Glutathione S-transferase and liver function in intrahepatic cholestasis of pregnancy and pruritus gravidarum
Abstract
Intrahepatic cholestasis of pregnancy (ICP) is a pregnancy-specific liver disease associated with poor maternal and fetal outcome. The diagnosis is based on pruritus with abnormal liver function in the absence of other pathological conditions. However, pruritus in pregnancy is common, and it may be the only presenting feature in ICP. No reliable test currently exists that can discriminate between those women destined to develop ICP and those with the benign condition of pruritus gravidarum (PG). The purpose of this prospective study was to investigate longitudinally the serum concentration of glutathione S-transferase alpha (GSTA, a specific marker of hepatocellular integrity) and to compare this with the temporal profile of conventional liver function markers in women with ICP (n = 63), PG (n = 43), and normal pregnant controls (n = 26). Blood was sampled on at least 3 separate occasions between 16 weeks of gestation and 4 weeks postpartum. Serum concentrations of GSTA increased with gestation in ICP, being significantly higher from 24 (±2) weeks compared with controls (400% difference; 95% CI, 240%–734%; P < .001). GSTA was also higher in ICP versus PG (433% difference; 95% CI, 228%-790%; P < .001) throughout the gestational period studied. Significant differences in the ICP compared with control and PG groups were also found for total bile acids, alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transpeptidase and alkaline phosphatase. In conclusion, the measurement of GSTA provides a test of liver dysfunction that distinguishes women with ICP from those with PG. Additionally, on the basis of this study, reference ranges for biochemical markers of liver function require reevaluation in pregnancy. (HEPATOLOGY 2004;40:1406–1414.)
Intrahepatic cholestasis of pregnancy (ICP), also known as obstetric cholestasis, is the most frequent cause of hepatic disease in pregnancy and is associated with fetal distress, spontaneous preterm labor, and stillbirth.1-7
ICP is a clinical syndrome of unknown origin, initially identified by persistent pruritus and confirmed by abnormal liver biochemistry (elevations in either total bile acids [tBA], alanine aminotransferase [ALT], aspartate aminotransferase [AST], and/or γ-glutamyl transpeptidase [γGT]).2, 6, 8 However, pruritus in pregnancy is reported in as many as 20% of women in early pregnancy,8-10 and the benign condition of pruritus gravidarum (PG) can be difficult to distinguish from a diagnosis of ICP, which generally is only confirmed in late pregnancy when liver function markers exceed normal reference ranges. As a result, women who present with pruritus and later develop ICP may frequently be overlooked in early pregnancy because of “normal” liver biochemistry at clinical presentation. Similarly, women with PG may be subjected to unnecessary surveillance and intervention, resulting in iatrogenic complications. Part of the problem lies in the absence of accurate definitions of gestational ranges for liver function tests and a lack of longitudinal studies in pregnancies affected by ICP. It is therefore important to (1) establish temporal gestational changes of indicators of conventional liver dysfunction in ICP and (2) identify serum markers with high specificity to allow for the early differentiation of women at risk of developing ICP amongst the much larger population of women with PG.
The phase II detoxification liver enzyme glutathione S-transferase alpha (GSTA) is a potentially useful candidate for the early identification of hepatocellular damage in ICP, since it is rapidly released into the circulation after acute liver damage. Serum concentrations have been shown to provide a more sensitive and specific evaluation of hepatic integrity than standard tests of liver function in a variety of clinical conditions associated with liver dysfunction.11-13
The aim of this prospective study was to longitudinally investigate serum concentrations of GSTA in comparison with conventional liver biochemical parameters (tBA, ALT, AST, γGT, alkaline phosphatase (ALP), total bilirubin, total protein, and albumin) in pregnant women who presented with pruritus. Comparison was made between women who developed ICP and those who did not (diagnosis of PG), and with a group of gestation-matched normal pregnant controls.
Abbreviations:
ICP, intrahepatic cholestasis of pregnancy; tBA, total bile acids; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γGT, γ-glutamyl transpeptidase; PG, pruritus gravidarum; GSTA, glutathione S-transferase alpha; ALP, alkaline phosphatase.
Patients and Methods
Patients.
