Concanavalin a injection activates intrahepatic innate immune cells to provoke an antitumor effect in murine liver
Abstract
Concanavalin A (ConA), directly injected into mice, induces T cell–mediated liver injury. However, it remains unclear whether ConA injection can activate innate immune cells, including natural killer (NK) cells and natural killer T (NKT) cells, both of which exist abundantly in the liver. Here we report that ConA injection stimulated interferon (IFN)-γ production from liver NKT cells as early as 2 hours after injection and augmented YAC-1 cytotoxicity of liver NK cells. ConA-induced NK cell activation required other types of immune cells and critically depended on IFN-γ. Because a nonhepatotoxic low dose of ConA was capable of fully activating both NKT cells and NK cells, we next addressed the possibility of ConA injection displaying an antitumor effect in the liver without liver injury. A nonhepatotoxic low-dose ConA injection augmented the cytotoxicity of liver NK cells against Colon-26 colon cancer cells and suppressed hepatic metastasis of Colon-26 cells in a NK cell– and IFN-γ–dependent manner. In conclusion, a nonhepatotoxic low dose of ConA might serve as an immunomodulator that can preferentially activate the innate immune cells to induce an antitumor effect against metastatic liver tumor. (HEPATOLOGY 2004;40:1190–1196.)
Concanavalin A (ConA) injection into mice leads to immunomediated liver injury.1 ConA has a high affinity toward the hepatic sinus, which results in activation of immune cells in the liver.1, 2 Several immune cell types such as T cells or Kupffer cells have been shown to be involved in ConA-induced liver injury.1, 3 Recently, it has been reported that natural killer T (NKT) cells could critically contribute to this liver injury, because Jα281 knockout mice and CD1 knockout mice—both of which lack NKT cells—were resistant to this type of liver injury, and adaptive transfer of NKT cells from wild-type mice into these knockout mice could cancel this resistance.4, 5 In addition, several proinflammatory cytokines such as interferon (IFN)-γ, tumor necrosis factor alpha, interleukin (IL)-1, IL-2, IL-12, and IL-18 were shown to be markedly upregulated in liver tissue after ConA injection.6-9 In particular, IFN-γ was reported to be critically involved in liver injury because its development was significantly reduced in mice deficient in IFN-γ or treated with IFN-γ–neutralizing monoclonal antibody (mAb).9-11 However, it remains to be fully elucidated what changes occur in liver lymphocytes—particularly innate immune cells, including natural killer (NK) cells as well as NKT cells—after ConA injection.
The liver contains an abundant proportion of NK cells and NKT cells compared with other immune organs.12 NK cells, which display nonspecific cytotoxicity against tumor cells,13 can be activated for increased cytotoxicity following stimulation by a variety of cytokines, including IL-2, IL-12, IL-15, or IFN-α/β.14, 15 NKT cells, which are characterized by the expression of surface markers of NK cells together with a single invariant T cell receptor for antigen,16 can be activated in a T cell receptor for antigen–dependent manner recognizing α-galactosylceramide (α-GalCer) bound on CD1d.17 In addition, NKT cells can be activated in a T-cell receptor for antigen–independent fashion through the action of several cytokines such as IL-2, IL-12, IL-15, and IL-18.18-20 It has been shown that NK cells and NKT cells can inhibit tumor formation in the murine liver and that these antitumor effects can be further enhanced by IL-2, IL-12, or α-GalCer administration,20-25 suggesting that these innate immune cells in the liver play a central role in the first-line defense against intrahepatic metastatic tumor cells.
In this study, we demonstrate that liver NKT cells and NK cells were activated after ConA injection. Interestingly, both types of cells were fully activated even on injection of a nonhepatotoxic low dose of ConA. Furthermore, ConA could cause an NK cell–dependent antitumor effect in the liver in vivo. This report focuses on the changes in the innate immune cells, NK cells as well as NKT cells, in the liver induced by ConA injection, and suggests the importance of these innate immune cells in the defense against intrahepatic metastasis of tumor cells.
