Oxidative stress stimulates proliferation and invasiveness of hepatic stellate cells via a MMP2-mediated mechanism†
Potential conflict of interest: Nothing to report.
Abstract
Experimental evidence indicates that reactive oxygen species (ROS) are involved in the development of hepatic fibrosis; they induce hepatic stellate cells (HSC) proliferation and collagen synthesis. To address the role of matrix metalloproteinase (MMP)-2 in promoting HSC proliferation during hepatic injury, we investigated whether oxidative stress modulates the growth and invasiveness of HSC by influencing MMP-2 activation. Cell invasiveness and proliferation, which were studied using Boyden chambers and by counting cells under a microscope, were evaluated after treatment with a superoxide-producing system, xanthine plus xanthine oxidase (X/XO), in the presence or absence of antioxidants and MMP inhibitors. Expression and activation of MMP-2 were evaluated via gel zymography, immunoassay, and ribonuclease protection assay. The addition of X/XO induced proliferation and invasiveness of human HSC in a dose-dependent manner. The addition of antioxidants as well as MMP-2–specific inhibitors impaired these phenomena. X/XO treatment increased MMP-2 expression and secretion appreciably and significantly induced members of its activation complex, specifically membrane-type 1 MMP and tissue inhibitor metalloproteinase 2. To study the intracellular signaling pathways involved in X/XO-induced MMP-2 expression, we evaluated the effects of different kinase inhibitors. The inhibition of extracellular signal-regulated kinase 1/2 (ERK1/2) and phosphatidyl inositol 3-kinase (PI3K) abrogated X/XO-elicited MMP-2 upregulation and completely prevented X/XO-induced growth and invasiveness of HSC. In conclusion, our findings suggest that MMP-2 is required for the mitogenic and proinvasive effects of ROS on HSC and demonstrate that ERK1/2 and PI3K are the main signals involved in ROS-mediated MMP-2 expression. (HEPATOLOGY 2005;41:1074–1084.)
Hepatic fibrosis is a major histological finding associated with the progression of chronic liver disease to cirrhosis; it is characterized by increased deposition of components of the extracellular matrix (ECM), in particular fibrillar collagens type I and type III.1 Hepatic stellate cells (HSC) are currently considered to be one of the major sources of ECM proteins in the liver; expansion of their pool is a key step in the fibrogenic process.2 Following hepatic injury, HSC develop a myofibroblast-like phenotype, characterized by a reduced content of vitamin A, increased proliferation and migration, enhanced expression of matrix protein, and production of matrix metalloproteinases (MMPs).3
Evidence from experimental and clinical studies indicates that production of reactive oxygen species (ROS) and lipid peroxidation of cell membranes are often associated with the development of hepatic fibrogenesis and suggests that oxidative stress may be a common factor in chronic liver diseases of different etiologies.4, 5
We have previously shown that the induction of lipid peroxidation in human HSC stimulates procollagen type I synthesis through interactions at the level of gene expression.6 Furthermore, cytochrome P450 2E1–derived diffusible oxidants induce proliferation and collagen synthesis in rodent HSC.7 Although several other studies have confirmed the role of oxidant stress in the pathogenesis of hepatic fibrosis, as well as the protective role of antioxidant administration in this context,8 the molecular events underlying the fibrogenic effects mediated by ROS in human HSC have not been completely elucidated.
The MMPs, a multidomain family of zinc-dependent endopeptidases, degrade all structural components of the ECM and many bioactive molecules and play an essential role in many physiological and pathological processes.9 Based on their structural organization and subcellular localization, the MMP family is divided into secreted and membrane-anchored enzymes.10 The membrane-type MMPs (MT-MMPs) constitute a subgroup of membrane-anchored MMPs that are the major mediators of pericellular proteolysis.11
During hepatic fibrogenesis, the expression of the MMPs involved in fibrillar collagen degradation (e.g., MMP-1 in humans and MMP-13 in rats) is very limited, whereas the expression of MMP-2 (gelatinase A, 72,000-kd gelatinase) is markedly increased.12 MMP-2 has been implicated in ECM turnover; in particular, it can degrade several components of the subendothelial matrix, including collagen IV, laminin, and fibronectin, and it may be important in the remodeling of matrix during tissue repair processes.13 MMP-2 is secreted as an inactive proenzyme, which is activated in the pericellular space through a trimolecular complex that includes MT1-MMP and tissue inhibitor metalloprotease 2 (TIMP-2). The MT1-MMP/TIMP-2 complex functions as a cell surface receptor for pro–MMP-2 by promoting its pericellular proteolysis and consequent activation.14
Several studies have suggested that expression of MMP-2 and its activator, MT1-MMP, is altered in the fibrotic liver.15, 16 HSC produce these enzymes during injury to the liver. In addition, both human and rat HSC increase expression of MMP-2 during activation in vitro.12, 17 Recently, in vitro studies have provided some clues relating to the possible profibrogenic mechanism mediated by MMP-2 by showing that this enzyme can modulate the proliferative response of rat HSC.18
We investigated whether oxidative stress can modulate the growth of HSC and their invasiveness by changing the degradation system for the ECM. Our results indicate that oxidative stress generated by the xanthine plus xanthine oxidase (X/XO) superoxide production system induces MMP-2 expression and increases the levels of components of its activation complex, MT1-MMP and TIMP-2. These effects were associated with the induction of proliferation of HSC and an increase in their invasiveness; these phenomena were blocked by specific MMP inhibitors and antioxidants.
