Plasticity in the adult rat pancreas: Transdifferentiation of exocrine to hepatocyte-like cells in primary culture
Abstract
Under certain experimental conditions, hepatocytes can arise in the pancreas. It has been suggested that the pancreas retains a source of hepatocyte progenitor cells. However, such cells have not been yet identified in the adult pancreas. We describe here the transdifferentiation of primary rat pancreatic exocrine cells into hepatocyte-like cells during 5 days of tissue culture in the presence of dexamethasone (DX). Using reverse-transcription polymerase chain reaction and immunocytochemistry, it was observed that DX treatment induced albumin RNA and protein expression in the cells. Coexpression of albumin and amylase, and the absence of cell proliferation, demonstrated a direct transdifferentiation of acinar cells to hepatocytic cells. CCAAT enhancer-binding protein-ß protein, a liver-enriched transcription factor that is considered to be the master switch in pancreatohepatic transdifferentiation, and α-fetoprotein were markedly upregulated in the cells after treatment with DX. We compared transcriptional profiles of freshly isolated exocrine cells and DX-treated cells using oligonucleotide microarrays and found that multiple liver-specific genes are induced along with albumin, and that certain pancreatic genes are downregulated in the DX-treated cells. In conclusion, these observations support the notion of plasticity in the adult pancreas and that exocrine cells can be reprogrammed to transdifferentiate into other cell types such as hepatocytes. (HEPATOLOGY 2004;39:1499–1507.)
Tissue regeneration in vertebrates generally is ascribed to the activity of stem cells, which are defined as undifferentiated cells with multipotential state. Yet, the more spectacular forms of regeneration, like that of amputated limbs in amphibians, are known to result from the process of transdifferentiation of fully differentiated cells. Transdifferentiation generally follows a dedifferentiation process whereby a reversal of the differentiated state occurs and adult cells reacquire fetal characteristics, including a multipotential state.1, 2 The phenomena of dedifferentiation and transdifferentiation also are believed to play a role in mammals, for instance in the regeneration of pancreatic and cardiac tissue2, 3 and the transdifferentiation from glial cells to astrocytes in the nervous system.4
Adult pancreas retains pronounced differentiation plasticity, the most remarkable kind of plasticity being represented by the formation of pancreatic hepatocytes. Development of foci of hepatocytes has been described, among others, in the injured pancreas of adult rats and hamsters5-7 and in transgenic mice overexpressing keratinocyte growth factor in the pancreas.8 Transplantation of pancreatic cells was shown to lead to liver repopulation9 and restoration of metabolic liver defects in the recipients.10 Although initially it was thought that acinar exocrine cells were the precursor cells of pancreatic hepatocytes,11 later studies suggested that duct, or “periductal” cells, or oval cells may be the precursors.12, 13 It is difficult, however, to establish conclusively from whole-animal experiments the progenitor cell types of pancreatic hepatocytes. An in vitro model remains to be developed to find out whether pancreatic hepatocytes can emerge from transdifferentiating exocrine acinar cells or duct cells, or to the alternative specification of an as yet undefined stem cell population.
Recently, the glucocorticoid dexamethasone (DX) was shown to induce hepatocyte differentiation in mouse embryonic dorsal pancreatic buds and in a rat pancreatic tumour cell line.14 Others demonstrated the existence of a population of bipotential hepatopancreatic stem cells in a region of mouse ventral foregut endoderm that can differentiate to either liver or pancreas, depending on a gradient of fibroblast growth factors.15 It is not known whether such a stem cell still resides in postnatal pancreas.
In previous studies, we reported that acinar exocrine cells isolated from adult rat pancreas can dedifferentiate into duct-like cells, which share a number of characteristics with fetal protodifferentiated pancreatocytes.16, 17 A very similar change in differentiation is observed in vivo, when adult pancreatic tissue is injured and tissue remodeling occurs with all surviving exocrine cells, acinar and ductal, switching to a similar fetal or protodifferentiated phenotype.17, 18 We hypothesize that the dedifferentiated pancreatocytes acquire a multipotential differentiation state and may act as tissue progenitors during regeneration. In the present study, we identified DX as an agent that interferes with acinar cell dedifferentiation and that promotes transdifferentiation to a hepatocyte-like phenotype in primary culture from adult exocrine tissue.