Pregnant women were recruited from the antenatal clinics of three London (United Kingdom) hospitals (Whipp's Cross University Hospital, West Middlesex University Hospital, and Guy's and St Thomas' Hospitals NHS Trust) from August 1999 to April 2001. Women with ICP and PG were identified through (1) a prospective questionnaire-based study designed to investigate the prevalence of pruritus and ICP in a nonselected antenatal population9 and (2) referral by clinical colleagues or self-referral in response to a poster advertisement in which women with pruritus were asked to volunteer for the study. Data and samples were prospectively collected with local ethics committee approval at each center, and all women gave their informed consent to participate in the research. Sixty-three women who presented with pruritus and later developed (n = 9) or had a diagnosis of ICP (n = 54) at the time of recruitment, 43 with PG (i.e., who did not develop ICP), and 26 healthy pregnant women (controls) were identified for longitudinal assessment of liver function.
Definition of Intrahepatic Cholestasis of Pregnancy.
The diagnosis of ICP was based on persistent pruritus in pregnancy (in the absence of dermatological pathological conditions), in combination with an elevation in one or more conventional liver function test (ALT, AST, tBA, and γGT) in the absence of other liver disease, which resolved postnatally. The clinical diagnosis of ICP was made when values were outside the reference ranges defined by the routine chemical pathology laboratories at the participating hospitals (the upper end of the normal range in pregnancy was assumed to be 80% of the value quoted for the nonpregnant population, consistent with other studies14). Serum tBA concentrations were considered abnormal if greater than 14 μmol/L. Severity of pruritus was measured by use of a tick box scoring system from 0 (no itching) to 5 (severe itching). On detection of abnormal liver function, possible alternative causes of liver disease were sought by testing for anti-smooth muscle antibodies, anti-mitochondrial antibodies, Epstein Barr virus, cytomegalovirus, hepatitis (A, B, and C) serology, and by performing a liver ultrasound.
Definition of Pruritus Gravidarum and Controls.
Women with pruritus, in the absence of a rash or a coincident medical cause, in whom results of liver biochemical tests were normal were defined as suffering from PG. Women with pruritus with a pregnancy-specific dermatosis were excluded. Controls were defined as pregnant women without persistent pruritus and with normal liver biochemistry.
Women in the PG or control group were excluded if they had a history of ICP, jaundice, pruritus caused by oral contraceptives, or any other disease affecting hepatobiliary function.
Treatment.
Women with ICP were offered palliation of their pruritus by administration of chlorpheniramine and topical emollients. All women received vitamin K supplementation, starting at 34 weeks of gestation or at the time of diagnosis. Twenty-one women with ICP received ursodeoxycholic acid and/or dexamethasone for further symptomatic control. As both are reported to affect liver function,2 only serum/plasma samples taken before treatment were included in this study. Elective delivery was offered at 37 to 38 weeks of gestation.
Longitudinal Blood Samples: Methods of Assessment.
Non-fasted venous blood samples were collected from each subject, and serum/plasma was stored at −80°C. Blood samples taken postprandially (<2 hours) were excluded because of their potential effect on tBA. From recruitment, repeated blood samples were taken where possible at 4 weekly intervals and at 4 weeks postpartum. Sampling was less frequent in some subjects, but a minimum of 3 samples were obtained from each women, with a mean of 4 samples (3 antenatal and 1 postnatal) per subject (total number of samples analyzed = 541; 250 from the ICP group, 174 from the PG group, and 117 from the control group). Liver function tests were reevaluated in the longitudinal samples on completion of the study. ALT, AST, γGT, ALP, total bilirubin, total protein, and albumin were measured by International Federation of Clinical Chemistry calibrated methods on an automated multichannel Beckman (LX20) analyzer (Beckman Instruments Inc, Fullerton, CA). Serum tBA concentrations were analyzed by an enzymatic assay using a commercial kit (Sigma-Aldrich Diagnostics, Poole, UK). Serum GSTA concentrations were measured by a commercially available quantitative high-sensitivity enzyme immunoassay according to the manufacturer's instructions (Biotrin International Ltd, Dublin, Ireland). The intra-assay and inter-assay coefficients of variation were below 8% for all assays.
Statistical Analysis.
All analytes, except albumin and total protein, had substantial positive skew and were subjected to a simple log transformation. A Box-Cox transformation test and normal distribution plot for each analyte confirmed that this improved the distributions. Results were expressed as geometric means ± SEM bars for the purposes of graphical display (Figs. 1, 2) and ratio of geometric means with 95% confidence intervals (CI) for statistical analysis. Serum albumin and protein results were expressed as arithmetic means. In regression models, gestation was categorized into intervals of 4 weeks in each group, and Stata version 8.0 (StataCorp, College Station, TX) was used for data analysis. Graphs were constructed by using Sigma Plot 6.0 (SPSS Inc, Chicago, IL). To allow for values censored at the limit of detection for a test, Tobit regression was used,15 with dummy variables appropriate for the statistical test to be conducted. Standard errors, 95% CI, and P values were adjusted for repeated measurements by using Hubers's method for clustering.16 Receiver operated characteristic curves were used to determine an optimum reference range for GSTA. Correlations were calculated by using Spearman's rank coefficient of correlation with a P value of less than .05 considered statistically significant.
