6 proteins via in vitro transcription and translation (Fig. 3B, right panel), which were then used for binding reactions with various GST-C/EBPα fusion proteins (Fig. 3A) immobilized on glutathione-sepharose beads as previously described.19 These GST-C/EBPα pull-down assays demonstrated that labeled HNF6 proteins that retained the cut domain sequences (Fig. 3C, WT HNF6, Cut-Homeo and Cut) bound to the GST-C/EBPα AD1/AD2 fusion proteins (Fig. 3C, GST-C/EBPα proteins 2 and 3), and that deletion of the HNF6 cut–homeodomain sequences abrogated this binding (Fig. 3C). None of the labeled HNF6 proteins interacted with GST-C/EBPα fusion proteins containing only the AD1, the basic leucine zipper, or basic domain sequences (Fig. 3A,C, proteins 1, 4, and 5). Furthermore, labeled WT HNF6 protein bound weakly to the GST-C/EBPα AD2 fusion protein (Fig. 3D, protein 6), suggesting that both the C/EBPα AD1 and AD2 sequences were required for efficient HNF6 binding. Taken together, these findings demonstrate that formation of the C/EBPα-HNF6 complex requires the HNF6 cut domain and the AD1/AD2 C/EBPα sequences.
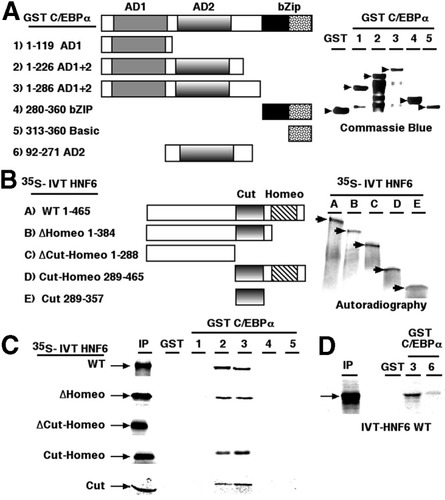
Formation of the HNF6 and C/EBPα protein complex requires the HNF6 cut domain sequence and C/EBPα AD2 sequence. (A) Schematic representation of the various GST-C/EBPα fusion proteins used for in vitro GST pull-down assays. The diagram depicts the GST-C/EBPα fusion proteins containing either transcriptional AD1 (1-119; construct 1), both AD1 and AD2 (1-226 or 1-286; constructs 2 and 3), the basic leucine zipper DNA binding domain (280-336; construct 4) or the basic domain (313-336; construct 5) or AD2 (92-271; construct 6). The right panel shows the expression levels of these GST-C/EBPα fusion proteins as visualized by Commassie blue staining following SDS-PAGE. (B) Diagram of HNF6 expression plasmids containing either the full-length HNF6 protein (WT), or N-terminal HNF6 protein that deleted either the homeodomain (ΔHomeo) or the cut–homeodomain (ΔCut-Homeo) from the C-terminus, the cut–homeodomain alone (Cut-Homeo), or the cut domain alone (Cut). These HNF6 deletion plasmids were used to synthesize radioactively labeled HNF6 proteins using in vitro transcription and translation with 35S-methionine, which were used in the in vitro GST-C/EBPα pull-down assays. The right panel shows the expression of these 35S-labeled HNF6 deletion proteins as visualized via autoradiography following SDS-PAGE. (C-D) The in vitro GST pull-down assays demonstrate that complex formation between the HNF6 and C/EBPα transcription factors requires the HNF6 cut domain sequences and the C/EBPα AD1 and AD2 sequences (amino acids 1-226). GST-C/EBPα fusion proteins (3 μg preabsorbed to 20 μL of glutathione-sepharose) were incubated with in vitro transcription and translation 35S-labeled full-length HNF6 or deletion mutants, then washed; boiling a sample in SDS loading buffer eluted bound proteins. Labeled bound HNF6 proteins were resolved via SDS-PAGE and visualized via autoradiography. GST, glutathione-S-transferase; C/EBPα, CCAAT/enhancer-binding protein α; AD, activation domain; bZip, basic leucine zipper; IVT, in vitro transcription and translation; HNF6, hepatocyte nuclear factor 6; WT, wild-type; Cut, cut domain; Homeo, homeodomain; IP, immunoprecipitated.
The C/EBPα Protein Co-immunoprecipitates With HNF6 in Transfected HepG2 Nuclear Extracts.