Abbreviations
DX, dexamethasone; RT-PCR, reverse-transcription polymerase chain reaction; PAI-1, plasminogen activator inhibitor-1; HNF, hepatocyte nuclear factor; C/EBP, CCAAT enhancer-binding protein.
Materials and Methods
Animals.
Male Wistar rats aged 10 to 12 weeks (weight, 250–300 g) were used for the isolations (Janvier, Le Genest-St-Isle, France). Experiments were approved by the ethical committee of the Vrije Universiteit Brussel, and animals received humane care as outlined in the Guide for the Care and Use of Laboratory Animals.
Cell Isolation and Culture.
After collagenase digestion of rat pancreas, the exocrine acini were separated from other tissue components by centrifugal elutriation as described.19 In brief, acini were recovered in the fraction with an average diameter of less than 100 μm with the rotor running at 250 rpm, at a counterflow rate of 240 mL/minute (JB6 Centrifuge; Beckman, Palo Alto, CA). Acini were cultured in bacteriological Petri dishes (Nunc, Naperville, IL) in RPMI-1640 glutamax medium (Gibco BRL, Paisley, Scotland) with penicillin (75 mg/L; Continental Pharma, Brussels, Belgium), streptomycin (100 mg/L; Sigma, St Louis, MO), and 10% fetal bovine serum (Gibco BRL). The cells were kept at 37°C in a humid atmosphere of 5% CO2 in air. For the study of cell replication, 10 μmol/L 5-bromo-2′-deoxyuridine (Sigma) was added 1 hour before harvesting the cells. Total DNA was measured as described.20 DX (Sigma) was added to the culture medium at a final concentration of 1 μmol/L. The glucocorticoid receptor antagonist RU-486 (Mifepristone; Sigma) was added at 2.5 μmol/L concentration 1 hour before addition of DX.
Freshly isolated hepatocytes were kindly provided by Dr. V. Rogiers (Free University of Brussels).
Immunocytochemistry.
Cell pellets were fixed for 1 hour in buffered 4% formaldehyde, entrapped in a 2% agarose gel (40°C; Sigma), and processed for paraffin embedding. Paraffin sections were immunostained with the streptavidin-biotin method and diaminobenzidine as chromogen.21 As primary antibodies, we used sheep polyclonal anti-albumin (Biogenesis, Poole, UK), rabbit polyclonal anti-α-amylase (Sigma), and mouse monoclonal anti-5-bromo-2′-deoxyuridine (ICN, Irvine, CA). For double immunofluorescence staining, we used an Alexa Fluor 488 conjugate (Molecular Probes, Eugene, OR) and a Sigma fluorexein isothiocyanate (FITC) conjugate as secondary antibody.
Electron Microscopy.
Cells were fixed and processed for transmission electron microscopy as described.20
Microarray Analysis.
For the micoarray analysis, freshly isolated cells and cells cultured for 5 days with DX were harvested, and total RNA (10 μg) was isolated using the Trizol RNA isolation method (Gibco BRL). Three RNA samples for each condition were analyzed. Microarray analysis were performed as described previously,22 except that we used the Enzo in vitro transcription kit containing biotin-labeled cytosine-5′-triphosphate (CTP) and uracyl-5′-triphosphate (UTP) (Enzo, Farmingdale, NY) and a fragmentation buffer containing 40 mmol/L potassium acetate and 30 mmol/L magnesium acetate. The microarray data were processed by the Affymetrix Gene Expression Analysis software and dChip (www.dchip.org).23 mRNA profiles were compared with Affymetrix (Santa Clara, CA) rat genome U34A oligonucleotide arrays. Genes were considered to be changed if they fulfilled the following criteria: (1) the average difference (AvDiff as defined by Affymetrix software on the basis of the difference between the perfect match and the mismatch probes) of 50 units or more in the condition of altered gene expression; (2) a mean fold change in expression level from three independent experiments of ≤−2 (decrease) or ≥2 (increase); (3) the expression level in each of the nine combinations having a ≤−1.5-fold or ≥1.5-fold change; or (4) P < .05.
RNA Isolation and Reverse-Transcription Polymerase Chain Reaction (RT-PCR).