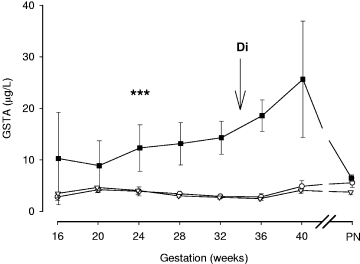
Longitudinal changes in serum GSTA concentrations during pregnancy. Intrahepatic cholestasis of pregnancy (ICP) group (n = 63, closed squares), pruritus gravidarum (PG) group (n = 43, open triangles) and control group (n = 26, open circles). Geometric mean ± SEM. PN, postnatal (4-6 weeks after delivery). When vertical error bar is not shown, the SEM value was less than the width of the symbol. Di, median gestational age at diagnosis of ICP; ***P < .001, comparison vs. control or PG.
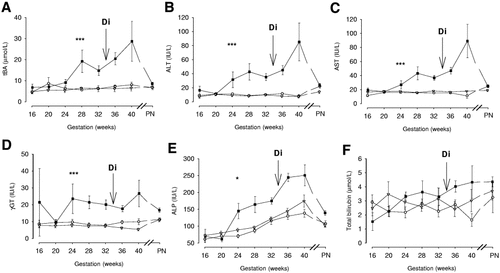
Longitudinal changes in plasma/serum standard biochemical tests of liver function during pregnancy: (A) tBA, (B) ALT, (C) AST, (D) γGT, (E) ALP, and (F) total bilirubin. Intrahepatic cholestasis of pregnancy (ICP) group (n = 63, closed squares), pruritus gravidarum (PG) group (n = 43, open triangles) and control group (n = 26, open circles). Geometric mean ± SEM. PN, postnatal (4–6 weeks after delivery). When vertical error bar is not shown, the SEM value was less than the width of the symbol. Di, median gestational age at diagnosis of ICP. *P < .05 and ***P < .001, comparison ICP vs. control or PG.
Results
Clinical Characteristics and Outcome.
The demographic and clinical characteristics of the women studied are shown in Table 1. The median gestation of clinical diagnosis of ICP was 33.6 (range, 12-40.7) weeks. Eighty-three percent of cases were diagnosed after 30 weeks of gestation. Only 2 cases were diagnosed at or before 24 weeks (12 and 24 weeks). The median gestation at onset of pruritus as recalled by women at recruitment was 30 (range, 4-39) weeks. The early samples were therefore predominantly from high-risk women before clinical onset of disease. Table 2 lists the number of samples obtained from each subject during the gestational period investigated. In the ICP group, data were available for 63 women (66 babies, three sets of twins). The following adverse events were recorded: postpartum hemorrhage (>500 mL blood loss), which occurred in 25% of ICP cases; meconium-stained liquor (14%); fetal distress (10%); preterm delivery (<37 weeks of gestation) (14%); and admission to a special care baby unit postpartum (11%). Thirty-one pregnancies (49%) resulted in no adverse events, with 1 adverse event in 33% (21 of 63), 2 in 13% (8 of 63), and 2 or more in 17% (11 of 63). There were no intrauterine deaths. Table 3 shows the concentrations of the liver function parameters measured in ICP according to adverse event(s).
