Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma
Abstract
Outcome predictors in patients with hepatocellular carcinoma (HCC) who are treated with percutaneous ablation are ill defined, and it is unknown if successful therapy is associated with improved survival. In our study, 282 cirrhotic patients with early nonsurgical HCC were treated with percutaneous ablation during a 15-year period. Single tumors were seen in 244 patients, and 2 to 3 nodules were seen in 38 patients. Initial complete response was achieved in 192 patients and was independently related to the size of the main tumor (P = .015) and tumor stage (P = .0001) (≤2 cm, 96%; 2.1-3 cm, 78%; >3 cm, 56%; 2-3 nodules, 46%). At the end of follow-up, 80 patients presented sustained complete response. The 1-, 3-, and 5-year survival rates were 87%, 51%, and 27%, respectively. The independent predictors of survival were Child-Turcotte-Pugh class (P = .0001) and initial complete response (P = .006). Child-Turcotte-Pugh class A patients with initial complete response achieved 42% survival at 5 years; this figure increased to 63% in patients with tumors 2 cm or smaller. In conclusion, our results demonstrate that initial complete response to percutaneous ablation is associated with an improved survival in both Child-Turcotte-Pugh class A and B patients with nonsurgical HCC. Accordingly, initial complete tumor necrosis should be considered a relevant therapeutic target irrespective of tumor size and liver function. (HEPATOLOGY 2004;40:1352–1360.)
Hepatocellular carcinoma (HCC) is a major health problem with a rising incidence worldwide.1 Surveillance plans in cirrhotics detect 30%-40% of patients at an early stage (single HCC ≤ 5 cm or 3 nodules ≤ 3 cm) when they can be treated with resection, liver transplantation (LT) or percutaneous ablation. Surgery is considered the first option and may offer 5-year survival rates above 50%.2-5 Percutaneous ablation, usually by percutaneous ethanol injection (PEI) or radiofrequency (RF), is proposed to nonsurgical patients,6 and encouraging results have been reported both in terms of treatment response (above 70% in tumors ≤ 3 cm7 but under 50% in tumors > 3 cm or multinodular)7-9 and survival (around 50% at 5 years in Child-Pugh A patients).2, 8-10 This has prompted some authors to suggest that percutaneous ablation could be preferred to resection and even avoid LT. However, this major change in the current treatment strategy should be based on randomized clinical trials comparing the available therapies. Because these studies are not available, the only tool for decision-making will come from the evaluation of large cohorts of treated patients in whom the efficacy of ablation has been assessed both immediately after treatment and in the long term.11 It should be noted that while the definitions to be used are well established for resection, the terms used after ablation are misleading. Consequently, some tumors initially thought to be completely necrotic after ablation display viable tumor nests during long follow-up; this is frequently described as local recurrence or local progression.12 However, it should be termed lack of local disease control or treatment failure, thus matching incomplete resection with residual disease. Furthermore, the mere description of survival without assessment of the major predictive factors of survival has limited value, because it is not feasible to identify the subgroup of individuals that benefit the most from therapy.
The current study defines all these critical aspects in a series of 282 patients treated with percutaneous treatments (PEI or RF) aiming to provide a rationale for establishing the optimal therapeutic policy.
Abbreviations:
HCC, hepatocellular carcinoma; LT, liver transplantation; PEI, percutaneous ethanol injection; RF, radiofrequency ablation; TAE, transarterial embolization; CT, computerized tomography; CR, complete response.
Patients and Methods
This study included 282 consecutive patients who were diagnosed, staged, and treated percutaneously in our Liver Unit during a 15-year period. Patients with early HCC (single nodule ≤ 5 cm or three nodules ≤ 3 cm each) were considered first for resection or transplantation. After 1995, we selected for resection only those subjects who had solitary HCC with normal bilirubin and absence of significant portal hypertension.13 Patients who did not fit into the surgical criteria were considered for percutaneous ablation. RF was initiated in 1998 and was considered the first technique in the absence of contraindications. These included cardiac arrhythmia, tumor location close to the perihiliar hepatic region, heart, gallbladder or stomach and bowel, tense ascites, and impaired clotting tests that precluded application of the RF. After 2001, contraindications also included subcapsular location of HCC impeding the puncture through a rim of nontumoral liver to prevent peritoneal seeding.14 Patients with large/ multinodular tumors were evaluated for transarterial chemoembolization or treatment with investigational agents. End-stage patients received symptomatic treatment.15
From May 1987 to March 2002, 2,114 HCC patients were evaluated. Among them, 592 (28%) received potentially curative treatments: resection (n = 124, 6%), LT (n = 186, 9%), or ablation (n = 282, 13%).