Abbreviations
ROS, reactive oxygen species; HSC, hepatic stellate cells; MMP, matrix metalloproteinase; X, xanthine; XO, xanthine oxidase; ERK, extracellular signal-regulated protein kinase; PI3K, phosphatidylinositol 3-kinase; ECM, extracellular matrix; MT-MMP, membrane-type MMP; TIMP, tissue inhibitor metalloproteinase; [3H]TdR, [methyl-3H]thymidine; SFIF, serum-free and insulin-free; mRNA, messenger RNA; RNase, ribonuclease.
Materials and Methods
Materials.
Culture media, trypsin, all restriction endonucleases, and DNA-modifying enzymes were obtained from GIBCO (Grand Island, NY). Stractan was obtained from Life Technologies (Milan, Italy). SB203580, PD98059, wortmannin, rapamycin, and MMP inhibitors (GM6001 and MMP-2 inhibitor I, OA-Hy) were obtained from Calbiochem (La Jolla, CA). Nitrocellulose membranes (Hybond) were obtained from Amersham (Milan, Italy). Pronase was obtained from Boehringer Mannheim (Monza, Italy). Antibodies against phosphorylated and diphosphorylated extracellular signal-regulated kinase (ERK1/2) and against 70-kd S6 kinase (p70S6K) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against MT1-MMP were obtained from Chemicon (Temecula, CA). Radioactive material was purchased from New England Nuclear (Boston, MA). All other reagents were obtained from Sigma Chemical Co. (Milan, Italy).
Isolation and Culture of HSC.
HSC were obtained from wedge sections of normal human liver that was unsuitable for transplantation after obtaining the approval of the appropriate local ethics committee. After combined 0.5% pronase/0.05% collagenase tissue digestion, human HSC were isolated by ultracentrifugation over four gradients (1.111/1.080/1.058/1.053) of stractan. They were characterized as previously described.19 The purity of HSC, estimated by autoflorescence of cells using UV-excited fluorescence microscopy, was beetwen 90% and 95%. Cells were cultured in Iscove's modified Dulbecco's Modified Eagle Medium supplemented with 20% fetal bovine serum, 2 mmol/L glutamine, 0.1 mmol/L nonessential amino acids, 1 mmol/L sodium pyruvate, 0.6 U/mL insulin, and 1% antibiotic-antifungal solution. Experiments were undertaken in triplicate using activated alpha smooth muscle–positive cells obtained between the first and third serial passage of cell lines obtained from five different patients.
Assay of DNA Synthesis and Cell Growth.
Cellular proliferation was evaluated by measuring DNA. The amount of [methyl-3H]thymidine ([3H]TdR) incorporated into trichloroacetic acid–precipitated material was determined, and cells were counted as previously described.19 Briefly, passage-activated HSC were seeded into 60-cm2 flasks until the monolayers were 75% to 80% confluent. Cell cultures were rendered quiescent by incubation in serum-free and insulin-free (SFIF) Iscove's medium for 48 hours and were next incubated with a superoxide anion donor [xanthine (500 μmol/L) plus xanthine oxidase (20-2,000 μU/mL) (X/XO)] for 20 hours; the cells were then pulsed for 4 hours with 1.0 μCi/mL [3H]TdR (6.7 Ci/mmol). Cells were disolved in 0.2 N NaOH. Their radioactivity was then measured in a scintillation counter. Six determinations were made to generate each experimental value.
Counting of hematoxylin and eosin–stained cells was undertaken after 48 hours of incubation with X/XO; a computerized video image analysis system (Leica Quantimet Q500MC; Leica Cambridge Ltd., Cambridge, UK) was used. Experiments were undertaken in triplicate using three different cell preparations. Production of superoxide anion was measured as SOD-inhibitable reduction of cytochrome c.6 Some experiments were undertaken in the presence of antioxidants (superoxide dismutase polyethylene glycol 250 U/mL or vitamin E 100 μmol/L), and MMP inhibitors (GM6001, 15 μmol/L or OA-Hy, 100 μmol/L) or kinase inhibitors (SB203580 10 μmol/L, PD98059 30 μmol/L, wortmannin 100 nm/L, rapamycin 5 ng/mL). Preliminary experiments indicated that cellular toxicity of the different treatments used could be disregarded for the following reasons: (1) lack of Typtan blue staining of the cells in the presence of relevant concentrations of test substances during the experimental period; (2) absence of lactate dehydrogenase leakage from HSC into the culture medium using a cytotoxicity detection kit (Boehringer Mannhein, Mannhein, Germany).
Invasion Assay.