| Variables | ICP n = 63 | PG n = 43 | Control n = 26 |
|---|---|---|---|
| Age, n | |||
| 19–35 yr | 49 | 37 | 21 |
| >35 yr | 14 | 6 | 5 |
| Age, yr ± SD | 31 ± 5 | 31 ± 4 | 31 ± 5 |
| Parity, n | |||
| 0 | 26 | 23 | 13 |
| >1 | 37 | 20 | 13 |
| Multiple gestation, n | |||
| Singleton | 60 | 42 | 26 |
| Twins | 3 | 1 | 0 |
| Ethnicity, n (%) | |||
| White European | 27 (43) | 23 (55) | 22 (85) |
| Black European | 1 (2) | 0 | 2 (8) |
| Black African | 5 (8) | 1 (2) | 0 |
| Black Caribbean | 4 (6) | 3 (7) | 0 |
| Indian | 4 (6) | 8 (19) | 0 |
| Bangladesh | 3 (5) | 3 (7) | 1 (4) |
| Pakistani | 7 (11) | 3 (7) | 0 |
| Asian other | 6 (10) | 0 | 0 |
| South American | 4 (6) | 0 | 0 |
| Other | 2 (3) | 2 (5) | 1 (4) |
| Maternal co-existing disease, n | |||
| Essential hypertension | 2 | 1 | 0 |
| Polycystic ovarian disease | 1 | 0 | 0 |
| Sjogrens. Anti-Ro Antibodies | 1 | 0 | 0 |
| Gestational age at delivery, wk ± SD | 37.6 ± 1.3 | 39.6 ± 1.7 | 39.9 ± 1.4 |
| No. (%) < 37 | 9 (14) | 2(5) | 0 (0) |
| Spontaneous | 3 | 2 | 0 |
| Iatrogenic | 6 | 0 | 0 |
| No. (%) ≥ 37 | 54 (86) | 41 (95) | 26 (100) |
| Spontaneous | 2 | 31 | 20 |
| Elective | 52 | 10 | 6 |
| Gestation (wk) | Subject Group | ||
|---|---|---|---|
| ICP (n = 63)* | PG (n = 43)* | Control (n = 26)* | |
| 16 ± 2 | 5 | 12 | 8 |
| 20 ± 2 | 9 | 9 | 11 |
| 24 ± 2 | 10 | 18 | 13 |
| 28 ± 2 | 20 | 23 | 17 |
| 32 ± 2 | 53 | 24 | 11 |
| 36 ± 2 | 61 | 24 | 19 |
| 40 ± 2 | 29 | 30 | 22 |
| PN | 63 | 34 | 16 |
- Abbreviation: PN, post-natal (4–6 weeks).
- * A minimum of 3 samples per subject were obtained.
| 0 Adverse Events, mean (95% CI) | 1 Adverse Event, mean (95% CI) | 2 Adverse Events, mean (95% CI) | ≥2 Adverse Events, mean (95% CI) | Comparison P Value | |
|---|---|---|---|---|---|
| GSTA, μg/L | 20.6 (14.6–29.2) | 36.8 (21.8–62.4) | 56.1 (29.6–106.5) | 42.8 (28.3–64.6) | *.072 |
| †<.01 | |||||
| ‡<.01 | |||||
| tBA, μmol/L | 24.4 (17.0–35.0) | 34.6 (24.5–48.8) | 28.5 (15.0–54.3) | 32.3 (23.6–44.3) | *.169 |
| †.676 | |||||
| ‡.244 | |||||
| ALT, IU/L | 49.5 (35.4–69.1) | 67.4 (44.0–103.2) | 99.6 (59.2–167.7) | 77.1 (55.1–107.8) | *.261 |
| †.028 | |||||
| ‡.066 | |||||
| AST, IU/L | 48.1 (36.4–63.4) | 62.0 (43.5–88.4) | 89.2 (61.5–129.2) | 70.6 (54.9–92.3) | *.263 |
| †.010 | |||||
| ‡.052 | |||||
| γGT, IU/L | 24.4 (17.4–34.2) | 21.2 (16.0–27.9) | 21.6 (13.6–34.4) | 21.3 (16.8–27.1) | *.519 |
| †.673 | |||||
| ‡.516 |
- Abbreviations: GSTA, glutathione S-transferase alpha; tBA, total bile acids; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γGT, γ-glutamyl transpeptidase.
- * 1 adverse event versus no adverse events.
- † 2 adverse events versus no adverse events.
- ‡ 2 adverse events versus no adverse events.
In the ICP group, no anti-smooth and/or anti-mitochondrial antibodies were detected, and there was no evidence of acute hepatic infection on serology or biliary obstruction on ultrasonography.
Glutathione S-Transferase Alpha.
The ICP group showed significantly higher serum GSTA concentrations compared with controls (400% difference; 95% CI, 240%-734%, P < .001) and the PG group (433% difference; 95% CI, 228%-790%, P < .001) at all gestational ages studied. However, GSTA concentrations were similar in control and PG groups (within 7%; 95% CI, –27%-20%, P = .614) (Table 4) and shared no gestation-related change. In the ICP group (see Fig. 1), serum GSTA concentrations increased significantly (P < .05) by 6.2% per week (95% CI, 1%-12.9%) from 20 ± 2 weeks, reaching maximal values at term. This amounted to a 3.4-fold (95% CI, 1.0-11.3-fold) increase over the last 20 weeks of gestation. Values were significantly greater in the ICP group, compared with the PG and control groups, by 24 weeks of gestation (with no overlap in 95% CI).