The C/EBPα protein is a potent inhibitor of cell proliferation23, 33; therefore, hepatoma cell lines do not express C/EBPα at high levels. To perform HNF6-C/EBPα co-immunoprecipitation (Co-IP) assays, we prepared nuclear extracts from HepG2 cells cotransfected with plasmids expressing either the GFP-tagged HNF6 protein19 or the HA-tagged C/EBPα protein.23 These transfected HepG2 nuclear extracts were subjected to immunoprecipitation with an HA-tag antibody followed by Western blot analysis using a monoclonal GFP antibody (Fig. 4A ). These Co-IP studies demonstrated that the GFP-HNF6 protein was able to associate with the HA-tagged C/EBPα protein (Fig. 4A, right panel). Co-IP assays with nuclear extracts prepared from HepG2 cells transfected with V5-tagged HNF621 and C/EBPβ22 expression vectors demonstrated that HNF6 protein failed to associate with the C/EBPβ protein (Fig. 4B, right panel). Western blot analysis of these transfected HepG2 nuclear extracts with the GFP, HA, V5, or C/EBPβ antibody showed protein expression from the transfected vectors (Fig. 4A-B, left panel, 10% input). These Co-IP results demonstrate that the HNF6 transcription factor associates with only the C/EBPα protein.
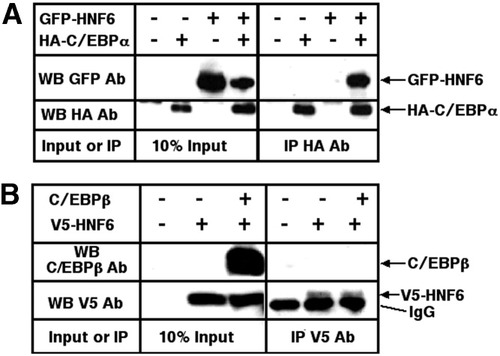
C/EBPα protein interacts with HNF6 protein in vivo. (A) Co-IP assays showed that the HNF6 and C/EBPα proteins form a complex in vivo. Nuclear protein extracts were prepared from HepG2 cells transfected with GFP-HNF6 and influenza HA epitope–tagged C/EBPα cDNA expression vectors. These nuclear extracts were subjected to immunoprecipitation with an HA monoclonal antibody followed by Western blot analysis using a monoclonal GFP or HA antibody (right panel). The transfected GFP-HNF6 or HA-tagged C/EBPα proteins were expressed as determined by Western blot analysis of the transfected HepG2 nuclear extracts with either the GFP or HA monoclonal antibody (left panel 10% input). (B) Co-IP assays showed that the HNF6 and C/EBPβ proteins fail to associate in vivo. Nuclear protein extracts were prepared from HepG2 cells transfected with the V5 epitope–tagged HNF6 protein and rat C/EBPβ cDNA expression vectors. These nuclear extracts were subjected to immunoprecipitation with a V5 monoclonal antibody followed by Western blot analysis using antibodies specific to either the V5 epiptope or C/EBPβ (right panel). The transfected V5-HNF6 or rat C/EBPβ proteins were expressed as determined via Western blot analysis of the transfected HepG2 nuclear extracts with antibodies specific to either the V5 epiptope or C/EBPβ (left panel, 10% input). Note that the antibody band migrates slightly faster than the V5-HNF6 protein in the control lanes. GFP, green fluorescent protein; HNF6, hepatocyte nuclear factor 6; HA, hemagglutinin; C/EBP, CCAAT/enhancer-binding protein; WB, Western blot; Ab, antibody; IP, immunoprecipitation; IgG, immunoblobulin G.
Functional Association Between the HNF6 and C/EBPα Transcription Factors and the CBP Coactivator.
To verify that HNF6 and C/EBPα transcription factors formed a complex with the CBP coactivator protein in vivo, we performed Co-IP assays with protein extracts from quiescent mouse liver or regenerating mouse liver isolated at 40 hours following partial hepatectomy, the latter of which represents the peak in hepatocyte DNA replication.34 Quiescent or regenerating mouse liver extracts were Co-IP with either the HNF6 or control rabbit serum; immunoprecipitated proteins were then subjected to Western blot analysis with either the C/EBPα or C/EBPβ antibody. This Co-IP experiment demonstrated that HNF6 and C/EBPα proteins formed a stable complex in extracts from both quiescent and regenerating liver, but HNF6 failed to interact with the related C/EBPβ protein (Fig. 5A ). Furthermore, Western blot analysis demonstrated that the CBP coactivator protein was Co-IP with C/EBPα in liver extracts using the HNF6 antibody (Fig. 5A). None of these transcription factors was immunoprecipitated by the control immunoblobulin G antibody (Fig. 5A). Taken together, these Co-IP studies with liver protein extracts demonstrated an association between the HNF6 and C/EBPα transcription factors and the CBP coactivator protein in vivo.