The ability of cells to invade through a Matrigel-coated filter was measured in Boyden chambers. Polyvinylpyrrolydone-free polycarbonate filters (pore size 8 μm) were coated with basement membrane Matrigel (200 μg/filter) (Collaborative Research Inc., Bedford, MA), as described in the standard protocol.20 Confluent cells were starved of serum for 48 hours. After washing and trypsinization, they were placed in the upper chambers at a density of 1 × 105 in serum-free medium with or without X/XO. Dulbecco's Modified Eagle Medium containing 0.1% fetal bovine serum was placed in the lower compartments of the Boyder chambers. Chambers were incubated for 12 hours in 5% CO2 at 37° C. At the end of the incubation, the cells on the upper surface were completely removed by wiping with a cotton swab. Filters were fixed in methanol and stained with hematoxylin and eosin. Cells from various areas of the lower surface were counted using a computerized video image analyzing system (Leica Quantimet Q500MC). Each assay was undertaken in triplicate.
RNA Extraction and RNase Protection Assay.
Total RNA was extracted from cultured human HSC using the guanidinium-phenol-chloroform method. Cellular levels of MMP-1, MMP-2, TIMP-1, TIMP-2, and MT1-MMP messenger RNA (mRNA) were determined using a previously described ribonuclease (RNase) protection assay.21
“Anti-sense” (complementary to mRNA) RNA probes were obtained by run-off transcription of pGEM1 (Promega, Madison, WI) plasmids that contained the following complementary DNA fragments: the 730-bp SstI-EcoRI complementary DNA fragment of pCllase1, which codes for human MMP-1 (ATCC 57685) and the 1,300-bp EcoRI-BglII fragment of pK121 (kindly provided by Dr. K. Tryggvason), which codes for human gelatinase A (MMP-2). For TIMP-1, TIMP-2, and MT-MMP, probes were generated using oligo(dT)-primed reverse transcription of total cellular RNA prepared from human placenta and HT144 melanoma cells as previously described.12, 21 A 110-bp complementary DNA fragment of the GAPDH gene, subcloned into the HindIII site of pGEM1, was used as a control. T7 or SP6 RNA polymerase (Promega, Milan, Italy) was used to run off the anti-sense [32P]-labeled RNA probe. The newly synthesized RNA probes (specific activity: approx. 1 × 109 cpm/μg) were hybridized overnight with RNA samples obtained from HSC. Protected fragments were separated by electrophoresis through a 7.0-mol/L urea/6% acrylamide gel and were then exposed to Kodak X-omat film at −80°C for 72 hours.
Gelatinase Activity Assay.
Aliquots (20 μg/protein/lane) of cell culture medium were electrophoresed in 7.5% SDS polyacrylamide gels containing 0.1% gelatin (Sigma Chemical Co., Milan, Italy). After electrophoresis, sodium dodecyl sulfate was removed by soaking the gels for 20 minutes each in buffers containing 50 mmol/L Tris (pH 7.6), 10 mmol/L CaCl, and 2.5% Triton X-100, followed by the same buffer containing 1% Triton. Digestion was allowed to occur at 37°C for 24 hours. Gels were then stained with Coomassie brilliant blue and destained until clear bands became evident.
ELISA.
Immunoreactive levels of MMP-2 in conditioned medium were measured using a Biotrak Human MMP-2 ELISA kit (Amersham Biosciences, Arlington Heights, IL), according to the manufacturer's instructions. MMP-2 levels could be measured in the range 1.5 to 24 ng/mL; the sensitivity of the assay was 0.37 ng/mL.
TIMP-2 levels in conditioned media were measured using a human TIMP-2 immunoassay kit (Chemicon International, Temecula, CA), according to the manufacturer's instructions. TIMP-2 could be measured in the range of 20 to 320 ng/mL; the sensitivity of the assay was 20 ng/mL. Experiments were undertaken in triplicate.
Western Blotting.
Cells were scraped and homogenized in ice-cold buffer (pH 7.4) that consisted of 50 mmol/L Tris (pH 7.4), 150 mmol/L KCl, 1% Triton X-100, 1 mmol/L ethylenediaminotetra-acetic acid, 5 mmol/L N-ethylmaleimide, and 0.2 mmol/L phenyl-methyl-sulfonyl-fluoride. Cell extracts (50 μg/lane) were separated via 12% gel electrophoresis and electroblotted onto nitrocellulose filters. Nonspecific binding sites were blocked by incubating nitrocellulose sheets for 1 hour in phosphate-buffered saline containing 5% low-fat dry milk. Blots were developed using an enhanced chemiluminescence detection system (ECL plus kit; Pharmacia Bioscience, Arlington Heights, IL).
Statistical Analysis.
Results are expressed as the mean ± SEM. Group means were compared via ANOVA followed by the Student-Newman-Keuls test, if the former was significant. A P value of less than .05 was considered significant.
Results
Effect of X/XO on HSC proliferation and Invasiveness of Activated HSC.
To evaluate the effects of X/XO on HSC proliferation, cells were starved of serum and insulin for 48 hours and then incubated for an additional 24 hours with different concentrations of the superoxide anion donor. Application of the [3H]TdR incorporation assay indicated that X/XO significantly stimulated DNA synthesis in HSC (Fig. 1A). The increase in [3H]TdR incorporation was associated with a significant dose-dependent increase in the number of cells after incubation for 48 hours (Fig. 1B). The effect of oxidative stress on the ability of HSC to invade through reconstituted basement membranes was assessed using invasion chambers coated with Matrigel as already described. Exposure to X/XO induced a dose-dependent stimulation of the invasiveness of HSC (Fig. 1C). Concentrations of XO higher than 200 μU/mL did not induce any further significant increase of either proliferation or invasiveness.