Overall, 203 patients received PEI and 49 received RF; 30 patients with single 3- to 7-cm nodules were treated with transarterial embolization (TAE) followed by PEI (TAE-PEI) in an unpublished phase II study. Patients receiving percutaneous ablation upon enlistment for LT were excluded.
Table 1 depicts patient characteristics. Mean age was 67 ± 7 years, and 181 patients were male. All had underlying cirrhosis due to hepatitis C virus (n = 234), hepatitis B virus (n = 11), alcohol use (n = 17), or other factors (n = 20). One hundred ninety-seven patients were Child-Turcotte-Pugh class A. Two hundred forty-four patients presented with solitary tumors (≤2 cm, n = 52; 2.1-3 cm, n = 99; 3.1-5 cm, n = 82; >5 cm, n = 11), and 38 had 2 to 3 lesions (≤3 cm, n = 24; 3.1-4 cm, n = 14).
| Variables | All Patients (n = 282) | PEI (n = 203) | RF (n = 49) | TAE-PEI (n = 30) | P Value |
|---|---|---|---|---|---|
| Age (yr) | 67 ± 7 | 67 ± 8 | 67 ± 8 | 67 ± 6 | NS |
| Sex (M/F) | 181/101 | 133/70 | 30/19 | 18/12 | NS |
| Etiology | |||||
| HCV | 234 | 167 | 39 | 28 | NS |
| HBV | 11 | 8 | 3 | — | |
| Alcohol | 17 | 12 | 4 | 1 | |
| Other | 20 | 16 | 3 | 1 | |
| Child-Turcotte-Pugh class (A/B) | 197/85 | 141/62 | 34/15 | 22/8 | NS |
| Ascites (yes/no) | 95/187 | 72/129 | 16/33 | 7/23 | NS |
| Bilirubin (mg/dL) | 1.4 ± 0.7 | 1.4 ± 0.7 | 1.6 ± 0.8 | 1.2 ± 0.4 | NS |
| Albumin (g/L) | 36 ± 5 | 36 ± 6 | 37 ± 5 | 36 ± 5 | NS |
| Prothrombin act (%) | 77 ± 15 | 77 ± 15 | 78 ± 17 | 82 ± 13 | NS |
| BUN (mg/dL) | 20 ± 9 | 20 ± 9 | 19 ± 8 | 18 ± 5 | NS |
| AFP (ng/mL) | NS | ||||
| ≤20/21-100/>100 | 189/45/31 | 137/31/22 | 32/7/6 | 20/7/3 | |
| Size of main tumor (mm) | 30 ± 10 (10–70) | 28 ± 8 (10–50) | 29 ± 9 (16–50) | 47 ± 11 (31–70) | .0001 |
| Tumor stage (cm) | .0001 | ||||
| Single (≤2/2.1–3/3.1–5/>5) | 52/99/82/11 | 41/82/51/— | 11/17/12/— | —/—/19/11 | |
| Multinodular | 38 | 29 | 9 | — | |
| Differentiation degree | NS | ||||
| Well/Moderate/Poor | 110/79/10 | 80/56/6 | 27/11/2 | 3/12/2 | |
| Okuda stage (I/II) | 172/110 | 116/87 | 35/14 | 21/9 | NS |
- Abbreviations: NS, not significant; M, male; F, female; HCV, hepatitis C virus; HBV, hepatitis B virus; BUN, blood urea nitrogen; AFP, alpha-fetoprotein.
Diagnosis was confirmed via needle biopsy in 227 patients or via noninvasive criteria11 in 55 patients. Biopsy was performed in other centers in 28 patients. Information regarding differentiation degree was not registered and was not rechecked. Disease extension was assessed using ultrasound and computed tomography (CT).
Treatment Procedures
Percutaneous Ethanol Injection.