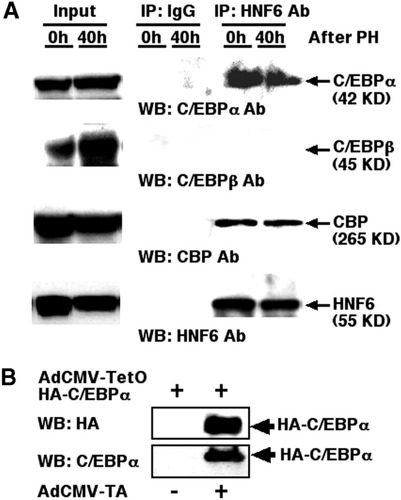
Co-IP assays with mouse liver extracts demonstrate functional association between the HNF6 and C/EBPα transcription factors and the CBP coactivator. (A) Co-IP assays with liver extracts and HNF6 antibody show association between HNF6 and the CBP coactivator protein. We performed Co-IP assays with protein extracts from quiescent mouse liver (0h) or regenerating mouse liver isolated at 40 hours (40h) following partial hepatectomy. Quiescent or regenerating mouse liver extracts were co-immunoprecipitated with either the HNF6 or rabbit serum control antibody, and immunoprecipitated proteins were then subjected to Western blot analysis with either C/EBPα, C/EBPβ, CBP, or HNF6 antibody as described in Materials and Methods. (B) Adenovirus vectors mediating inducible expression of HA-tagged human C/EBPα protein. Coinfection of HepG2 cells with both AdCMV-TetO-HA-C/EBPα and AdCMV-TA induces expression of the HA-C/EBPα as visualized via Western blot analysis with either the HA epitope or C/EBPα antibody. IP, immunoprecipitation; IgG, immunoblobulin G; HNF6, hepatocyte nuclear factor 6; Ab, antibody; PH, partial hepatectomy; C/EBP, CCAAT/enhancer-binding protein; WB, Western blot; Ab, antibody; CBP, CREB binding protein; AdCMV-TetO, adenovirus containing the 7 copies of the tetracycline operator sequence linked to the minimal CMV promoter; HA, hemagglutinin; AdCMV-TA, adenovirus expressing tetracycline transcriptional activator.
Increased Expression of HNF6 and C/EBPα Stimulates Their Binding and CBP Recruitment to the Endogenous Mouse Foxa2 Promoter.
To increase expression of C/EBPα in hepatoma cells, we generated an adenovirus vector that conditionally expressed the HA-tagged human C/EBPα protein under the control of CMV-TetO. Expression of HA-C/EBPα protein is induced by coinfection with a separate adenovirus containing the CMV promoter driving expression of AdCMV-TA, which is active in the absence of doxycycline (Tet-Off system). Coinfection of hepatoma cells with both AdCMV-TetO-HA-C/EBPα and AdCMV-TA (AdC/EBPα) is sufficient to induce expression of the HA tagged C/EBPα protein, as evidenced by Western blot analysis with antibody specific to either the HA tag or the C/EBPα protein (Fig. 5B). The proximal mouse Foxa2 promoter region contains a high affinity binding site for the HNF6 (−138 and −126 bp) and C/EBP (−84 to −74 bp) proteins as previously described.18, 22 To determine whether increased expression of both C/EBPα and HNF6 protein enhances association of these transcription factors and the CBP coactivator protein with the endogenous mouse Foxa2 promoter region, we used ChIP assays as previously described.30, 31
We prepared cross-linked chromatin from mouse hepatoma Hepa1-6 cells 24 hours after infection with AdHNF6 or AdC/EBPα, either alone or combined, and then sonicated chromosomal DNA into fragments between 500 to 1,000 nucleotides in length. We also prepared cross-linked and sonicated chromatin from Hepa1-6 cells that were efficiently transfected with CMV-HNF6 or CMV-C/EBPβ expression vector alone or combined by electroporation as described in Materials and Methods. This chromatin was then immunoprecipitated with antibodies specific to HNF6, CBP, C/EBPα, C/EBPβ or rabbit serum (control) and the immunoprecipitated genomic DNA was analyzed for the amount of mouse Foxa2 promoter DNA using quantative real-time PCR with primers specific to the mouse Foxa2 promoter region. We also performed ChIP assays with Hepa1-6 cells either infected with AdGFP or electroporated with CMV-GFP expression plasmid as normalization controls.