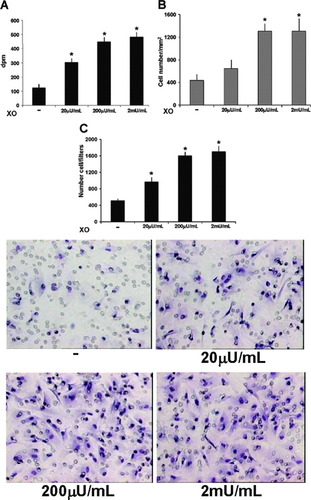
Oxidative stress induces proliferation and invasiveness of human HSC. (A) For [3H]TdR incorporation, subconfluent cells were rendered quiescent by incubation in SFIF medium for 48 hours and were then incubated with xanthine (500 μmol/L) in the presence or absence of various concentrations of XO (20-2,000 μU/mL) for 20 hours. Data are expressed as the mean ± SEM from four experiments, each of which was performed sixfold. *P < .05 versus control. (B) Cell counts after 48 hours of incubation in the presence of X/XO. Data are expressed as the mean ± SEM from four experiments, each of which was performed in triplicate. *P < .01 versus control. (C) Invasion assay. Confluent cells were rendered quiescent through incubation in SFIF medium for 48 hours; they were then trypsinized, suspended in SFIF medium containing xanthine (500 μmol/L) in the presence or absence of various concentrations of XO (20-2,000 μU/mL), and placed in the upper compartment of a Boyden chamber. After 12 hours of incubation, filters were stained and migrating cells that adhered to the underside of the membrane were counted (lower panel; original magnification × 200). Data are expressed as the mean ± SEM from four experiments, each of which was performed in triplicate. *P < .05 versus control (upper panel). XO, xanthine oxidase.
X/XO Effects Are Associated With Increased MMP-2 Expression.
Gelatin zymography of culture media of unstimulated HSC revealed a gelatinolytic band of 72 kD that represented latent MMP-2 (Fig. 2A). After addition of X/XO to HSC and culture for 24 hours, MMP-2 levels were increased. In addition, less intense bands of 66 to 64 kD were present in zymograms of supernatants collected after 3 and 6 hours of incubation. These bands corresponded to partially or completely activated forms of MMP-2.
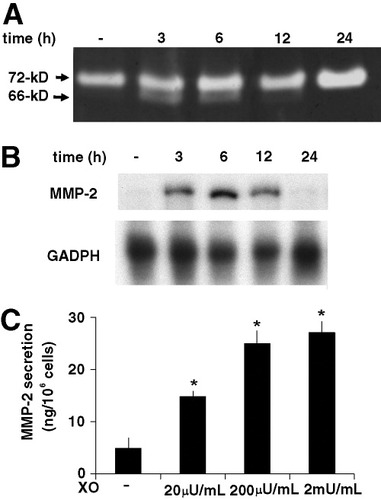
X/XO induces MMP-2 expression and activity in human HSC. HSC were rendered quiescent through incubation in SFIF medium for 48 hours; they were then incubated with X/XO for the indicated periods. (A) For a gelatinolytic activity assay, equal amounts of protein (20 μg) were separated on a gelatin-containing polyacrylamide gel. Representative data from three independent experiments, each of which was performed in triplicate, are shown. (B) RNase protection assay. After incubation with X/XO for the indicated periods, total RNA was extracted and 10 μg were hybridized with antisense RNA probes specific for MMP-2 and GAPDH mRNA. Representative data from three independent experiments, each of which was performed in duplicate, are shown. (C) MMP-2 secretion was measured in conditioned media from HSC that had been cultured with X (500 μmol/L) in the presence or absence of various concentrations of XO (20-2,000 μU/mL) for 24 hours using an ELISA kit. Data are expressed as the mean ± SEM from three experiments, each of which was perfomred in duplicate. *P < .01 versus control. MMP-2, matrix metalloproteinase 2; XO, xanthine oxidase.
The increase of MMP-2 synthesis, suggested by gelatin zymography, was confirmed by a marked upregulation of MMP-2 mRNA expression 3 to 12 hours after X/XO treatment (Fig. 2B). In contrast, MMP-1 mRNA levels were not affected by X/XO treatment (data not shown). Furthermore, immunoreactive MMP-2, measured via ELISA, was markedly increased in the medium of cultured HSC that had been stimulated by X/XO for 24 hours, and the increase was dose-dependent (Fig. 2C).
Incubation with antioxidants, such as vitamin E and superoxide dismutase polyethylene glycol, abolished increased MMP-2 gene expression and secretion induced by X/XO (Fig. 3A–B) and impaired X/XO-induced proliferation and migration of activated HSC (Fig. 3C).