Patients were hospitalized and ethanol injection was performed on separate days under real-time ultrasound guidance and local anesthesia. A 22-gauge needle was advanced into the tumor and absolute alcohol was injected. Injections were repeated in different tumor areas until complete tumor infiltration was achieved. The procedure was repeated for up to 4 or 5 sessions completing 1 treatment cycle. One month after discharge a CT scan was performed. If viable residual tumor was detected, another treatment cycle was given, up to a maximum of 3 cycles. If the tumor was still viable after the third cycle, PEI was considered to have failed. Overall, 203 patients treated with PEI received 1.6 ± 0.7 cycles, including 7 ± 4 sessions with a mean volume of alcohol of 22 ± 13 cm3.
Radiofrequency Thermal Ablation.
RF was performed under conscious sedation and ultrasound guidance. Two instruments were used: a 17-gauge internally cooled-tip electrode with an exposed tip of 2 or 3 cm connected to an electrical generator (100 W) (Radionics, Inc., Burlington, MA) and a 10–curved prong device (RF 2000 system, RadioTherapeutics Corp., Mountain View, CA). For tumors 2 cm or smaller in diameter, the electrode was placed into the center of the lesion. For tumors 2.1 to 5 cm in diameter, several insertions were made (mean insertions, 2.1 ± 1.2). Tissue impedance was monitored and the maximum power was adjusted accordingly. At the end of tumor ablation, thermocoagulation was performed along the needle track. Another RF session was performed if CT revealed a residual tumor. Overall, 49 patients treated with RF received 1.4 ± 0.6 cycles; 22 of these patients also received PEI sessions to ablate minor residual foci after initial RF.
Arterial Embolization and Ethanol Injection.
Thirty patients were treated with TAE-PEI. TAE was performed as described elsewhere16: after identifying the nodule, the catheter was advanced to obtain the most selective arterial occlusion. At that level small gelatin cubes (1 × 1 mm) were injected without associated chemotherapy. PEI was performed 1 month after TAE and repeated until complete tumor infiltration was achieved (number of sessions, 7 ± 3; mean volume of alcohol, 23 ± 2 cm3; cycles, 1.5 ± 0.6).
Assessment of Treatment Response and Follow-Up
Treatment response was defined following the World Health Organization (WHO) criteria taking into account tumor necrosis.11
Complete response (CR) was defined as the absence of enhanced tumoral areas reflecting complete tissue necrosis assessed by CT scan 1 month after treatment. Initial CR refers to the status after completing all treatment sessions. Sustained CR refers to the status of being free of disease at the end of follow-up.
Initial treatment failure was defined as the presence of viable tumor at the end of treatment. Late treatment failure refers to the detection of viable tumor during follow-up either within the treated nodule or within 2 cm of the surrounding parenchyma as previously reported.17, 18 Therefore, treatment failure includes early and late failure and represents the failure of treatment to achieve local disease control. Disease recurrence was defined as the appearance of HCC foci beyond this area in the absence of failure.
Patients were followed every 3 months by means of clinical examination, blood analysis including alpha-fetoprotein and imaging techniques (ultrasound and spiral CT, alternatively). Upon detection of failure or recurrence, patients were considered for new treatment sessions.
Statistical Analysis
The main end point of the study was survival. Secondary end points were treatment efficacy and recurrence. Baseline characteristics of patients are expressed as mean ± SD. Comparison among groups was performed using ANOVA for continuous variables and the chi-square test with Yates correction for categorical variables. Follow-up length and survival are expressed as the median (range). Follow-up was computed as starting from the beginning of the treatment and was maintained until death or the last visit before September 30, 2002.