Abbreviations
ConA, concanavalin A; NK, natural killer; NKT, natural killer T; IFN, interferon; IL, interleukin; mAb, monoclonal antibody; α-GalCer, α-galactosylceramide; PBS, phosphate-buffered saline; ALT, alanine aminotransferase; LMNC, liver mononuclear cell; Ig, immunoglobulin; E/T, effector to target; anti-AGM1, anti-asialo GM1 antibody.
Materials and Methods
Cells and Animals.
YAC-1, a mouse lymphoma cell line sensitive to NK cells, was purchased from American Type Culture Collection (ATCC, Rockville, MD). Colon-26, a mouse colon cancer cell line derived from BALB/c mice, was provided by Dr. Takashi Tsuruo (Institute of Molecular and Cellular Biosciences, University of Tokyo, Tokyo, Japan).26 Female BALB/c mice and BALB/c nu/nu (nude) mice were obtained from Clea Japan, Inc. (Tokyo, Japan). BALB/c background IFN-γ−/− mice were provided by Dr. Yoichiro Iwakura (Institute of Medical Science, University of Tokyo, Tokyo, Japan).10 These mice were maintained in a temperature-controlled, specific pathogen–free room at the Institute of Experimental Animal Science, Osaka University Medical School, and treated with humane care according to National Institutes of Health guidelines. Seven- to nine-week-old mice were used in this study.
Injection of ConA and Evaluation of Liver Injury.
ConA, purchased from Sigma (St. Louis, MO), was dissolved in pyrogen-free phosphate-buffered saline (PBS) and intravenously injected to mice through the tail vein. Serum and liver from individual mice were obtained 15 hours after injection. Serum alanine aminotransferase (ALT) activities were measured with a standard UV method using a Hitachi type 7170 automatic analyzer (Tokyo, Japan). Removed livers were fixed in 10% formalin, embedded in paraffin, thin sectioned, and stained with hematoxylin-eosin.
Preparation of Liver Mononuclear Cells.
Mice were anesthetized with pentobarbital sodium and their abdomens were opened. The inferior caval vein and the portal vein were cut to enable blood outflow. The liver was removed and passed through a stainless steel mesh. The liver cell suspension was collected and parenchymal cells were separated from liver mononuclear cells (LMNCs) by centrifugation at 50g for 5 minutes. LMNC populations were purified by centrifugation through a Percoll gradient as described previously.21
Flow Cytometric Analysis.
LMNCs were suspended in PBS supplemented with 0.3% wt/vol bovine serum albumin and 0.1% wt/vol sodium azide. To avoid the nonspecific binding of Abs to the Fcγ receptor, the cells were preincubated with anti-CD16/32 mAb. Cells were then incubated with a saturating amount (1 μg/106 cells) of fluorescein isothiocyanate–conjugated anti-CD69 mAb or isotype-matched control immunoglobulin (Ig); peridinin chlorophyll protein–conjugated anti-CD3e mAb; and either phycoerythrin-conjugated anti–NK cell (DX5) mAb, anti-CD4 mAb, anti-CD8a mAb, or α-GalCer–loaded CD1d tetramers. For intracellular IFN-γ staining, the cells were stained with fluorescein isothiocyanate–conjugated anti–IFN-γ mAb or isotype-matched control Ig after staining of cell surface markers followed by fixation and permeabilization with a Cytofix-Cytoperm kit according to the manufacturer's instructions (BD Pharmingen, San Diego, CA). The stained cells were analyzed with a FACScan (Becton Dickinson, Mountain View, CA), and the data were processed using CELLQuest software (Becton Dickinson). CD69 expression and IFN-γ production of CD4+ T cells, CD8+ T cells, NK cells, or NKT cells were analyzed on electronically gated CD3e+ CD4+, CD3e+ CD8a+, CD3e− DX5+ or CD1d-tetramer–reactive cells, respectively. All mAbs and control Ig were obtained from BD PharMingen; phycoerythrin-conjugated α-GalCer–loaded CD1d tetramers were provided by Dr. Richard S. Blumberg (Brigham and Women's Hospital, Boston, MA) and Dr. Mitchel Kronenberg (La Jolla Institute for Allergy and Immunology, San Diego, CA).27
Cytotoxicity Assay.