Total RNA was isolated from cells and tissues using the Trizol RNA isolation method (Gibco BRL). For the semiquantitative analysis of transcripts encoding albumin, α-fetoprotein, mitochondrial cytochrome P450, α-2-macroglobulin, plasminogen activator inhibitor-1 (PAI-1), transferrin, hepatocyte nuclear factor-6 (HNF-6), cationic trypsinogen, lipase, glycosylated membrane-associated lipase, and β-actin, the total RNA was reversed transcribed and amplified as described by the manufacturer (Gibco BRL) with blanks in each assay. Primers were designed to anneal to specific sequences of rat albumin-5′: atacacccagaaagcacctc and albumin-3′: cacgaattgtgcgaatgtcac, mitochondrial cytochrome P450-5′: gcccctgctaaaagctgtgat and mitochondrial cytochrome P450-3′: ccttgacagcaggagttgcat, α-2-macroglobulin-5′: gatgcctcagctccacaaaaa and α-2-macroglobulin-3′: ttcttaaccagcaccgtgctc, PAI-1-5′: ccctctatttcaacggccaat and PAI-1-3′: cagcctggtcatgttgctctt, transferrin-5′: tgccttgacaatacccgca and transferrin-3′: ccttcccgctgatttcgaat, HNF-6-5′: agccctggagcaaactcaagt and HNF-6-3′: tggacggacgcttattttcc, cationic trypsinogen-5′: ccctcaattctcgagtgtcca and cationic trypsinogen-3′: acagccataaccccaggaaac;, lipase-5′: cgcatcattatccacggctt and lipase-3′:tcccaatagctccgaatgtcc; and glycosylated membrane-associated lipase-5′: tggaatgagccaaaaggtcg and glycosylated membrane-associated lipase-3′: gcggttttcccctcaaattg. Specific primers for control amplifications were: β-actin-5′: actatcggcaatgagcggttc and β-actin-3′: agagccaccaatccacacaga. For detection of α-fetoprotein RNA, a nested PCR was performed as described previously.24 The cycling profile for albumin was: 4 minutes at 94°C followed by 0.5 minute at 94°C, 1.5 minutes at 62°C, and 1 minute at 72°C for 30 cycles. For the other primers, the cycling profile was: 1.5 minutes at 94°C followed by 0.5 minute at 94°C, 0.5 minutes at 60°C, and 1 minute at 72°C for 10 cycles and 0.5 minute at 94°C, 0.5 minute at 58°C, and 1 minute at 72°C for 16 to 20 cycles (total of 26 cycles for transferrin; of 28 cycles for α-2-macroglobulin, PAI-1, cytochrome P450, and β-actin; and 30 cycles for HNF-6, cationic trypsinogen, trypsinogen I, lipase, and glycosylated membrane-associated lipase). Analysis of the amplified fragments was performed on ethidium bromide-stained agarose gels. All analyses were performed at least three times.
Western Blot.
Protein extraction from pancreatic cells and immunoblotting were performed as described.25 Fifty micrograms of sample were loaded on the gel. Anti-CCAAT enhancer-binding protein-β (C/EBP-β) antibody (1/100; C-19 antibody; Santa Cruz Biotechnology, Santa Cruz, CA) and horseradish peroxidase-coupled anti-mouse antibody (1/3000; Amersham, Roosendaal, The Netherlands) were used, respectively, as the first and secondary antibody. Normal rat hepatocytes were used as a positive control for C/EBP-β. All analyses were performed at least three times.
Results
Albumin and α-Fetoprotein Expression in DX-Treated Acinar Cells.