Predictors of initial treatment efficacy were identified via logistic regression. Cox regression was used to identify predictors of survival, sustained CR, late and overall treatment failure, and recurrence. Probability curves obtained via the Kaplan-Meier method were compared using the Mantel-Cox test. Twenty-four variables were assessed in the univariate analysis: sex; age; cause of underlying cirrhosis; type of treatment; Okuda stage; Child-Turcotte-Pugh class; ascites; previous decompensation; differentiation degree; tumor stage (single ≤ 2 cm, 2.1-3 cm, > 3 cm; multinodular); alpha-fetoprotein levels, both as a continuous variable and categorized (≤20/21-100/>100 ng/mL); prothrombin activity; platelets; creatinine; blood urea nitrogen; bilirubin; albumin; γ-glutamyl transpeptidase; alanine aminotransferase; aspartate aminotransferase; alkaline phosphatase; size of main tumor, both continuous and categorical (≤2, 2.1-3, 3.1-5, >5 cm); initial CR (vs. failure); and sustained CR (vs. initial/late failure or recurrence). For continuous variables, the cutoff level was their median value. Significant variables (P < .05) were included in a stepwise Cox regression analysis. Probability of recurrence or late failure was calculated in patients with initial CR in whom one of these events occurred as the first event during follow-up. Calculations were performed with SPSS package (SPSS Inc., Chicago, IL).
Results
Treatment Efficacy
Assessment of treatment efficacy was feasible in 276 patients; those who died within the first 30 days (n = 5) or who were lost to follow-up (n = 1) were excluded. Overall, 192 patients (70%) achieved an initial CR, 144 patients (73%) were treated with PEI, 31 (65%) were treated with RF, and 17 (57%) were treated with TAE-PEI (Table 2). Among patients who achieved an initial CR, 80 had a sustained CR (61 PEI, 15 RF, and 4 TAE-PEI), 76 had late treatment failure, and 36 had recurrence (Fig. 1).
| According to Treatment | All Patients N = 276 | PEI n = 198 | RF n = 48 | TAE-PEI n = 30 | ||
|---|---|---|---|---|---|---|
| Initial response | ||||||
| Complete response | 192 (70%) | 144 (73%) | 31 (65%) | 17 (57%) | ||
| Initial failure | 84 (30%) | 54 (27%) | 17 (35%) | 13 (43%) | ||
| Response end follow-up | ||||||
| Late failure | 76 (22%) | 55 (28%) | 11 (23%) | 10 (33%) | ||
| Recurrence | 36 (28%) | 28 (15%) | 5 (10%) | 3 (10%) | ||
| Sustained CR | 80 (29%) | 61 (31%) | 15 (31%) | 4 (13%) | ||
| Failure/recurrence | 196 (71%) | 137 (69%) | 33 (69%) | 26 (87%) | ||
| According to Size/Stage | ≤2 cm (n = 52) | 2.1–3 cm (n = 96) | 3.1–5 cm (n = 80) | >5 cm (n = 11) | Multinodular (n = 37) | |
| Initial response | ||||||
| Complete response (n = 192) | 50 (96%) | 75 (78%) | 45 (57%) | 5 (45%) | 17 (46%) | |
| Initial failure (n = 84) | 2 (4%) | 21 (22%) | 35 (43%) | 6 (55%) | 20 (54%) | |
| Response end follow-up | ||||||
| Late failure (n = 76) | 16 (31%) | 29 (30%) | 21 (26%) | 5 (45%) | 5 (13%) | |
| Recurrence (n = 36) | 11 (21%) | 14 (15%) | 8 (11%) | 0 (0%) | 3 (8%) | |
| Sustained CR (n = 80) | 23 (44%) | 32 (33%) | 16 (35%) | 0 (0%) | 9 (24%) | |
| Failure/recurrence (n = 196) | 29 (56%) | 64 (67%) | 64 (65%) | 11 (100%) | 28 (76%) | |

Study flowchart showing overall treatment efficacy. *Four patients retreated achieved CR at the end of follow-up. **Eight patients retreated achieved CR at the end of follow-up. CR, complete reponse.
Initial CR was significantly associated with tumor stage, size of main tumor, differentiation degree, γ-glutamyl transpeptidase, and prothrombin activity. Size of the main tumor (odds ratio, 1.04; 95% CI, 1.01-1.07; P = .015) and tumor stage (odds ratio, 2.18; 95% CI, 1.50-3.17; P = .0001) were independent predictors of initial CR. This varied according to tumor stage: tumors 2 cm or smaller, 96% (50 of 52 patients); tumors 2.1-3 cm, 78% (75 of 96 patients); tumors 3.1-5 cm, 57% (46 of 80 patients); tumors larger than 5 cm, 45.5% (5 of 11 patients); and multinodular, 46% (17 of 37 patients) (see Table 2). There were no differences among the type of treatments.