The cytotoxicity of prepared LMNCs against YAC-1 cells or Colon-26 cells was measured by a standard 51Cr release assay as previously described.21 Target cells (1 × 106) were labeled for 60 minutes at 37°C with 100 μCi of Na2 51CrO4 in 500 μL of RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum. Labeled targets (5 × 103 cells/well) were incubated for 4 hours at 37°C in 96-well plates containing medium (total volume of 100 μL) and LMNCs at various effector to target (E/T) ratios.
Separation of LMNCs via DX5 Marker.
Prepared LMNCs were used to isolate DX5-positive and DX5-negative cells via magnetic cell sorting using MACS (Miltenyi Biotec, Gladbach, Germany) according to the manufacturer's protocol. The purity of the fraction of both DX5-positive and DX5-negative cells was more than 90%. The fraction of DX5-positive cells consisted of mainly CD3e−/DX5+ NK cells (more than 90%).
Experimental Liver Metastasis of Colon-26 Cells.
Mice were anesthetized, and the spleen was exposed to allow direct injection of 3 × 104 viable Colon-26 cells in 150 μL of PBS. The spleen was then returned, and the abdomen and skin were surgically sutured. Two days after the tumor injection, the mice were randomly assigned to the ConA treatment group or the control group. ConA (0.05 mg/200 μL) or 200 μL of PBS was intravenously administered to each group of mice, respectively. Two weeks after the tumor injection, the livers of ConA-treated or PBS-treated mice were removed, and the weight was measured to examine intrahepatic tumor growth. The removed livers were fixed in 10% formalin, embedded in paraffin, thin sectioned, and stained with hematoxylin-eosin. The numbers of tumor foci in the broadest sections including the portion of the liver hilus were counted under light microscopy to evaluate intrahepatic tumor formation.
In Vivo Depletion of NK Cells.
To deplete NK cells in vivo, we used 50 μL of anti-asialo GM1 antibody (anti-AGM1) (Wako, Osaka, Japan), which we previously showed can selectively deplete NK cells.21 Mice were intrasplenically injected with Colon-26 cells (3 × 104/mouse) and, on the next day, intraperitoneally administered either 50 μL of anti-AGM1 or the same amount of control normal rabbit Ig (DAKO, Copenhagen, Denmark). Two days after the tumor burden, 0.05 mg of ConA or 200 μL of PBS was injected to each group of anti-AGM1–administered mice and that of control Ig-administered mice. Two weeks after the splenic injection, the mice of each group were sacrificed to evaluate tumor growth as described above.
Statistical Analysis.
The statistical significance of differences between the two groups was determined by applying the Mann-Whitney U test. We defined the statistical significance as a P value less than .05.
Results
Dose Dependence of ConA-Induced Liver Injury.
ConA-induced liver injury has been reported to peak 8 to 24 hours after injection.2, 7 To examine the degree of liver injury, we assessed the serum ALT activities of BALB/c mice 15 hours after various doses of ConA injection. Serum ALT activities were considerably elevated in the mice injected with more than 0.2 mg of ConA (Fig. 1). The elevation of serum ALT activity was accompanied by histological hepatocyte damage (data not shown). When the injected dose of ConA was lower than 0.1 mg, neither elevation of serum ALT activity nor histological hepatocyte damage was observed (Fig. 1; data not shown). For further analysis, a dose of 0.05 mg was considered to be a nonhepatotoxic low dose, and a dose of 0.4 mg was considered to be a hepatotoxic dose.
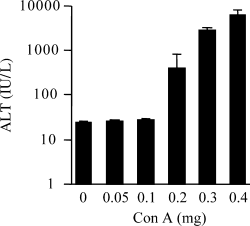
Dose dependence of ConA-induced liver injury. BALB/c mice (n = 3) were injected intravenously with 200 μL of PBS containing the indicated doses of ConA. Serum ALT activity was determined 15 hours after the injection. Data are represented as the mean ± SE. ALT, alanine aminotransferase; Con A, concanavalin A.
Activation of Liver CD4+ T and CD8+ T Cells by ConA Injection.
We investigated liver T cell activation after a nonhepatotoxic low dose as well as hepatotoxic dose of ConA injection. To this end, we evaluated the CD69 expression, an early activation marker,28 and the IFN-γ production of liver CD4+ T and CD8+ T cells 15 hours after ConA injection. Although both doses of ConA upregulated CD69 expression and intracellular IFN-γ production of liver CD4+ T cells and CD8+ T cells, the upregulation induced by the nonhepatotoxic low dose (0.05 mg) was weaker, by about half, than that of the hepatotoxic dose (0.4 mg) (Fig. 2A, 2B).