Exocrine acini from adult rat pancreas were purified by centrifugal elutriation and were cultured in bacteriological dishes to prevent cell adhesion. In medium supplemented with 10% fetal bovine serum, the acini aggregate to form cell spheroids after a few days.16 When cells were cultured for 5 or 10 days in the presence of DX, albumin RNA became readily detectable by RT-PCR, whereas it was undetectable in cells cultured without DX (Fig. 1A). When normalized for β-actin mRNA abundance, the relative expression of albumin mRNA was approximately four times lower than that in liver. In whole pancreas and freshly isolated cells, albumin was undetectable. As shown in Fig. 1B, the fetal liver marker α-fetoprotein also becomes detectable after treatment of freshly isolated exocrine cells with DX. Albumin protein expression in DX-treated cells was demonstrated by immunocytochemistry (Fig. 2A,B), whereas there was no immunoreactivity when DX was absent from the culture medium. After 5, 10, and 15 days of culture in the presence of DX, approximately 50%, 55%, and 60%, respectively, of the cells were immunoreactive for albumin. Albumin immunoreactivity showed a granular cytoplasmic staining very similar to what was observed in freshly isolated liver hepatocytes or in liver sections (Lardon J, unpublished data, 2002). Also, the intensity of staining was comparable with that of liver hepatocytes. When cells were first cultured for 5 days in the presence of DX, followed by a culture period of another 5 days without DX, they still retained albumin expression (Lardon J, unpublished data, 2002). Thus, the albumin expression induced by DX seems to be stable even in the absence of further incubation with glucocorticoids. In the presence of the glucocorticoid receptor antagonist RU486, DX failed to induce expression of albumin (Lardon J, unpublished data, 2002). Because albumin is considered to be a hepatocyte-specific gene product, our observations suggest transdifferentiation of pancreatic cells to hepatocytes. In control cultures, amylase immunoreactivity was lost within 4 days, as we reported previously, because of dedifferentiation of the cells.16 However, the acinar cells cultured in the presence of DX retained their amylase expression. We demonstrated cells coexpressing albumin and amylase by double immunofluorescence (Fig. 3A,B). After 2 weeks of culture, amylase immunoreactivity became weak, whereas albumin staining remained intense.
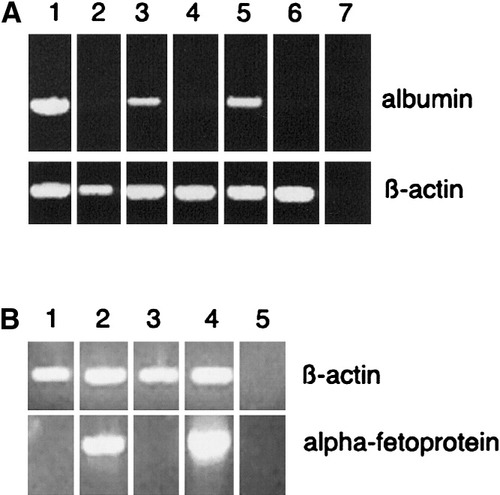
(A) Reverse-transcription polymerase chain reaction (RT-PCR) result of albumin expression in rat: (1) normal rat liver, (2) freshly isolated pancreatic exocrine cells, exocrine cells cultured in (3 and 5) the presence or (4 and 6) absence of dexamethasone (DX) for (3 and 4) 5 days or (5 and 6) 10 days and (7) water. (B) RT-PCR result of α-fetoprotein expression in rat: (1) freshly isolated pancreatic exocrine cells, (2) exocrine cells cultured in the presence of DX for 5 days, (3) normal rat pancreas, (4) fetal rat liver, and (5) water.
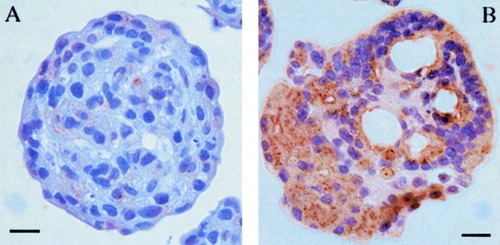
Immunocytochemistry for albumin on pancreatic exocrine cells cultured for 5 days in (A) the absence or (B) the presence of dexamethasone. Scale bar, 15 μm.
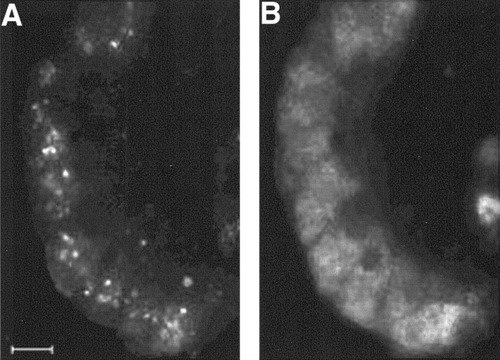
Immunocytochemistry for (A) albumin and (B) amylase on pancreatic exocrine cells cultured for 5 days in the presence dexamethasone. Scare bar, 15 μm.