Late treatment failure in the absence of distant recurrence was registered in 76 patients, this representing a 1-, 3-, and 5-year probability of 26%, 56%, and 74%, respectively. Only 8 out of 41 retreated cases achieved CR after repeated therapy.
Recurrence in the absence of failure was registered in 36 patients. Sixteen of these patients were retreated, but only 4 remained free of disease at the end of follow-up. Probability of recurrence at 1, 3, and 5 years was 16%, 36%, and 51%, respectively (Fig. 2). Male sex (odds ratio, 2.8; 95% CI, 1.19-6.38; P = .017) and cirrhosis associated with hepatitis C virus (odds ratio, 5.7; 95% CI, 1.36-24.08; P = .017) were independent predictors of recurrence. Consequently, male patients with hepatitis C virus infection (n = 59) had a probability of recurrence of 25%, 50%, and 77% at 1, 3, and 5 years, respectively.
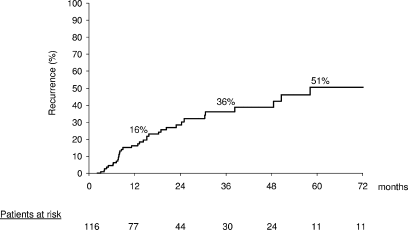
Actuarial probability of recurrence after initial CR.
Sustained CR was achieved at the end of follow-up in 80 patients. Probability of treated patients to achieve and maintain a sustained CR at 1, 3, and 5 years was 87%, 54%, and 31%, respectively. Sustained CR was independently associated with tumor stage (odds ratio, 1.53; 95% CI, 1.2-1.8; P = .0001), hepatitis C virus infection (odds ratio, 1.79; 95% CI, 1.04-3.07; P = .017) and baseline alpha-fetoprotein levels (odds ratio, 1.4; 95% CI, 1.4-1.82; P = .012).
Survival.
After a median follow-up of 25 months (range, 0.1-151), 1-, 3-, and 5-year survival was 87%, 51%, and 27%, respectively, (median survival, 36 months) (Fig. 3). There were 5 deaths (1.7%) within the first 30 days. One patient presented with arterial mesenteric vascular occlusion unrelated to ethanol injection, another patient with minor hemoperitoneum suffered massive myocardial infarction, and a third one died of cardiac arrhythmia. Finally, two early deaths were due to progressive liver failure not related to treatment.
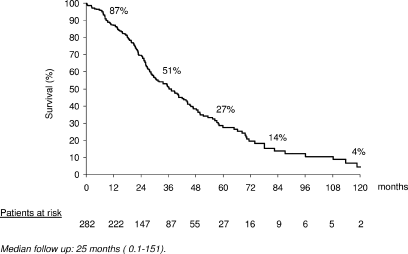
Overall probability of survival.
At the time of the analysis, 162 patients had died and 17 (6%) had been lost to follow-up. Death was due to tumor progression in 97 cases (60%) and to liver failure in 43 (27%), without differences among treatments. Death related to liver failure was significantly more frequent in Child-Turcotte-Pugh B patients (38%) compared with Child-Turcotte-Pugh A (18%) (P = .009). Similarly, tumor-related death was more frequent in patients with initial treatment failure than in patients with initial CR (80% vs. 53%; P = .0001).
Predictors of Survival
Twelve variables were associated with survival. They reflected liver function (Child-Turcotte-Pugh class, ascites, previous decompensation, prothrombin activity, albumin, blood urea nitrogen), tumor extent (tumor stage, size of main tumor, alpha-fetoprotein, differentiation degree, Okuda stage) and initial CR. Multivariate analysis disclosed 3 independent predictors: Child-Turcotte-Pugh class (odds ratio, 1.9; 95% CI, 1.3-2.9; P = .0001), initial CR (odds ratio, 1.71; 95% CI, 1.1-2.5; P = .006) and blood urea nitrogen (odds ratio, 1.54; 95% CI, 1.1-2.2; P = .02) (Table 3).