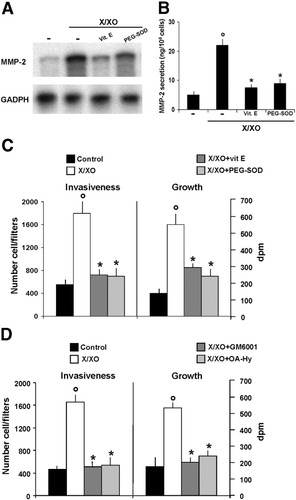
Antioxidants and MMP inhibitors impair X/XO-induced proliferation and invasiveness of HSC. (A) For the RNase protection assay, confluent HSC were rendered quiescent through incubation in SFIF medium for 48 hours; they were then incubated with X/XO in the presence or absence of antioxidants for 6 hours. Representative data from three independent experiments, each of which was performed in duplicate, are shown. (B) MMP-2 secretion was measured in conditioned media from quiescent HSC that had been treated with X/XO in the presence or absence of antioxidants for 24 hours using an ELISA kit. Data are expressed as the mean ± SEM from five experiments, each of which was performed in duplicate. *P < .002 versus cells treated with X/XO only. °P < .001 versus control. (C,D) For [3H]TdR incorporation, HSC were incubated with X/XO (500 μmol/L/2 mU/mL) for 20 hours in the presence or absence of antioxidants or MMP inhibitors. Data are expressed as the mean ± SEM from four experiments, each of which was performed sixfold. *P < .01 versus cells treated with X/XO only. °P < .002 versus control. For the invasion assay, confluent cells were rendered quiescent through incubation in SFIF medium for 48 hours; they were then trypsinized and suspended in SFIF medium containing X/XO (500 μmol/L/2 mU/mL) in the presence or absence of antioxidants (C) or MMP inhibitors (D). Migrating cells were counted. Data are expressed as the mean ± SEM from four experiments, each of which was performed in triplicate. *P < .01 versus cells treated with X/XO only. °P < .001 versus control. X/XO, xanthine/xanthine oxidase; Vit. E, vitamin E; PEG-SOD, superoxide dismutase polyethylene glycol; MMP-2, matrix metalloproteinase 2.
To confirm the role of MMP-2 in the regulation of proliferation and invasiveness of HSC, we added two chemically different MMP inhibitors. Both the broad spectrum MMP inhibitor, GM6001,22 and the specific MMP-2 inhibitor, 1 (OA-Hy),23 completely abrogated the effects of X/XO treatment on the growth and motility of HSC (Fig. 3D).
Effect of X/XO on Expression of TIMP-2 and MT1-MMP by HSC.
To examine the influence of oxidative stress on the expression of components of the MMP-2 activation complex, we assessed TIMP-2 and MT1-MMP expression in HSC treated with X/XO.
Figure 4A and C indicate a significant time-dependent upregulation of steady-state levels of TIMP-2 and MT1-MMP mRNA in X/XO-treated HSC. A similar induction of their protein products was found, as shown by the results of applying an ELISA assay for TIMP-2 and Western blotting for MT1-MMP. X/XO treatment induced a dose-dependent increase in TIMP-2 secretion (Fig. 4B) and the expression of both latent (65-kD) and activated (63-kD) forms of MT1-MMP; expression of the 63-kD form was greater than that of the 65-kD form (Fig. 4D). These findings suggest that oxidative stress can induce MT1-MMP activation that is required for the mediation of cell surface pro–MMP-2 activation. Pretreatment with vitamin E and superoxide dismutase polyethylene glycol abolished the increase of TIMP-2 and MT1-MMP mRNA levels induced by X/XO (Fig. 4E).
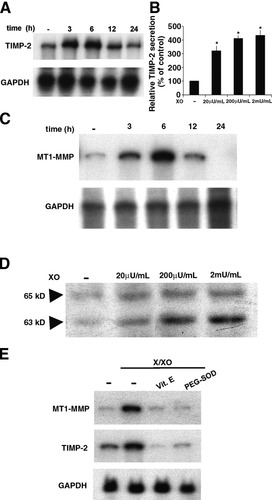
Oxidative stress induces TIMP-2 and MT1-MMP expression. (A,C,E) For the RNase protection assay, HSC were rendered quiescent through incubation in SFIF medium for 48 hours; they were then incubated with X/XO. Representative data from three independent experiments, each of which was performed in duplicate, are shown. (B) TIMP-2 secretion was measured in conditioned media from cultures of HSC that had been treated for 24 hours using an ELISA kit. Data are expressed as the mean ± SEM from three experiments, each of which was performed in duplicate. *P < .05 versus control. (D) Protein extracted from HSC that had been treated for 24 hours with X/XO were tested for MT1-MMP via Western blot analysis. Representative data from three independent experiments, each of which was performed in duplicate, are shown. TIMP-2, tissue inhibitor metalloproteinase 2; MT1-MMP, membrane-type 1 matrix metalloproteinase; XO, xanthine oxidase; X/XO, xanthine/xanthine oxidase; Vit. E, vitamin E; PEG-SOD, superoxide dismutase polyethylene glycol.
ERK1/2 and p70S6K Activation Is Involved in X/XO-Mediated Induction of MMP-2.
To assess the mechanism of action of X/XO in the regulation of MMP-2 expression, we undertook experiments to assess the intracellular signaling involved. To assess the role of the PI3K and ERK1/2 signal transduction pathways in the regulation of MMP-2 expression,24, 25 we determined the effects of specific kinase inhibitors on the expression of MMP-2 induced by X/XO. MMP-2 mRNA expression (Fig. 5A) and protein secretion (Fig. 5B) induced by X/XO were blocked by wortmannin, a PI3K inhibitor, and by rapamycin, a mTOR/ p70S6K inhibitor. Similarly, PD98058, a MEK inhibitor, significantly abrogated the effect of X/XO treatment on MMP-2 expression, whereas SB203580, a p38 inhibitor, had a negligible effect on this system.