Proliferation and Survival of DX-Treated Acinar Cultures.
Neither the control nor the DX-treated cells showed signs of proliferative activity, with 5-bromo-2′-deoxyuridine-labelling indices of less than 1%. The possibility of an overgrowth of a putative subset of stem cells in these cultures thus could be ruled out. The lack of proliferation and the presence of amylase-albumin double-positive cells indicate a direct transdifferentiation from acinar cells to hepatocytes. Although the total amount of DNA greatly varied between cultures, we observed consistently a better cell survival in the presence of DX. After 5, 10, and 15 days of culture, there was a survival of 38%, 29%, and 10%, respectively, of the DX-treated cells versus 25%, 8%, and 4% in the untreated cells. Microscopic observations confirmed that there was less cell death in the presence of DX and that the DX-treated cells could be kept in culture for a longer period.
mRNA Profiles of DX-Treated Acinar Cultures.
To search for other hepatocyte genes that may become activated, we compared the mRNA profile of the cells using U34A Affymetrix GeneChips. We compared the freshly isolated pancreatic exocrine cells with the cells cultured for 5 days in the presence of DX from three independent experiments. From the 8700 transcripts covered by oligo-probes on the chip, 1831 mRNAs were detectable above threshold values in the freshly isolated cells and 2825 were detectable in the DX-cultured cells. According to the criteria we specified in Methods, a total number of 263 transcripts encoding for known proteins and 217 encoding expressed sequence tags were upregulated, whereas 119 transcripts and 69 encoding expressed sequence tags were downregulated in the DX cultures when compared with the freshly isolated exocrine cells. From the upregulated genes encoding for known proteins in the DX culture, 34 (recognized by 40 probe sets) can be considered to be liver specific. These liver-specific genes can be divided over different functional classes, including plasma proteins and acute-phase proteins produced by liver, carbohydrate, amino acid and lipid metabolism, detoxification and drug metabolism, iron metabolism, urea cycle, and transcription factors (Table 1).
| GenBank Accession Number | Name Transcript | Fold |
|---|---|---|
| Plasma proteins/ acute phase proteins | ||
| D00753 | Contrapsin-like protease inhibitor- related protein (Cpi-26) | +20,3 |
| L00191 | Fibronectin | +15,8 |
| X05834 | Fibronectin | +15,8 |
| M23566 | Alpha-2-macroglobulin | +139,6 |
| M24067 | Plasminogen activator inhibitor-1 | +33,5 |
| M29866 | Complement component C3 | +17,3 |
| X71127 | Complement protein C1q | +35,1 |
| Complement protein C1q | +5,8 | |
| X82445 | C15 | +4,5 |
| Carbohydrate, amino acid, and lipid metabolism | ||
| AB017544 | Peroxisomal membrane anchor protein Pex14 | +7,0 |
| D10354 | Alanine aminotransferase | +21,2 |
| D90038 | Liver 70-kDa peroxisomal membrane protein (PMP70) | +2,7 |
| L07736 | Carnitine palmitoyltransferase 1 | +5,1 |
| L25387 | C-type phosphofructokinase | +10,6 |
| M33648 | Mitochondrial 3-hydroxy- 3-methylglutaryl-CoA synthase | +90,4 |
| M57263 | Protein-glutamine gamma- glutamyltransferase | +4,3 |
| M91652 | Glutamine synthetase (glnA) | +3,6 |
| Glutamine synthetase (glnA) | +3,5 | |
| S63233 | Phosphoglycerate mutase type B subunit | +4,5 |
| Phosphoglycerate mutase type B subunit | +2,5 | |
| S81497 | Lysosomal acid lipase | +4,6 |
| U08976 | Peroxisomal enoyl hydratase- like protein (PXEL) | +34,1 |
| U09256 | Transketolase | +3,4 |