| Variable | Values | Patients | Median Survival (months) | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|---|
| Univariate analysis | |||||
| Liver function | |||||
| Previous decompensation | Yes | 117 | 28 | .0038 | |
| No | 159 | 42 | |||
| Ascites | Yes | 93 | 26 | .0041 | |
| No | 183 | 40 | |||
| Child-Turcotte-Pugh class | A | 194 | 44 | .00001 | |
| B | 82 | 26 | |||
| Albumin (g/L) | ≤36 | 149 | 30 | .0073 | |
| >36 | 119 | 44 | |||
| BUN (mg/dL) | ≤17 | 131 | 50 | .006 | |
| >17 | 120 | 32 | |||
| Prothrombin activity (%) | ≤78 | 135 | 31 | .013 | |
| >78 | 140 | 42 | |||
| Tumor status | |||||
| AFP (ng/mL) | ≤20 | 186 | 44 | .0086 | |
| 21–100 | 44 | 23 | |||
| >100 | 31 | 31 | |||
| Tumor stage (cm) | ≤2 | 52 | 50 | .0046 | |
| 2.1–3 | 96 | 26 | |||
| >3 | 91 | 40 | |||
| Multinodular | 37 | 32 | |||
| Size of main tumor (cm) | ≤2 | 60 | 50 | .017 | |
| 2.1–3 | 111 | 30 | |||
| 3.1–5 | 94 | 42 | |||
| >5 | 11 | 37 | |||
| Okuda stage | I | 169 | 43 | .0014 | |
| II | 107 | 28 | |||
| Differentiation degree | Well | 108 | 40 | .015 | |
| Moderate | 79 | 40 | |||
| Poor | 8 | 13 | |||
| Treatment response | |||||
| Initial response | Complete | 192 | 43 | .0002 | |
| Failure | 84 | 28 | |||
| Multivariate analysis | |||||
| Child-Turcotte-Pugh class (A/B) | 1.9 (1.3–2.9) | .0001 | |||
| Initial complete response (yes/no) | 1.71 (1.1–2.5) | .006 | |||
| BUN (≤17/>17) mg/dL | 1.54 (1.1–2.2) | .02 |
- Abbreviations: BUN, blood urea nitrogen; AFP, alpha-fetoprotein.
Predictors of Survival in Child-Turcotte-Pugh A Patients.
Survival in 196 Child-Turcotte-Pugh A patients was 93%, 60%, and 37% at 1, 3, and 5 years, respectively (median, 44 mo). Multivariate analysis disclosed initial CR (odds ratio, 1.83; 95% CI, 1.1-3.1; P = .020) and blood urea nitrogen (odds ratio, 1.68; 95% CI, 1.1-2.7; P = .027) as independent variables associated with survival. Survival in patients with initial CR (n = 138) was 97%, 63%, and 42% at 1, 3, and 5 years, respectively, compared with 83%, 50%, and 18%, respectively, in patients with initial failure (n = 56) (P = .0136) (Fig. 4). Survival of Child-Turcotte-Pugh A patients achieving sustained CR (n = 54) was 96%, 71%, and 57% at 1, 3, and 5 years, respectively (median 66 mo), compared with that of patients with failure/recurrence (n = 140): 92%, 57%, and 31% at 1, 3, and 5 years, respectively (median, 42 months) (P = .042) (Fig. 5).
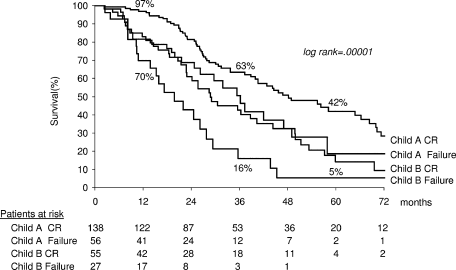
Actuarial survival of patients divided according to Child-Turcotte-Pugh class and initial CR. Child, Child-Turcotte-Pugh; CR, complete response.
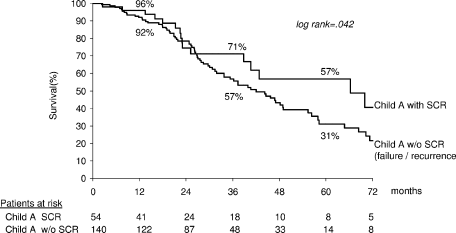
Actuarial survival of Child-Turcotte-Pugh A patients divided according to sustained CR and failure or recurrence. Child, Child-Turcotte-Pugh; SCR, sustained complete response.