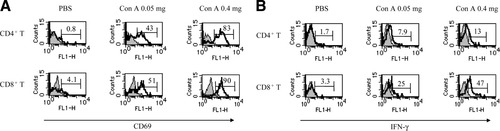
Induction of CD69 expression and IFN-γ production of liver CD4+ T cells and CD8+ T cells by ConA injection. LMNCs were isolated from mice injected with 200 μL of PBS or the indicated doses of ConA 15 hours after injection. Cells were subjected to examination of CD69 expression and IFN-γ production of liver CD4+ T cells and CD8+ T cells. Open and closed histograms indicate staining with anti-CD69 mAb (A) or anti–IFN-γ mAb (B) and isotype-matched control Ig, respectively. Numbers indicate the percentage of (A) CD69+ cells or (B) intracellular IFN-γ+ cells. All experiments were done 3 times and representative data are shown. PBS, phosphate-buffered saline; Con A, concanavalin A; IFN-γ, interferon gamma.
Activation of Liver NKT Cells and NK Cells by ConA Injection.
We investigated liver NKT cell activation after ConA injection of the nonhepatotoxic low dose as well as the hepatotoxic dose by comparison with that after the administration of 2 μg α-GalCer, provided by Kirin Brewery (Gumma, Japan), which has been shown to fully activate NKT cells in a CD1d-restricted manner.21, 29, 30 Liver NKT cells began to decrease in number 2 hours after ConA- or α-GalCer injection and disappeared almost completely 12 hours after injection (data not shown). Therefore, CD69 expression and IFN-γ production of liver NKT cells were determined 2 hours after injection. Intracellular IFN-γ production of liver NKT cells was considerably upregulated by ConA injection of a nonhepatotoxic low dose as well as a hepatotoxic dose, both to the same degree as that by α-GalCer administration (Fig. 3A). Both doses of ConA injection upregulated CD69 expression at levels similar to that of α-GalCer administration (Fig. 3B).
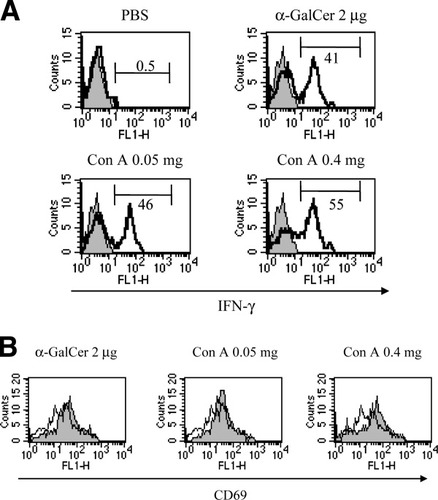
Induction of intracellular IFN-γ production and CD69 expression of liver NKT cells by ConA injection. LMNCs were isolated from mice injected with 200 μL of PBS, 2 μg of α-GalCer, or the indicated doses of ConA 2 hours after injection. Cells were subjected to examination of (A) IFN-γ production and (B) CD69 expression of liver NKT cells. (A) Open and closed histograms indicate staining with anti–IFN-γ mAb and isotype-matched control Ig, respectively. Numbers indicate the percent of intracellular IFN-γ+ cells. (B) Open and closed histograms indicate the CD69 expression of PBS-injected mice and that of α-GalCer–injected or ConA-injected mice, respectively. Data are representative of 3 experiments. PBS, phosphate-buffered saline; α-GalCer, α-galactosylceramide; Con A, concanavalin A; IFN-γ, interferon gamma.