These hepatoma cell ChIP assays demonstrated that increased expression of both HNF6 and C/EBPα proteins stimulated association of either HNF6 (Fig. 6A) or C/EBPα (Fig. 6B) protein to the endogenous Foxa2 promoter region compared with hepatoma cells infected separately with AdHNF6 or AdC/EBPα (Fig. 6A-B). Interestingly, ChIP assays revealed that increased levels of both HNF6 and C/EBPα stimulated recruitment of the CBP coactivator protein to the endogenous Foxa2 promoter region compared with control hepatoma cells infected singly with AdHNF6 or AdC/EBPα (Fig. 6C). Although we were unable to demonstrate that HNF6 and C/EBPβ proteins interact using Co-IP assays with transfected cells or liver protein extract, cotransfection of both HNF6 and C/EBPβ expression vectors stimulated HNF6 binding to the endogenous Foxa2 promoter region (Fig. 6D), suggesting that these factors may interact in the context of the chromatin associated Foxa2 promoter region. However, cotransfection of both HNF6 and C/EBPβ expression vectors was unable to efficiently recruit either C/EBPβ (Fig. 6E) or the CBP coactivator protein (Fig. 6F) to the endogenous Foxa2 promoter region. ChIP assays with cross-linked and sonicated mouse liver tissue showed that HNF6, C/EBPα, and C/EBPβ bound at similar levels to the endogenous mouse Foxa2 promoter region (Fig. 6G). Interestingly, we observed reduced levels of CBP coactivator recruitment to the mouse liver Foxa2 promoter compared with that observed in Hepa1-6 cells with increased levels of HNF6 and C/EBPα proteins (compare Fig. 6C and 6G). These results are consistent with the inability of the HNF6-C/EBPβ complex to efficiently recruit the CBP coactivator protein to the endogenous Foxa2 promoter region. These studies support our hypothesis that formation of the C/EBPα-HNF6 complex stimulated binding of these transcription factors and recruitment of the CBP coactivator protein to the chromatin-associated Foxa2 promoter region.
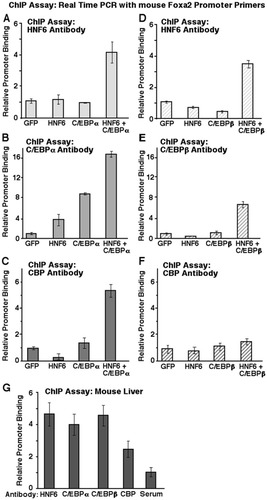
ChIP assays demonstrated that increased expression of HNF6 and C/EBPα stimulates their binding and CBP recruitment to the endogenous mouse Foxa2 promoter. Mouse hepatoma Hepa1-6 cells were infected with AdHNF6 or AdC/EBPα either separately or together or with AdGFP control virus. We also used the Nucleofector II apparatus and buffers recommended by the manufacturer (Amaxa, Gaithersburg, MD) to electroporate Hepa1-6 cells with CMV expression vectors containing either mouse HNF6 cDNA or rat C/EBP cDNA separately, together, or with the CMV-GFP expression plasmid alone. At 24 hours after infection or electroporation, the cells were processed for ChIP assay as described in Materials and Methods. The cross-linked and sonicated chromatin was immunoprecipitated with blocked protein A–sepharose CL4B and antibodies specific to HNF6 (A, D), C/EBPα (B), C/EBPβ (E), CBP (C, F), or immunoglobulin G (control) and then washed. Protein was then digested and removed from chromosomal DNA as described in Materials and Methods. The immunoprecipitated genomic DNA was analyzed for the amount of mouse Fox2 promoter region using a MyiQ single-color real-time PCR detection system and primers designed for the proximal mouse Foxa2 promoter region (−166 to −67 base pair; in triplicate). Normalization was performed using the ΔΔCT method as previously described.19, 29 These relative values were then normalized to either the AdGFP-infected or CMV-GFP–electroporated samples and shown graphically as relative promoter binding ± SD. (G) ChIP assays for Foxa2 binding factors with mouse liver. WT livers from 3 distinct mice were cross-linked and sonicated; immunoprecipitated with antibodies specific for HNF6, C/EBPα, C/EBPβ, CBP, or rabbit serum; and analyzed for the mouse Foxa2 promoter region via real-time PCR (performed in triplicate) as described in Materials and Methods. These relative values were averaged from 3 distinct mouse livers, normalized to rabbit serum value (value set at 1), and shown graphically as relative promoter binding ± SD. ChIP, chromatin immunoprecipitation; HNF6, hepatocyte nuclear factor 6; GFP, green fluorescent protein; C/EBP, CCAAT/enhancer-binding protein; CBP, CREB binding protein.