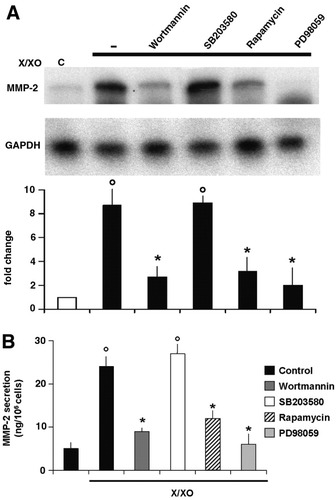
Effect of different kinase inhibitors on X/XO-induced MMP-2 expression. (A) For the RNase protection assay, HSC were rendered quiescent through incubation in SFIF medium for 48 hours; they were then incubated with X/XO. The effect of kinase inhibitors was evaluated by preincubating cells with each inhibitor for 30 minutes before X/XO treatment. Representative data from three independent experiments, each of which had been undertaken in duplicate, are shown. The histogram summarizes desitometric data from all the experiments undertaken; the control was assigned a value of 1. Data are expressed as the mean ± SEM. °P < .001 versus control. *P < .01 versus cells treated with X/XO only. (B) MMP-2 secretion was measured in conditioned media from HSC that had been treated with X/XO for 24 hours using an ELISA kit. Data are expressed as the mean ± SEM from three experiments, each of which was performed in duplicate. °P < .01 versus control. *P < .01 versus cells treated with X/XO only. X/XO, xanthine/xanthine oxidase; MMP-2, matrix metalloproteinase 2.
It has been shown that PI3K and ERK, both of which can activate p70S6K, play a key role in regulating the proliferation and migration of HSC via external stimuli.26-29 Western blot analysis was undertaken to evaluate the effects of X/XO on p70S6K and ERK1/2 activation. p70S6K activation is associated with increased phosphorylation of serine and threonine residues that results in reduced mobility of the protein on SDS-PAGE. The effects of X/XO on ERK1/2 phosphorylation were determined using antibodies that specifically recognize the active tyrosine-phosphorylated forms of ERK1 and ERK2.
X/XO induced p70S6K phosphorylation; it caused an appreciable decrease in the mobility pattern of the 70-kD and 85-kD protein triplets relative to untreated controls (Fig. 6A, upper panel). This change in gel mobility pattern became evident 10 minutes after X/XO treatment; it was sustained at 30 minutes, but was lost by 60 minutes (Fig. 6B). Also, X/XO induced an increase in ERK1/2 phosphorylation after incubation for 10 minutes; the increase was less at 30 minutes and was no longer present at 60 minutes (Fig. 6A, middle panel; Fig. 6B). The equivalent loading of protein in each lane was confirmed by probing membranes with anti-ERK1/2 antibodies (Fig 6A, lower panel).
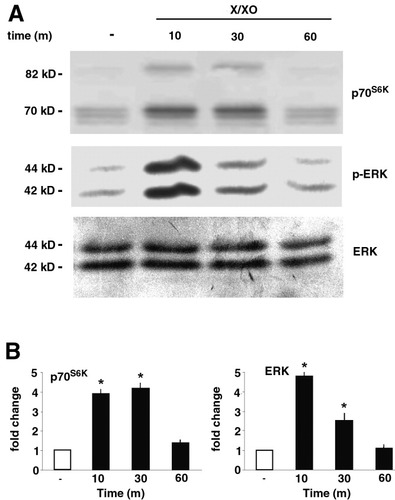
Effect of X/XO treatment on p70S6K and ERK1/2 phosphorylation. HSC were cultured for 48 hours in SFIF medium and than treated with X/XO (500 μmol/L/2 mU/mL) for the indicated periods. Cell lysates were obtained. Proteins (50 μg/lane) were separated via SDS-PAGE, transferred to nitrocellulosa, and then incubated with specific antibodies. Molecular weight markers are represented on the left-hand side of each panel. (A) The upper and middle panel indicate p70S6K and ERK1/2 phosphorylation, respectively. Equal loading of proteins in each lane was confirmed by probing the membranes with anti-ERK antibody (lower panel). Representative data from three independent experiments, each of which was performed in duplicate, are shown. Densitometric data shown in (B) are average values (± SEM) from all of the experiments. *P < .01 versus control. X/XO, xanthine/xanthine oxidase; ERK, extracellular signal-regulated protein kinase.
Effect of Intracellular Pathway Inhibitors on X/XO-Induced Proliferation and Invasiveness of HSC.
The signal transduction pathways involved in the regulation of MMP-2 expression induced by X/XO, induced us to evaluate the effects of different kinase inhibitors on the number of cells and chemoinvasion of cells after X/X0 treatment. Cellular proliferation and chemoinvasion of cells induced by X/XO were significantly reduced by addition of the MEK inhibitor, PD98059 (Fig. 7A–B). Similarly, the addition of either the PI3K inhibitor, wortmannin, or the mTOR/p70S6K inhibitor, rapamycin, reduced X/XO-induced proliferation and invasiveness in HSC. No similar effect occurred when cells were treated with the p38 inhibitor, SB203580.