| ICP vs. Control | ICP vs. PG | PG vs. Control | ||||
|---|---|---|---|---|---|---|
| % Change (95% CI) | P Value | % Change (95% CI) | P Value | % Change (95% CI) | P Value | |
| GSTA | 400 (240 to 734) | <.001 | 433 (228 to 790) | <.001 | −7 (−27 to 20) | .614 |
| tBA | 161 (96 to 249) | <.001 | 186 (91 to 427) | <.001 | −8 (−24 to 10) | .355 |
| ALT | 344 (222 to 515) | <.001 | 431 (234 to 743) | <.001 | −16 (−33 to 5) | .122 |
| AST | 130 (89 to 179) | <.001 | 101 (56 to 158) | <.001 | 14 (1 to 29) | .029 |
| γGT | 176 (109 to 264) | <.001 | 255 (129 to 451) | <.001 | −22 (−40 to 0) | .050 |
| ALP | 47 (29 to 68) | <.001 | 30 (5 to 59) | .013 | 13 (−1 to 29) | .053 |
| Bilirubin | 20 (−21 to 83) | .381 | 8 (−43 to 104) | .821 | 12 (−17 to 51) | .467 |
- Abbreviations: GSTA, glutathione S-transferase alpha; tBA, total bile acids; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γGT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase.
Receiver operated characteristic curves were constructed to determine the optimal GSTA concentration as a biochemical marker for ICP in women presenting with pruritus. At first available sample (obtained within 2 weeks) after pruritus was reported, a GSTA concentration of 5.5 μg/L had a sensitivity of 84% (95% CI, 73%-92%), specificity of 81% (95% CI, 67%-92%), positive predictive value for identification of disease of 87% (95% CI, 76%-94%), negative predictive value of 78% (95% CI, 63%- 89%), and a likelihood ratio of 4.5 for a positive test that differentiated ICP from PG. Of the 9 high-risk women in whom blood samples were obtained before diagnosis of ICP (using the current definition), 8 (88.9%) had at least one positive (>5.5 μg/L) GSTA measurement.
Total Bile Acids.
In the ICP group, serum tBA values increased 2.6-fold (95% CI, 1.4-4.7-fold) from 20 weeks of gestation until term (Fig. 2A). During the gestational period studied, tBA values were substantially (P < .001) higher in ICP cases with concentrations 161% (95% CI, 96%-249%) and 186% (95% CI, 91%-427%) greater than control and PG groups, respectively (Table 4). Concentrations of tBA were greater from 28 weeks of gestation with no overlap of 95% CI in the ICP compared with the control or PG groups. However, mean values were not consistently over the reference value of 14 μmol/L until after 32 ± 2 weeks of gestation and, at the time of appearance of first clinical symptoms, concentrations were not always elevated above this reference value. There was no statistically significant difference in tBA concentrations or change with gestation between the control and PG groups. Mean concentrations in the control group and PG group were 5.6 μmol/L (95% CI, 5.2-6.7; range, 2.2-11.4 μmol/L) and 5.8 μmol/L (95% CI, 5.3-6.7; range, 2.2-11.5 μmol/L), respectively.
Alanine Aminotransferase.
Plasma ALT activities over the gestation studied were significantly higher in the ICP group compared with the control (344%; 95% CI, 222%-515%) and PG (431%; 95% CI, 234%-743%) groups (Table 4). Plasma activities rose progressively with gestation from 20 ± 2 weeks in the ICP group, increasing 3.6 times (95% CI, 1.8-7.1 times), and reached maximal values at term (see Fig. 2B). No difference (P = .122) in ALT levels between PG (mean, 9 IU/L; 95% CI, 8-10; range, 2-32 IU/L) and control (mean, 10 IU/L; 95% CI, 9-11; range, 2-32 IU/L) groups was detected, and values remained stable with gestation in both groups. Although a detectable difference could be established from 24 ± 2 weeks of gestation in the ICP group, mean values were not elevated beyond the reference value (Table 5) until 28 ± 2 weeks of gestation compared with the other groups.
| Test | ICP With 80% of Abnormal Values (%)* | ICP With Abnormal Values (%) | Definition of Abnormal Value† | Median | 95% CI | Range |
|---|---|---|---|---|---|---|
| GSTA | X | 94 | ≥5.5 μg/L | 26.2 | 15.1–43.8 | 5.5–143 |
| tBA | X | 63 | ≥14 μmol/L | 19 | 14–38 | 4–190 |
| ALT | 64 | 54 | ≥55 IU/L | 63 | 45–109 | 6–339 |
| AST | 80 | 69 | ≥35 IU/L | 62 | 41–73 | 13–307 |
| γGT | 18 | 11 | ≥72 IU/L | 19 | 14–31 | 7–338 |
| ALP | X | 95 | ≥116 IU/L | 240 | 231–258 | 72–540 |
| Bilirubin | 2 | 2 | ≥22 μmol/L | 5 | 4–7 | 1–38 |
| Albumin | X | 6 | <35 g/L | 39 | 39–40 | 33–46 |
| Protein | X | 34 | <64 g/L | 67 | 65–69 | 3–95 |
- NOTE. X, reference value was already derived for use in pregnancy or no pregnancy adjusted value was available.