Among Child-Turcotte-Pugh A patients with initial CR, the best outcomes were achieved in patients with a single nodule 2 cm or smaller in diameter (n = 34): 1-, 3-, and 5-year survival was 97%, 72%, and 63%, respectively, compared with 96%, 56%, and 32%, respectively, in patients with single tumors 2.1-5 cm (n = 87) (P = .0075) (Fig. 6).
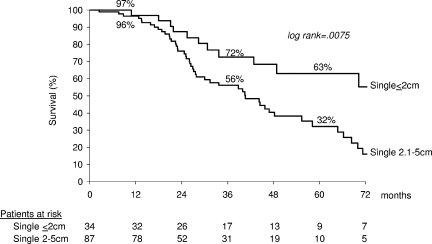
Actuarial survival of Child-Turcotte-Pugh A patients with initial CR divided according to tumor size.
Predictors of Survival in Child-Turcotte-Pugh B Patients.
Survival in 85 Child-Turcotte-Pugh B patients was 76%, 34%, and 11% at 1, 3, and 5 years, respectively. The sole variable associated with survival was initial CR (odds ratio, 1.93; 95% CI, 1.14-3.29; P = .014). Patients with initial CR (n = 54) achieved 1-, 3-, and 5-year survival of 81%, 42%, and 14%, respectively, compared with those with failure (n = 28): 70%, 16%, and 5%, respectively (P = .0127) (see Fig. 4).
Discussion
Percutaneous ablation is an effective therapy for patients with early HCC.2, 8, 9, 19-21 Small tumors might be fully necrosed and in some series patient survival might be as good as that of surgical cases.2, 9, 19, 22 However, the available information does not establish if effective percutaneous treatment improves survival or if the benefit is restricted to a specific subgroup. Because most HCCs arise in a liver with cirrhosis, severe liver function impairment may already define a poor prognosis, and HCC treatment will not change the outcome.11 This is the case in Child-Turcotte-Pugh C patients, and the uncertainty affects Child-Turcotte-Pugh A and B patients. Clarification of this issue would require a randomized clinical trial comparing therapy and no treatment, but this is ethically unfeasible. Thus, the sole approach to evaluate the benefits of percutaneous therapy is to analyze large cohorts of treated individuals to define the prognostic predictors of survival and assess if effective therapy emerges as significant. This demonstration and the definition of the parameters related to effective treatment would ultimately define the optimal candidates for treatment, as has been done for LT and resection.23
These comments emphasize the novelty of our data, which unequivocally show that achievement of an initial CR is a major predictor of survival because it is associated with a significant outcome improvement and is obtained in more than half of treated patients. The impact of extensive tumor necrosis on survival has also been observed in patients treated with transarterial chemoembolization.24 Thus, effective antitumoral therapy that is not simultaneously hampered by severe side effects improves patient survival. This need to avoid side effects is well known in the surgical setting, where restrictive criteria are applied to prevent postoperative liver failure.6 Because percutaneous treatment is reasonably safe, it can be applied in Child-Turcotte-Pugh A and B patients. Interestingly, we have identified initial CR as a significant survival predictor in both classes. In Child-Turcotte-Pugh A patients, a 20% difference is achieved at 5 years (42% in responders vs. 18% in nonresponders), while in Child-Turcotte-Pugh B patients, the difference is achieved at 3 years (42% in responders vs. 16% in nonresponders). This finding is highly relevant from a clinical perspective. Until now, it was suggested that Child-Turcotte-Pugh B patients would benefit from surveillance if LT was an option.11 Our data show that if the tumor is effectively ablated, this translates into an improved outcome, and thus surveillance to allow early detection is justified in Child-Turcotte-Pugh B patients.