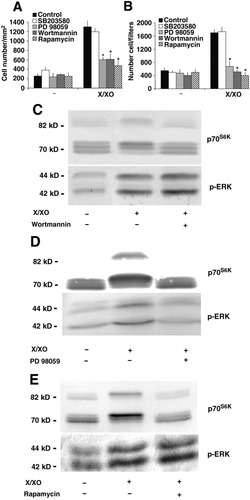
Effect of different kinase inhibitors on proliferation, invasiveness, and signaling pathways of HSC induced by X/XO. (A) HSC were rendered quiescent through incubation in SFIF medium for 48 hours; they were then incubated in X/XO for 48 hours. The effect of kinase inhibitors was evaluated by preincubating cells with each inhibitor for 30 minutes before X/XO treatment. Cell counts were undertaken. Data are expressed as the mean ± SEM from four experiments, each of which was performed in triplicate. *P < .05 versus X/XO-treated cells in the absence of inhibitors. (B) For the invasion assay, confluent cells were rendered quiescent through incubation in SFIF medium for 48 hours. Data are expressed as the mean ± SEM from four experiments, each of which was performed in triplicate. *P < .01) versus X/XO-treated cells in the absence of inhibitors. (C-E) HSC were cultured in SFIF medium for 48 hours and were then treated with X/XO (500 μmol/L/2 mU/mL) in the presence or absence of inhibitors for 10 minutes. Molecular weight markers are shown on the left of each panel. Representative data from three independent experiments, each of which was performed in duplicate, are shown. X/XO, xanthine/xanthine oxidase.
The effects of various kinase inhibitors on p70S6K and ERK1/2 activation were also evaluated (see Fig. 7). It has been shown that p70S6K can be a downstream component of PI3K pathways, which may also contribute to ERK1/2 activation in HSC.29 We tested the effect of the PI3K inhibitor, wortmannin, on p70S6K and ERK1/2 phosphorylation in HSC incubated with X/XO. Wortmannin completely blocked X/XO-induced p70S6K phosphorylation, but it did not affect ERK1/2 activation (Fig. 7C). We also studied the effect of the addition of the MEK inhibitor PD98059 on control and X/XO-treated HSC. The increase in p70S6K and ERK1/2 phosphorylation induced by X/XO was completely abrogated by PD98059 (Fig. 7D). In contrast, rapamycin did not have any effect on ERK phosphorylation, but it completely abrogated phosphorylation of its specific substrate, p70S6K (Fig. 7E).
Discussion
In this study, we have shown that ROS generated by the X/XO system can promote proliferation and invasiveness of human HSC and that these effects correlate with MMP-2 expression and activation. Previous findings have already shown that oxidative stress may provide a profibrogenic stimulus for HSC5, 30, 31 as a consequence of increased collagen synthesis and cellular proliferation.6-8 Our results extend these findings and indicate that oxidative stress may enhance the fibrogenic cascade by directly interacting with the MMP-2 matrix degrading system.
Benyon et al. have shown that the increased rate of proliferation of rat HSC during activation correlates with MMP-2 activity mediated by the induction of components of its activation system, specifically MT1-MMP and TIMP-2.18 Recently, the central role of MMP-2 in the growth and invasiveness of HSC was underlined by Olaso et al., who showed that collagen type I activation of the discoidin domain receptor 2 can increase proliferation and invasiveness of HSC, phenomena that directly reflect the increased expression of active MMP-2.32
Consistent with our findings, regulation of MMP-2 by oxidative stress has also been shown in nonhepatic cells. Oxygen increases expression of MMP-2 and MMP-9 mRNA in rat lungs,33 and X/XO and H2O2 increase expression and activation of MMP-2 in dermal fibroblasts.34 Siwik et al. demonstrated that ROS induce both pro–MMP-2 expression and MMP-2 activation in cardiac fibroblasts,35 findings that suggest that the effects of ROS are mediated at both transcriptional and posttranscriptional levels. In agreement with these findings, we found that X/XO treatment of HSC induced the rapid appearance of active forms of MMP-2 after 3 to 6 hours. The mechanism leading to the rapid posttranscriptional activation of MMP-2 remains unclear. It might involve the activation of MT1-MMP by rapid clustering of cell-surface glycoproteins and tyrosine phosphorylation.11
Several studies have demonstrated that activation of pro–MMP-2 by MT1-MMP depends on the presence of critical amounts of TIMP-2, which is required for the formation of the ternary complex that facilitates pro–MMP-2 activation.9 In our stellate cell system, we also demonstrated that both MT1-MMP and TIMP-2 levels were induced by X/XO treatment and abrogated by antioxidants. Therefore, ROS may promote matrix synthesis6-8 and also act as an autocrine growth factor by modulating MMP-2 expression and activation.