- Abbreviations: GSTA, glutathione S-transferase alpha; tBA, total bile acids; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γGT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase; bilirubin, total bilirubin; protein, total protein.
- * 80% of non pregnant range as suggested by Girling et al. (1997) for pregnancy specific ranges.14
- † Based on non-pregnant reference ranges used at St. Thomas' Hospital, except tBA range quoted by Selly Oak Hospital Birmingham (non-fasted) and GSTA derived from ROC curve analysis optimising for sensitivity and specificity; see text.
Aspartate Aminotransferase.
In ICP cases, plasma AST activity increased with gestation by 2.9 times (95% CI, 1.9-4.4 times), peaking at term (Fig. 2C), and was significantly (P < .001) greater than other groups (difference from controls, 130% [95% CI, 89%-179%], and from the PG group, 101% [95% CI, 56%-158%], see Table 4). Mean values exceeded the reference value (Table 5) from 28 ± 2 weeks, although a significant difference was observed from 24 ± 2 weeks gestation between ICP cases and the other two groups. AST did not change with gestation in the control (mean, 15 IU/L; 95% CI, 14-16; range, 2-27 IU/L) (P = .983) or PG (mean, 17 IU/L; 95% CI, 17-19; range, 8-27 IU/L) (P = .801) groups. However, a slight but significant difference (P = .029) was detected between the control and PG groups throughout pregnancy (Table 4).
γ-Glutamyl Transferase.
Serum γGT activity remained stable throughout gestation in the control group and fell from 32 ± 2 weeks in the PG group (Fig. 2D). Overall, serum γGT activities in the ICP group remained constant (P = .373) over the gestational range studied, apart from a transient decrease at 20 weeks, but were significantly greater (Table 4) than controls (176%; 95% CI, 109%-264%) and PG groups (255%; 95% CI, 129%-451%) throughout pregnancy.
Alkaline Phosphatase.
Plasma ALP activities increased progressively from 20 ± 2 weeks until term in the ICP, PG, and control groups (see Fig. 2E). However, the increment was considerably greater in the ICP group (47%, 95% CI, 29%-68%; and 30%, 95% CI, 5%-59%) compared with the control and PG groups, respectively (Table 4). No statistical difference was detected between control and PG groups (P = .053).
Total Bilirubin.
Total bilirubin concentrations showed marked variation between subjects in each group (see Fig. 2F). Although values were higher in ICP cases, no significant difference was detected between groups (see Table 4). However, the mean total bilirubin concentration showed a significant increase (P < .01) with gestation in the ICP group, represented by a 2.5-fold (95% CI, 1.3- to 4.6-fold) rise during the study period. In contrast, no significant change with gestation was detected in the control or PG groups.
Albumin and Total Protein.
Serum albumin values significantly decreased (P < .01) with gestation in all groups, but were attenuated greater in the ICP group compared with controls (by −1.3 [95% CI, −2.4 to −0.3] g/L; P = .015). Albumin concentrations were not significantly different in the ICP group compared with PG (difference of −0.5 [95% CI, −2.4 to 1.5] g/L; P = .633) or PG versus control (difference of −0.9 [95% CI, −2.0-0.3] g/L; P = .143) groups during the pregnancy period investigated. Although total protein concentrations were higher in ICP cases across gestation, this did not reach statistical significance compared with control (difference of 2.5 [95% CI, −2.7 to 7.7] g/L; P = .344) or PG (difference of 4.0 [95% CI, −5.4 to 13.4] g/L; P = .403) groups. No difference was observed between PG and control (difference, −1.5 [95% CI, −6.8 to 3.8] g/L; P = .575) total protein concentrations during the study period. Total protein concentrations significantly fell with advancing gestation in controls (−18.3 [95% CI, −29.6 to −6.9] g/L; P = .002), but remained stable in ICP (P = .668) and PG (P = .091) groups.
Relationship Between Different Liver Function Markers in the ICP Group.