Next, we investigated liver NK cell activation after ConA injection of the nonhepatotoxic low dose as well as the hepatotoxic dose. To this end, we evaluated the number, CD69 expression, and IFN-γ production of liver NK cells and the cytotoxicity of LMNCs against YAC-1 cells, which are sensitive to NK cell cytotoxicity, 15 hours after injection. The number of liver NK cells increased after ConA injection at doses of 0.05 mg and 0.4 mg, compared with that after PBS injection (0.99 ± 0.11, 1.19 ± 0.12, 0.41 ± 0.04 × 106 cell/mouse, respectively). The CD69 expression and the intracellular IFN-γ production of liver NK cells were upregulated after ConA injection, and the upregulations caused by the 0.05-mg dose were nearly of the same degree as those caused by the 0.4-mg dose (Fig. 4A). The cytotoxicity against YAC-1 cells of LMNCs was remarkably augmented after ConA injection of both doses, and the augmentation caused by the 0.05-mg dose was nearly of the same degree as that of the 0.4-mg dose (Fig. 4B). Both doses of ConA injection augmented splenic mononuclear cell cytotoxicity, but to a much lesser extent (data not shown).
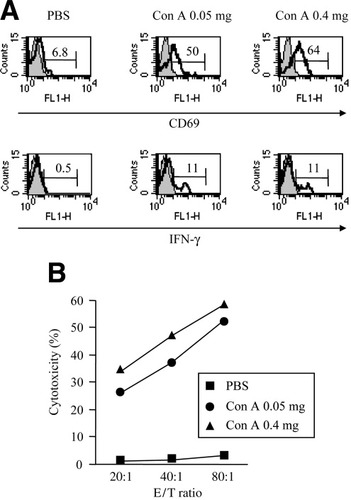
Induction of CD69 expression and IFN-γ production of liver NK cells and cytotoxicity against YAC-1 cells of LMNCs by ConA injection. LMNCs were isolated from mice injected with 200 μL of PBS or the indicated doses of ConA 15 hours after injection. Cells were subjected to examination of CD69 expression and IFN-γ production of liver NK cells and examination of cytotoxicity of LMNCs against YAC-1 cells. (A) Open and closed histograms indicate staining with anti-CD69 mAb or anti–IFN-γ mAb and isotype-matched control Ig, respectively. Numbers indicate the percentage of CD69+ cells or intracellular IFN-γ+ cells. (B) Cytotoxicity of LMNCs against 51Cr-labelled YAC-1 cells at the indicated E/T ratios. Data are representative of 3 experiments. PBS, phosphate-buffered saline; Con A, concanavalin A; IFN-γ, interferon gamma; E/T, effector to target.
IFN-γ Dependence of Liver NK Cell Activation Induced by ConA Injection.
Using nude mice, which have been shown to possess NK cells but not NKT cells or T cells (data not shown), we investigated whether NK cells were solely sufficient for their own activation after ConA injection. The cytotoxicity of LMNCs against YAC-1 cells in nude mice showed no augmentation after ConA injection at doses of 0.05 mg and 0.4 mg compared with that after PBS injection (Fig. 5A), suggesting the involvement of NKT cells and/or T cells in ConA-induced NK cell activation.
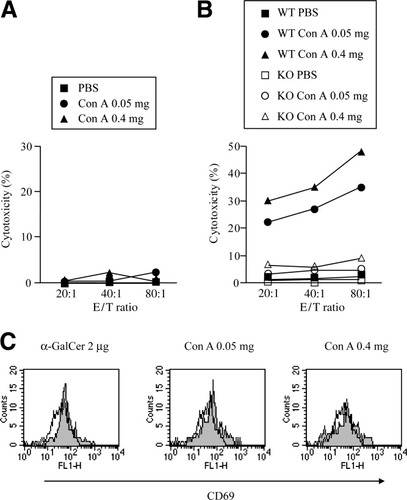
Involvement of IFN-γ in liver NK cell activation by ConA injection. (A) Nude mice were injected with 200 μL of PBS or the indicated doses of ConA. Fifteen hours after the injection, LMNCs were isolated to examine the cytotoxicity against 51Cr-labelled YAC-1 cells at the indicated E/T ratios. (B) IFN-γ−/− mice or wild-type mice were intravenously injected with 200 μL of PBS or the indicated doses of ConA. Fifteen hours after the injection, LMNCs were isolated from knockout or wild-type mice to examine the cytotoxicity against 51Cr-labelled YAC-1 cells at the indicated E/T ratios. (C) LMNCs were isolated from IFN-γ−/− mice injected with 200 μL of PBS, 2 μg of α-GalCer, or the indicated doses of ConA 2 hours after injection. Cells were subjected to examination of CD69 expression of liver NKT cells. Open and closed histograms indicate the CD69 expression of PBS-injected mice and that of α-GalCer–injected or ConA-injected mice, respectively. Data are representative of 3 experiments. PBS, phosphate-buffered saline; Con A, concanavalin A; E/T, effector to target; WT, wild-type; KO, knockout; α-GalCer, α-galactosylceramide.