| X07467 | Glucose-6-phosphate dehydrogenase | +5,6 |
| X58865 | L-type phosphofructokinase | +2,4 |
| Detoxification/ drug metabolism | ||
| D83796 | UDP-glucuronosyltransferase UGT1 | +55,9 |
| J03752 | Glutathione S-transferase | +10,0 |
| J05132 | 3-Methylcholanthrene-inducible UDP-glucuronosyltransferase | +17,8 |
| L19998 | Minoxidil sulfotransferase | +15,6 |
| M10068 | NADPH-cytochrome P450 oxidoreductase | +4,2 |
| M38566 | Mitochondrial cytochrome P450 | +14,2 |
| M60753 | Catechol-O-methyltransferase | +11,9 |
| M93257 | Catechol-O-methyltransferase | +6,3 |
| Iron metabolism | ||
| D38380 | Transferrin | +16,1 |
| Transferrin | +9,0 | |
| J02722 | Heme oxygenase | +9,0 |
| J03190 | 5-Aminolevulinate synthase | +7,0 |
| X57523 | mtp1 | +6,4 |
| Transcription factor | ||
| Y14933 | Hepatocyte nuclear factor 6 beta | +6,3 |
| Urea cycle enzyme | ||
| D13978 | Argininosuccinate lyase | +4,1 |
For some of the genes, RT-PCR was used to confirm the results of the DNA microarrays. RNA encoding α-2-macroglobulin, PAI-1, transferrin, mitochondrial cytochrome P450, and HNF-6 was expressed in the DX-treated cells, whereas it was undetectable in freshly isolated exocrine cells (Fig. 4A,B). Adult rat liver was taken as a positive control. For PAI-1, there was no RNA detectable in the normal liver; however, it is known that PAI-1 is only present in the liver during regeneration.26 We also investigated whether pancreatic-specific genes were downregulated in the DX-cultured cells. Table 2 shows that 11 of 119 downregulated transcripts are pancreas specific. We used RT-PCR to confirm the expression pattern for some of the genes. RNA encoding cationic trypsinogen, glycosylated membrane-associated lipase, and lipase was downregulated in the DX-treated cells and in the liver, where it was highly expressed in the pancreas and in the freshly isolated exocrine cells (Fig. 5). Normal rat pancreas was used as a positive control.
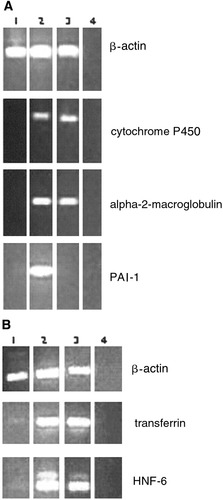
(A) Reverse-transcription polymerase chain reaction (RT-PCR) result of mitochondrial cytochrome P450, α-2-macroglobulin, and plasminogen activator inhibitor-1 (PAI-1) expression in rat: (1) freshly isolated pancreatic exocrine cells, (2) exocrine cells cultured in the presence of dexamethasone (DX) for 5 days, (3) normal rat liver, and (4) water. (B) RT-PCR result of transferrin and hepatocyte nuclear factor 6 (HNF-6) expression in rat: (1) freshly isolated pancreatic exocrine cells, (2) exocrine cells cultured in the presence of DX for 5 days, (3) normal rat liver, and (4) water.
| GenBank Accession Number | Name Transcript | Fold |
|---|---|---|
| Pancreas-specific genes | ||
| D50608 | Cholcysto-kinin type-A receptor | −7,4 |
| J00758 | Pancreatic preprokallikrein | −24,54 |
| L00131 | Pancreatic trypsinogen II | −83,97 |
| L09216 | Glycosylated membrane-associated lipase | −2,24 |
| M16624 | Pancreatic cationic trypsinogen | −15,1 |
| M35299 | Pancreatic secretory trypsin inhibitor-like protein | −55,54 |
| M58716 | Zymogen granule membrane protein GP-2 | −2,53 |
| U58279 | Mist1 bHLH protein | −2,34 |
| X15679 | Preprotrypsinogen IV | −4,24 |
| X59012 | Trypsin V | −38,69 |
| X99773 | Serpin, ZG-46p | −104,28 |
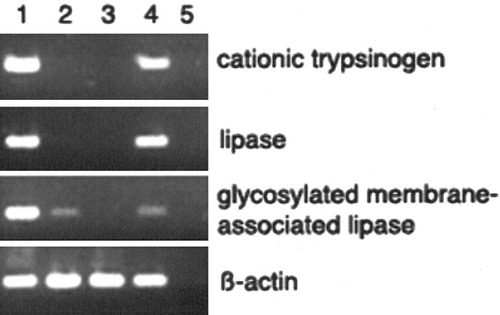
Reverse-transcription polymerase chain reaction result of cationic trypsinogen, lipase and glycosylated membrane-associated lipase expression in rat: (1) freshly isolated pancreatic exocrine cells, (2) exocrine cells cultured in the presence of dexamethasone for 5 days, (3) normal rat liver, (4) normal rat pancreas, and (5) water.