Correlations between all the biochemical parameters in the ICP group were investigated by using single measurements obtained on the day of delivery. There was a highly significant (P < .001) positive correlation between serum concentrations of GSTA and tBA (Spearman's rank correlation, r = 0.62), ALT (r = 0.76), and AST (r = 0.75) with a lower but significant (P < 0.01) association with total bilirubin (r = 0.33) and total protein (r = 0.24). Total bilirubin was also significantly (r = 0.42-0.51, P < .001) correlated to markers of liver function (tBA, ALT, AST). Similarly, a strong (P < .001) positive correlation was observed between tBA and ALT (r = 0.65), AST (r = 0.66), and ALP (r = 0.56), but not to γGT (r = 0.12, P = .197). A positive (P < .01) correlation existed between severity of itch and tBA (r = 0.27), ALT (r = 0.27), AST (r = 0.24), γGT (r = 0.26), and ALP (r = 0.24). There was no association of liver function markers with pruritus in the PG group.
Postpartum Biochemical Characteristics.
All aberrations in liver biochemistry resolved to within normal reference limits by 4 to 6 weeks postpartum in the ICP group (see Figs. 1, 2).
Discussion
This study presents a prospective longitudinal evaluation of GSTA and liver function in ICP and PG. In ICP, we report substantive increases in the cytosolic, mitochondrial, and membrane-associated enzymes studied compared with both PG and control groups.
GSTA has been reported to be a more specific and sensitive serum indicator of hepatic dysfunction than ALT, ALP, and AST,11-13 because it exhibits a specific but ubiquitous hepatic distribution (2%-5% of soluble cytosolic protein in hepatocytes), has a short serum in vivo half life (< 1 hour) and is rapidly and substantively released into the circulation in large amounts after acute hepatocellular damage.12, 17, 18 Longitudinal assessment of GSTA in the current study clearly showed that this liver enzyme is a novel and useful serum indicator of liver dysfunction in ICP. GSTA concentrations were significantly elevated in ICP pregnancies, compared with women with PG and normal pregnant controls, and correlated with changes in conventional indicators of liver dysfunction. Furthermore, using the cutoff value derived in this study, GSTA was raised in 94% of women in the ICP group at the time of diagnosis. This compares favorably with the triad of liver function markers (ALT, AST, and tBA) routinely used to diagnose ICP, which individually only identified 63% to 80% of women. The upper normal reference value for GSTA determined in our study (5.5 μg/L) is in agreement with Mulder et al., who derived a value of 5.9 μg/L from plasma samples of 350 healthy nonpregnant controls.19 Elevated GSTA serum concentrations were also detected from 24 weeks of gestation, and 88.9% of the high-risk women who developed ICP had an abnormal value before diagnosis. This suggests that evaluation of GSTA may allow for earlier diagnosis of ICP. Although this may in part be because of the limitations of the reference ranges currently employed for aminotransferases (see later discussion), our data strongly suggest that assessment of serum GSTA concentrations in pregnancy may provide significant clinical benefits over conventional tests in ICP. In particular, GSTA provides a potentially useful tool for distinguishing between women in early pregnancy who report pruritus in the absence of conventional liver dysfunction (but who subsequently develop ICP)8 and women with benign PG. Since as many as 1 in 5 women report pruritus in pregnancy,9, 10 earlier identification of women at risk for ICP could have economic benefits by targeting surveillance and thereby reducing the time through which pruritic women would require intensive monitoring. Early detection also could impact on perinatal morbidity and mortality. As several inexpensive methodologies exist (e.g., enzyme-linked immunosorbent assay and immunofluorometric assays) for the evaluation of GSTA in the routine clinical setting, its determination could be both efficient and cost effective. A larger prospective cohort study in unselected pregnant women is now indicated to fully evaluate the clinical use of GSTA in ICP.
Controversy exists as to which conventional biochemical test is the most sensitive in ICP, although ALT and tBA are generally accepted to be the most reliable markers for diagnosis.2;20-22 Overall, ALT, AST, and to a much lesser extent, ALP were good indicators of disease in this study. The lower percentage of ICP cases identified by ALT and AST compared with GSTA may reflect the high specificity of GSTA for hepatocellular damage and the relatively wider tissue distribution of the others.12, 13, 23 This also may account for the small but significant difference between AST values in the control and PG groups across pregnancy. The high values of ALP in all groups is considered to represent the placental contribution to plasma concentrations and thus limits the use of ALP in pregnancy. Longitudinal evaluation showed the conventional diagnostic liver function markers (ALT, AST, and γGT) were significantly higher than controls (or PG) much earlier in ICP than indicated by current reference values (adjusted for pregnancy14). This implies that reference values should be reevaluated in pregnancy to facilitate earlier diagnosis of the disease and that serial measurements be considered for women reporting pruritus.