IFN-γ was produced considerably from liver NKT cells and to a lesser extent from liver T cells after ConA injection (Fig. 2B, 3A). We therefore investigated the contribution of IFN-γ to liver NK cell activation after ConA injection. ConA injection (at a dose of either 0.05 mg or 0.4 mg) had only a marginal effect on cytotoxicity against YAC-1 cells of LMNCs in IFN-γ−/− mice (Fig. 5B). In contrast, liver NKT cells in IFN-γ−/− mice upregulated CD69 expression and began to decrease in number 2 hours after ConA injection at doses of both 0.05 mg and 0.4 mg (Fig. 5C) to the same degree as that after α-GalCer administration, observations that were identical to those in wild-type mice (Fig. 3B). These results demonstrate that either dose of ConA could activate liver NKT cells, but not liver NK cells, in IFN-γ−/− mice.
Antitumor Effect Against Metastatic Colon-26 Cells in the Liver by Nonhepatotoxic Low-Dose ConA Injection.
Based on the findings that a nonhepatotoxic low-dose ConA injection could induce liver NK cell and NKT cell activation, we hypothesized that ConA injection might induce an antitumor effect in the liver without liver injury. We administered a nonhepatotoxic low dose of ConA into mice displaying 2-day intrahepatic metastasis of Colon-26 cells. Two weeks later, the number of tumor foci in the liver section of the mice in the ConA treatment group was significantly smaller than that in the control treatment group (Fig. 6A). Also, the liver weight of the mice in the ConA treatment group was significantly lighter than that in the control treatment group (Fig. 6B).
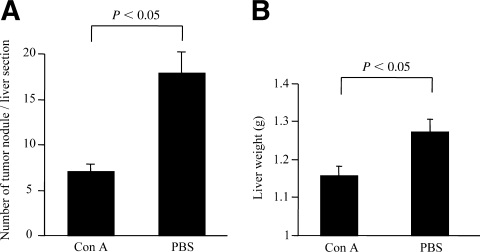
Antitumor effect of nonhepatotoxic low-dose ConA injection in vivo. Tumor growth in the liver was estimated by (A) the number of tumor foci in the liver section and (B) the liver weight (n = 7). Data are represented as the mean ± SE and are representative of 3 experiments. Con A, concanavalin A; PBS, phosphate-buffered saline.
To examine whether NK cells were involved in this ConA-mediated antitumor effect, BALB/c mice were preinjected with anti-AGM1 to selectively deplete NK cells and were analyzed for the antitumor effect. There was no significant difference between the liver weights of the anti-AGM1–administered ConA treatment group and the anti-AGM1–administered PBS treatment group (n = 5; 3.8 ± 0.6 g, 3.0 ± 0.7 g, respectively). Because ConA-induced NK cell activation is critically dependent on IFN-γ (Fig. 5B), we also examined the antitumor effect in IFN-γ–deficient mice. There was no significant difference between the liver weights of the ConA-treated IFN-γ−/− mice group and the PBS-treated IFN-γ−/− mice group (n = 6; 3.4 ± 0.1 g, 4.0 ± 0.4 g, respectively).
Augmentation of the Cytotoxicity of Liver NK Cells Against Colon-26 Cells by Nonhepatotoxic Low-Dose ConA Injection.
Finally, we examined the cytotoxicity of liver NK cells against Colon-26 cells. The intrahepatic lymphocytes considerably augmented their cytotoxicity against Colon-26 cells after a nonhepatotoxic low-dose ConA injection compared with the control PBS injection (Fig. 7A). To characterize the lymphocyte subsets involved in this elevated cytotoxicity, the NK cell marker DX5-positive cells were separated from LMNCs and analyzed for their killing activity. DX5-positive cells killed Colon-26 cells much more efficiently than did preseparated LMNCs, while DX5-negative cells did not kill Colon-26 cells at all (Fig. 7B). These results agreed with the finding that NK cells were involved in the antitumor effect of a nonhepatotoxic low-dose ConA injection in the liver in vivo.