Expression of C/EBP-β Transcription Factor.
We analyzed the presence of C/EBP-β, a liver-selective transcription factor that was previously shown to be the “master switch” for transdifferentiation of the acinar tumour cell line AR42J into hepatocytes.14 Using Western blotting, we found that C/EBP-β protein was absent in the freshly isolated cells, but we found one protein band with the expected molecular weight of C/EBP-β, that is 36 kDa, in the cells treated with DX (Lardon, unpublished data).
Microscopic Phenotype of the Transdifferentiated Cells.
When analyzing sections from paraffin-embedded cell pellets by light microscopy, the cells in the DX-treated cultures were seen to be arranged in various ways. Sometimes a single layer of polarized cells was conspicuous at the periphery of the spheroids (Fig. 3A,B). With electron microscopy, the cells were seen to contain abundant rough endoplasmic reticulum, and in approximately 50% of the cells, zymogen granules could be found. The observed ultrastructure is not typical for acinar cells nor for hepatocytes, but may represent an intermediate between both (Lardon, unpublished data).
Discussion
We previously showed that purified exocrine acinar cells from adult rat pancreas spontaneously transdifferentiate to duct-like cells in primary culture, and that this phenomenon can be inhibited or reversed by agents like nicotinamide or inhibitors of histone deacetylation.16, 17 We demonstrate herein that the synthetic glucocorticoid DX in the culture medium induces, in the same starting cell preparation, a program of partial hepatocytic transdifferentiation. After a culture period of only 5 days, DX causes induction of the albumin gene as well other liver-specific genes such as α-fetoprotein, α-2-macroglobulin, transferrin, and alanine aminotransferase. Microarrays demonstrated the presence of 34 transcripts associated with differentiated liver function and that normally are not expressed in the pancreas. Besides the upregulated liver-specific genes, 11 pancreatic-specific genes as cationic trypsinogen and lipase are downregulated in the exocrine cells after 5 days of culture in the presence of DX as shown by the microarray data and RT-PCR.
As to the mechanism of the observed transdifferentiation, we found that a glucocorticoid receptor inhibitor completely inhibited DX-induced hepatocytic transdifferentiation, indicating that the effect is mediated via the glucocorticoid receptor. This is a well-known gene regulatory protein that first must form a complex with a glucocorticoid hormone to bind to regulatory sites in DNA. In hepatocytes shortly after birth, and later in life during periods of starvation or intense physical activity, glucocorticoids increase the expression of different genes involved in gluconeogenesis.27, 28 One possibility is that all of the upregulated genes are regulated directly by the binding of the hormone–glucocorticoid receptor complex to a regulatory site in their DNA. A glucocorticoid receptor-binding site also exists in the α-amylase29 and lipase30 genes and mediates functional responses of pancreatic acinar cells to glucocorticoid hormones. In the case of hepatocytes, it is known that when the hormone is no longer present, the expression of all of the genes involved in gluconeogenesis drops back to its normal level. In our experiments with pancreatic cells, albumin expression remained stable after withdrawal of DX from the culture medium. This means that DX induced a stable change in gene expression or cell differentiation. Glucocorticoid hormones have been found to regulate DNA demethylation within a key enhancer of the rat liver-specific tyrosine aminotransferase (Tat) gene. In hepatoma cells, genomic footprinting analysis showed that the glucocorticoid receptor uses local DNA demethylation as one of several steps to recruit transcription factors. This demethylation was shown to be stable after hormone withdrawal.31 It remains to be investigated whether the transdifferentiation effect of DX in our model is mediated via DNA demethylation. Another possibility is that DX treatment induces a master switch of gene transcription that, in turn, alters the expression of many other genes. The transcription factor C/EBP-ß was upregulated significantly in the exocrine cells after treatment with DX, as shown by Western blotting. C/EBPs are known to play important roles in regulating the expression of multiple hepatocyte-specific genes.32 C/EBP-ß normally is not expressed in the pancreas, but is known to be involved in liver regeneration and in the acute phase reaction.33 C/EBP-ß is considered to be the master switch of the transdifferentiation from a pancreatic tumour cell line into hepatocytes,14 it becomes expressed in embryonic pancreatic buds cultured in the presence of DX,34 and it is upregulated in the model of copper depletion leading to the appearance of pancreatic hepatocytes.6, 7
HNF-6, another liver-enriched transcription factor, also was increased in the DX-treated cells. HNF-6 is considered to be a regulator of glucose homeostasis through its role during endocrine pancreatic development and its activity on enzymes of the hepatic glucose metabolism.35
Acute phase proteins are secreted into the blood by hepatocytes in response to trauma, inflammation, or disease. Several acute-phase proteins, such as α-2-macroglobulin, were found to be upregulated in the DX-treated exocrine cells. These results are in concordance with the study of Kurash et al.,36 in which the authors found that acute phase proteins are induced in a pancreatic cell line and embryonic pancreatic buds cultured in the presence of DX.