How oxidants act to initiate these events is unclear. It has been suggested that ROS may mimic the ligand receptor interaction that directly leads to activation of growth factor receptors36 or, alternatively, they may inactivate critical glutathione-sensitive membrane-bound phosphatases that are necessary to turn off growth factor receptor signaling.37 Furthermore, it has been demonstrated that certain proliferation-associated signaling pathways, including those leading to activation of ERK1/2 and PI3K, are activated by oxidative stress in a growth factor receptor-–dependent manner and may promote cell survival.38 Thus, it appears that at least some signaling pathways involved in the regulation of proliferation can participate in the cellular response to oxidative stress. In this context, it is interesting to note that the selective inhibitor of the epidermal growth factor receptor tyrosine kinase activity, AG1478, but not inhibitors of platelet derived growth factor receptor and insulin-like growth factor 1 receptor tyrosine kinase activities, prevented X/XO-mediated induction of MMP-2 expression by reducing ERK1/2 and PI3K activation (Galli et al., unpublished observations, 2004).
The ERK1/2 and PI3K pathways are central to many signal transduction processes and constitute the major systems through which growth factor receptors transduce proliferative signals to the nucleus in HSC.39 We have shown that inhibition of these pathways by specific inhibitors abrogated the effects of oxidative stress on MMP-2 expression and secretion and inhibited X/XO-induced growth and invasiveness of HSC. Pan et al. have shown that ERK1/2 activity can modulate MMP-2 expression via the redox-regulated transcription factor, Sp1, and that nonsteroidal anti-inflammatory drugs can inhibit MMP-2 via ERK/Sp1-mediated transcription.40 In addition, overexpression of constitutively active MEK1 can induce ERK1/2 activity and stimulate MMP-2 expression in transfected cells.41 The involvement of PI3K/mTOR/p70S6K signaling in MMP-2 activation has been demonstrated in the EphB4 receptor pathways that mediate endothelial cell migration and proliferation.42
In different cell types, ERK1/2 and PI3K activation is also essential for cell migration via MT1-MMP activation.43, 44 Recent evidence indicates that in HT1080 fibroblasts MT1-MMP plays a central role in the activation of ERK1/2, which in turn upregulates MT1-MMP and MMP-2.45
It is interesting to speculate that oxidative stress, through activation of MT1-MMP, leads to a positive feedback loop that results in sustained ERK1/2 activity and subsequent accumulation of MT1-MMP/MMP-2, phenomena that together promote the migration and growth of HSC. Accordingly, inhibition of ERK1/2 and PI3K by specific inhibitors completely prevents X/XO-induced cellular invasion and proliferation. This finding is in agreement with previous studies that suggest that activation of these signaling pathways plays a key role in mediating proliferation and migration of HSC.46, 47
In this study, we also demonstrate that X/XO treatment stimulates p70S6K in HSC. This kinase can be activated by both ERK1/2 and PI3K and plays a primary role in progression of the cell cycle in different cells, including HSC.28, 48 Interestingly, the PI3K inhibitor wortmannin completely abrogated X/XO-induced p70S6K phosphorylation, but had no effect on ERK1/2 activation. These findings suggest that, under the experimental conditions used, ERK1/2 does not represent a component of the PI3K pathway. It has been suggested that enzymes other than PI3K may regulate p70S6K phosphorylation; ERK1/2 might be a plausible mediator (Fig. 8).
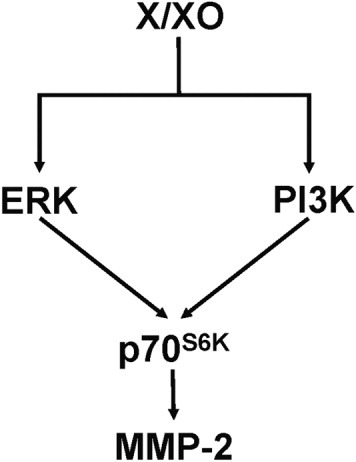
Proposed pathways involved in X/XO-mediated regulation of MMP-2 expression in HSC. X/XO, xanthine/xanthine oxidase; ERK, extracellular signal-regulated protein kinase; PI3K, phosphatidylinositol 3-kinase; MMP-2, matrix metalloproteinase 2.
Different reports have indicated that the stress pathways p38 and SAPK/JNK are activated by oxidant injury; these pathways may influence survival of HSC.49, 50 We found that the p38 pathway, but not SAPK/JNK, was weakly activated by the high levels of superoxide ion obtained by the highest concentration of XO used (data not shown). Conversely, inhibition of p38 by the SB203580 inhibitor did not alter expression and secretion of MMP-2 or growth and invasiveness of X/XO-treated HSC. These findings further suggest that the ultimate outcome of the effects on HSC may depend on the biological response generated by cross-talk between various pathways; activation of ERK1/2 and PI3K, observed in our experimental setting, may override the proapoptotic signals usually initiated by the activation of p38 kinase.
In conclusion, our results demonstrate that MMP-2 activation is a critical pathophysiological mechanism relating to survival of HSC mediated during the response to acute oxidative stress. They also suggest that ERK1/2 and PI3K pathways are the main signals involved in ROS-mediated MMP-2 expression. Our findings provide further insight into the processes that lead to hepatic fibrosis, and may facilitate the future development of more rational therapeutic strategies for liver disease.
Acknowledgements
The authors are most grateful to Professor W. Bosron and Dr. T. Mello for their many helpful comments and suggestions.