Discussion
Our previous cotransfection studies demonstrated that formation of complexes between the HNF6 and Foxa2 DNA binding domains stimulated Foxa2 transcriptional activity through recruitment of the CBP coactivator protein by the HNF6 cut–homeodomain.19 In the present study, we used cotransfection assays in human hepatoma HepG2 cells to demonstrate that HNF6 transcriptional activity was stimulated by C/EBPα, but not by the related C/EBPβ or C/EBPδ proteins. Formation of the C/EBPα-HNF6 protein complex required the HNF6 cut DNA binding domain sequences and the C/EBPα AD1/AD2 transcriptional activation domain sequences (Fig. 7A ). Structure–function cotransfection assays demonstrated that stimulation of HNF6 transcriptional activity required the C/EBPα AD1/AD2 sequences, which were shown to mediate transcriptional activation by the p300/CBP coactivators (Fig. 7A-B). Consistent with these studies, cotransfection of the adenovirus E1A protein, which inhibits the activity of the p300/CBP histone acetyltransferase, abrogated C/EBPα-HNF6 transcriptional synergy. Co-IP experiments with liver protein extracts and ChIP assays with hepatoma cells allowed us to demonstrate a functional association between the HNF6 and C/EBPα transcription factors to stimulate recruitment of the CBP coactivator promoter to the endogenous Foxa2 promoter region. The p300/CBP coactivators are known to stimulate transcription by acetylating positively charged lysine residues on histone proteins, causing their dissociation from DNA regulatory regions, and by interacting with the basal transcriptional machinery.32
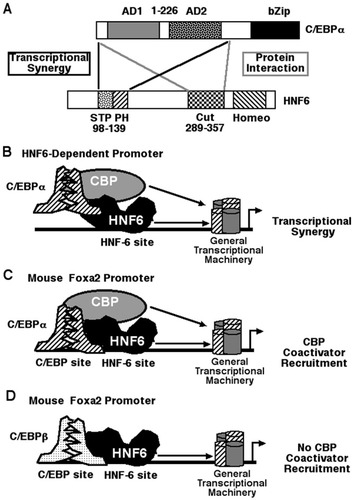
Model for C/EBPα-HNF6 transcriptional synergy on an HNF6-dependent promoter. (A) Schematically depicted are the C/EBPα and HNF6 sequences required for protein interaction and transcriptional synergy. Structure–function studies demonstrated that formation of the C/EBPα-HNF6 complex required the HNF6 cut domain and C/EBPα AD1 and AD2 sequences (gray lines). C/EBPα-HNF6 transcriptional synergy required the HNF6 STP box and PH sequences and the C/EBPα AD1/AD2 sequences (black lines). (B) On a HNF6-dependent promoter, formation of the C/EBPα and HNF6 protein complex synergistically stimulated HNF6-dependent transcription, which is likely mediated by C/EBPα AD1 recruitment of the CBP coactivator protein. (C) On a promoter containing both C/EBP and HNF6 sites (Foxa2 promoter), C/EBPα and HNF6 associate on the endogenous promoter region and recruit the CBP coactivator protein. (D) On a promoter containing both C/EBP and HNF6 sites (Foxa2 promoter) C/EBPβ and HNF6 associate on the endogenous promoter region, and this complex is unable to recruit the CBP coactivator protein. AD, activation domain; bZip, basic leucine zipper; C/EBP, CCAAT/enhancer-binding protein; HNF6, hepatocyte nuclear factor 6; STP, serine/threonine/proline; PH, polyhistidine; Cut, cut domain; Homeo, homeodomain; CBP, CREB binding protein.