Serum tBA was also a good biomarker of ICP, but significant elevations in concentrations of this parameter occurred later in gestation (28 weeks as opposed to 24 weeks) than that observed for GSTA and aminotransferases. The delayed elevation of tBA compared with ALT, AST, and GSTA contrasts with reports suggesting that tBA is the first parameter to increase.20, 21, 24, 25 We found tBA to be increased in 63%, ALT in 64%, and AST in 80% of women with ICP (at diagnosis), whereas others have reported tBA and ALT to be elevated in 92% to 100% and 50% to 100% of ICP cases, respectively.1, 20, 21, 26 Part of this variability highlights the problem of a consistent definition for ICP, particularly in earlier series. The magnitude of elevations in tBA were lower than those of ALT activity or GSTA concentrations in our study, further supporting the use of the latter. It has been suggested that a diagnosis of ICP can be made clinically on pruritus alone in pregnancy.27, 28 In contrast, the current study confirms that pregnancy-associated pruritus, PG, does not result in biochemical cholestasis.29
Conflicting reports on the correlations of tBA with liver function have been published. Raised tBA may reflect another aspect of the disease process in ICP and could provide additional diagnostic information.26, 30, 31 Our data show a positive correlation of tBA with other markers of liver function, suggesting that tBA provides similar information. Bile acid–induced toxicity may play a role with adverse events in ICP,26, 32-35 therefore, tBA evaluation has been suggested to be useful in predicting adverse outcome. However, comparison of mean concentrations of liver biochemical parameters with respect to adverse events in ICP showed that GSTA was the best discriminator of adverse outcome. GSTA also could prove to be useful in predicting adverse outcome and in the management of affected pregnancies.
The usefulness of γGT for the diagnosis of ICP is debatable. In the current study, γGT was only increased in 18% of ICP cases. Some authors have suggested that elevated serum activities of γGT are exceptional in ICP6; however, in our study, γGT activities were consistently higher than in PG or control groups, suggesting that the reference value used may be inappropriate. Previously, γGT has been reported to be elevated in 30% to 50% of women with ICP when substantially lower reference ranges were used (between 17 and 35 IU/L).26, 36-38
Suppression of γGT synthesis in pregnancies complicated by ICP has also been suggested39 but is not supported by our data. The lower percentage of women diagnosed with abnormal γGT compared with other liver function markers may reflect heterogeneity in the underlying cause of the disease. Recent reports show that mutations in genes coding for a range of transporter proteins (including multidrug resistant 3) and progressive familial intrahepatic cholestasis type 3, may predispose to elevated γGT concentrations and ICP.37, 40 However, until reference ranges for γGT are standardized and related to relevant gene mutation frequency in ICP, this issue will remain unresolved.
Only 2% of ICP cases showed an elevation in the total bilirubin concentration and, despite a rise with gestation, there was no significant difference between the groups, which confirms results from previous studies.1, 26 There are reports of elevated bilirubin concentrations in ICP, predominantly association with a rise in conjugated bilirubin (61%-91% of cases).21, 41, 42 In the current study, total bilirubin showed a positive correlation with GSTA, tBA, ALT, and AST and a weaker association with ALP, suggesting bilirubin is influenced by ICP. However, it clearly has no value in the early diagnosis or follow-up of women with this disorder.
Serum albumin concentrations were attenuated with advancing gestation in all groups, and both albumin and total protein significantly increased postnatally, reflecting hemodilution in pregnancy. Albumin concentrations were significantly lower in the ICP group compared with controls, as reported previously.43 This may be a consequence of impaired hepatic function in ICP but is of little diagnostic value.
This comprehensive longitudinal study of liver function in women with ICP has clearly demonstrated that a detailed assessment of liver biochemistry provides a means by which to distinguish between diagnoses of ICP and PG. The study suggests a need to reassess the current approach for the diagnosis of ICP. Since pruritus can predate derangements in tBA, aminotransferases, and γGT, the measurement of GSTA could contribute to targeted clinical surveillance and management in high-risk pregnancies. The serial measurement of liver function markers in women reporting pruritus is recommended to correctly identify ICP. Furthermore, reference ranges for conventional liver function markers require reevaluation during pregnancy. In conclusion, GSTA may prove to be the most sensitive and specific marker of early disease, and increased concentrations appear to be a better indicator of disease severity and adverse outcome.
Acknowledgements
The authors thank all the women and clinical staff who participated in the study. We are particularly grateful for the input and support of Joanna Girling, Dr. Catherine Nelson-Piercy, and Dr. Catherine Williamson.