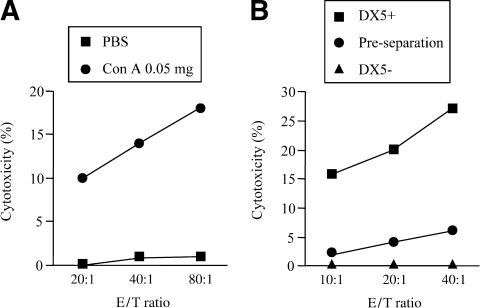
Induction of cytotoxicity against Colon-26 colon cancer cells of liver NK cells by nonhepatotoxic low-dose ConA injection. Mice were injected with 200 μL of PBS or 0.05 mg of ConA. (A) Fifteen hours after the injection, LMNCs were isolated to examine the cytotoxicity against 51Cr-labelled Colon-26 cells at the indicated E/T ratios. Also, prepared cells from 0.05 mg of ConA-injected mice (n = 15) were separated using NK cell marker DX5. (B) DX5-positive cells and DX5-negative cells as well as preseparated LMNCs were analyzed for cytotoxicity against 51Cr-labelled Colon-26 cells at the indicated E/T ratios. Data are representative of 3 experiments. PBS, phosphate-buffered saline; Con A, concanavalin A; E/T, effector to target.
Discussion
In this study, we demonstrated that ConA injection into mice could induce the activation of innate immune cells, including NK cells and NKT cells, as well as T cells in the liver. Interestingly, liver NK cells and NKT cells could be fully activated by ConA injection even at a nonhepatotoxic low dose, while T cells were activated to a lesser degree than that observed after a hepatotoxic dose. This result is in line with mounting lines of evidence indicating that the activation of T cells plays an essential role in ConA-induced liver injury.
We have demonstrated here that liver NK cell activation after ConA injection could not be observed in nude mice in which NK cells are present but NKT cells and T cells are absent. This finding suggested that NK cells could not be directly activated by ConA injection and that NKT cells and/or T cells could be critically involved in NK cell activation after ConA injection. Although we did not directly address the question of which immune cell subset was involved in NK cell activation after ConA injection, we speculate that NKT cells are important for the following reasons. First, among the immune cell subsets tested, NKT cells were the earliest activated after ConA injection. Secondly, NKT cells produced the highest level of IFN-γ after ConA injection among the cell subsets tested. Importantly, NKT cells, but not NK cells, could also be activated after ConA injection in IFN-γ−/− mice. These findings suggested that IFN-γ produced from liver NKT cells might contribute to liver NK cell activation after ConA injection. However, further study is needed to clarify this point.
An important question is whether these activated NK cells were involved in ConA-induced liver injury. We observed that liver NK cell activation could be induced by ConA injection at a nonhepatotoxic low dose as well as a hepatotoxic dose to almost the same degree. Toyabe et al. reported that in vivo depletion of NK cells by anti-AGM1 administration did not inhibit ConA-induced liver injury,31 which is consistent with our unpublished findings. Taken together with our findings and this report, we speculate that liver NK cell activation does not critically contribute to ConA-induced liver injury.
We have demonstrated that injection of a nonhepatotoxic low dose of ConA elicits an antitumor effect against metastatic Colon-26 colon cancer cells in the murine liver. This effect was critically dependent on the activation of NK cells, because the ConA injection could not suppress tumor growth in NK cell–depleted mice or IFN-γ–deficient mice in which NK cells were not activated after the injection. Mounting lines of evidence suggest the therapeutic usefulness of α-GalCer, IL-2, and IL-12 administration for inhibiting intrahepatic metastasis of tumor cells, which depends, at least in part, on their ability to activate NKT cells and/or NK cells. However, overdosing of these agents is well known to be potentially toxic to the liver,32-35 similar to ConA injection. Our present study suggests that a low dose of ConA is capable of preferentially activating innate immune cells in the liver to provoke an antitumor effect without causing liver injury.