The critical point is to define the best candidates for percutaneous treatment and determine if this approach could substitute for LT or resection in a subgroup of patients. As has been reported previously,7-9 the most important predictors for an initial CR are tumor size and stage. Consequently, an initial CR was obtained in 96% of the tumors 2 cm or smaller, while this figure decreased to almost 50% in multinodular/large HCCs, a figure that is similar to that reported by most referral groups.7-9 However, after a median follow-up of 25 months, only one third of the patients had remained disease-free. Accordingly, patients with tumors 2 cm or smaller are the patients most likely to benefit from therapy, but almost half of them will present with late failure or recurrence during follow-up. Thus, in 50% of the optimal candidates, percutaneous ablation will represent a curative option again reproducing figures from other groups.2, 8, 9, 19-21 All cohort studies assessing percutaneous ablation report the appearance of viable tumor tissue within the treated nodule or its vicinity after an initial complete response. However, this event is heterogeneously defined (distance from the main nodule, location in the same segment) and named.17, 25-27 Although we have called it late failure, late recognition of failure, or lack of local disease control, it is usually termed local recurrence or local tumor progression.12 Whatever term is used, it is obvious that the available imaging techniques cannot assure that a tumor has been successfully treated. In our series, 76 of the 192 patients with initial CR presented with late failure with a 1-, 3-, and 5-year probability of 26%, 56%, and 74%, respectively. Most were detected early during follow-up, but 34% were registered at 2 years and 13% were detected beyond 3 years. Even tumors 2 cm or smaller can present with late failure, and studies in resected tumors explain this: one third of tumors 2 cm or smaller present microscopic vascular invasion or satellites, which will most likely not be affected by ablation and emerge as failure early or late during follow-up.28 At the same time, these characteristics are the most accurate predictors of distant recurrence after resection.4, 29-32 Thus, ablation will only be able to locally control the disease and diminish the risk of distant dissemination in the long term if these minute tumors lack these pathological markers and hence belong to the so-called “carcinoma in situ” or stage 0 in the last Barcelona Clínic Liver Cancer staging scheme.15 In that sense, we have shown that HCC patients treated by means of resection in whom pathology discloses vascular invasion or additional sites benefit from enlistment for LT, because recurrence will surely appear during follow-up.33 Contrarily, in the absence of pathological predictors of recurrence the dissemination risk is low, and new HCC sites will represent de novo oncogenesis.34 In these very early tumors ablation competes with resection; unfortunately, current imaging techniques are not able to identify the pathological markers.
It could be argued that the end point of treatment is survival and that this might be very similar between surgical and ablated cases. Our results indicate that the best outcomes will be achieved in Child-Turcotte-Pugh A patients with tumors 2 cm or smaller who achieve a 50% sustained CR rate and a 60% 5-year survival. These encouraging figures have also been reported by others,9, 19, 22, 35 and if (local or initial) treatment response were 100%, percutaneous ablation would surely reproduce that of surgical candidates in whom death during follow-up is due mostly to tumor recurrence.36 Thus, maximal antitumoral effect of therapy should translate into maximal long-term survival. Accordingly, optimal candidates for resection should still be offered this option, and only those patients for whom surgery implies a higher risk of mortality should receive percutaneous ablation as a first treatment option.
Furthermore, solitary tumors 2 cm or smaller are an infrequent subset of patients. Large tumors are more commonly encountered, and the probability of achieving initial and sustained CR in these cases is reduced. However, if initial CR is achieved, survival will be improved. Thus, attempts to fully ablate large tumors should not be disregarded.
One last scenario that should be considered is the role of ablation in patients enlisted for LT. It has been proposed that patients who respond to ablation could avoid LT, because the neoplasm might be eliminated after ablation treatment or, at least, they should not receive any priority as their risk of drop-out4 due to progression would be almost nil. However, because the assessment of therapeutic response can be wrong in a large proportion of patients with theoretical CR, we strongly discourage this policy.
In summary, our results indicate that the best candidates for percutaneous ablation are those patients with very small HCC who are highly likely to achieve complete tumor necrosis. If these patients belong to Child-Turcotte-Pugh class A, they may achieve a 5-year survival beyond 60%. In these patients percutaneous ablation could almost compete with resection. However, segmental surgical resection still has relevant advantages because it eliminates both the tumor and the surrounding tissue where vascular invasion and satellite dissemination may have already taken place. If percutaneously treated, these characteristics will surely translate into treatment failure and patients will have missed the opportunity of obtaining complete tumor removal by surgery and potential enlistment for salvage LT due to recurrence risk. Accordingly, until imaging techniques reach enough sensitivity to rule out the predictors of failure/recurrence and accurately define very small HCC as carcinoma in situ (stage 0), surgical resection should still be considered the first line treatment.