We also used ChIP assays with mouse hepatoma Hepa1-6 cells to demonstrate that increased levels of both the C/EBPα and HNF6 proteins were required to stimulate binding of these transcription factors and recruitment of the CBP coactivator protein to the endogenous mouse Foxa2 promoter region (Fig. 7C). Although elevated levels of both C/EBPβ and HNF6 proteins increased binding of these transcription factors to the endogenous Foxa2 promoter, they were unable to stimulate recruitment of the CBP coactivator to this endogenous promoter region as determined via ChIP assay (Fig. 7D). ChIP assays with cross-linked and sonicated mouse liver tissue showed that HNF6, C/EBPα, and C/EBPβ bound at similar levels to the endogenous mouse Foxa2 promoter region. Interestingly, we observed reduced levels of CBP coactivator recruitment to the mouse liver Foxa2 promoter when C/EBPα and C/EBPβ proteins are recruited equally well to the Foxa2 promoter region. One possible explanation for this result is that C/EBPβ and C/EBPα proteins are forming heterodimers to bind to the endogenous FoxA2 promoter region, and HNF6 protein forms a complex with C/EBPα protein of this heterodimer complex. Recent studies using mouse livers that are conditionally deleted in the Foxa2 gene demonstrated that Foxa2 is required for transcriptional activation of genes encoding gluconeogenic enzymes during fasting and that Foxa2 stimulated recruitment of CREB transcription factor and glucocorticoid receptor to these chromatin-associated promoter regions.35 Our ChIP data suggest that formation of the HNF6-C/EBPα complex stimulates recruitment of the CBP coactivator protein to the endogenous Foxa2 promoter region, and that increased Foxa2 levels by these transcription factors may stimulate expression of hepatocyte Foxa2 target genes involved in gluconeogenesis.35
We also showed that the C/EBPα AD1/AD2 sequences and the HNF6 cut domain motif were sufficient to mediate association between the C/EBPα and HNF6 proteins (Fig. 7A-B). This result implies that interaction between the C/EBPα AD1/AD2 and cut domain DNA binding domain sequences does not inhibit the HNF6 cut–homeodomain motif from binding to a high affinity HNF6 recognition site and thus allows this C/EBPα-HNF6 complex to provide HNF6 transcriptional synergy. Furthermore, we showed that C/EBPα-HNF6 transcriptional synergy required both the N-terminal HNF6 STP box transcriptional activation domain and the PH sequences and the C/EBPα AD1 sequences (Fig. 7A), the latter of which have been shown to mediate transcriptional activation of C/EBPα by the p300/CBP coactivators.11 This result suggests that these HNF6 mutant proteins are unable to productively synergize with the C/EBPα transcription factor, indicating that the HNF6 STP and PH sequences play an important role in mediating transcriptional synergy with the C/EBPα protein. Previous structure–function cotransfection studies have determined that deletion of the N-terminal HNF6 PH caused only a slight elevation in HNF6 transcriptional activity, suggesting that the PH sequence did not contribute significantly in regulating HNF6 transcriptional activity.16 The current study enabled us to identify an important function of the HNF6 PH sequence: retention of the PH sequence is essential for mediating the C/EBPα-HNF6 transcriptional synergy. These results suggest that the C/EBPα-HNF6 transcriptional synergy involves interaction between the activation domains of each of these transcription factors.This is in contrast to the Foxa2-HNF6 transcriptional synergy, in which the HNF6 cut–homeodomain sequences alone were sufficient to potentiate transcriptional activity of the Foxa2 protein.19
In conclusion, we demonstrated that formation of the C/EBPα and HNF6 protein complex stimulate HNF6 transcriptional activity through C/EBPα AD1/AD2-mediated recruitment of the p300/CBP coactivators and required the N-terminal HNF6 STP box and PH sequences (Fig. 7A-B). Complex formation between the C/EBPα and HNF6 transcription factors required the HNF6 cut DNA binding domain sequences and the C/EBPα AD1/AD2 region (Fig. 7A). Consistent with this model, Co-IP experiments with liver protein extracts and ChIP studies with hepatoma cells allowed us to demonstrate a functional association between the HNF6 and C/EBPα transcription factors to stimulate recruitment of the CBP coactivator promoter to the endogenous Foxa2 promoter region (Fig. 7C).
Acknowledgements
We thank V. V. Kalinichenko, P. Raychaudhuri, and members of the Costa laboratory for critical review of this manuscript.