A previous study showed that DX can induce hepatocyte differentiation in mouse embryonic dorsal pancreatic buds and in a rat pancreatic tumor cell line.14 Similar to our study, the authors found that the transdifferentiated cells express liver-specific markers such as cytochrome P450 and glutamine synthase.37
Many of the albumin-immunoreactive cells still contained amylase, an acinar cell marker, which demonstrates a transdifferentiation from acinar to hepatocytic cells. We also were able to exclude overgrowth of putative stem cells, because there were practically no proliferating cells in these cultures. It is clear, however, that the transdifferentiation from acinar pancreatocytes to hepatocytes was not complete, given the continued presence of amylase and zymogen granules. It can be expected, however, that the culture conditions that we used are not optimal for obtaining a complete hepatocytic differentiation. Also, complete transdifferentiation may require longer culture time, but this was not feasible with the present conditions. It is known that acinar cells survive poorly after isolation, and the conditions of, for instance, extracellular matrix or other survival factors still need to be determined. If we can determine the optimal culture conditions to obtain differentiated hepatocytes, these cells may be useful in studies of organ repopulation after acute or chronic liver injury.
Our results indicate that adult acinar cells could be the precursors of pancreatic hepatocytes. Although earlier work suggested that pancreatic hepatocytes in vivo were derived from acinar cells,11 later papers suggest that cells from ductular epithelium were the cells of origin.12, 13 In the latter studies, however, the treatments induced not only the appearance of hepatocytes but also caused massive acinar cell death and tissue remodeling.5 Reddy et al.38 proposed that the adult pancreas contains bipotential stem cells that can differentiate to either pancreatic or hepatic cells. In general, when exocrine tissue is injured, acinoductal metaplasia is observed that includes transdifferentiation of the surviving acinar cells into ductal cells.39 In vitro, we found that this conversion from acinar to ductal cells requires only 3 to 4 days.16 Therefore, it remains possible that the ducts from which hepatocytic cells seemed to arise in vivo in their turn originated from acini. Interestingly, in the transplantation experiments leading to liver repopulation and restoration of metabolic liver disease, whole pancreas dissociates were effective, whereas purified ducts were not.10 This also indicates that the hepatocyte progenitors were present in acini rather than in ducts. The available evidence concerning in vivo generation of pancreatic hepatocytes thus may be reconciled with our in vitro demonstration of acinar cells as the cells of origin.
The adult pancreas has regenerative potential,40, 41 and it has been argued that because the exocrine and endocrine pancreatic cell types and hepatocytes are the offspring of a common stem cell pool during embryogenesis, adult pancreas may retain hepatopancreatic stem cells with a broad differentiation potential, including differentiation to endocrine islet cells.15 However, adult stem cells have not yet been identified in the pancreas. This leaves open the possibility that the precursor cells are not necessarily actual or potential stem cells,42, 43 but may be fully differentiated cells with transdifferentiation potential, as in the case of acinar cells.40 Acinar cells have the potential to renew and regenerate the exocrine tissue,44 they can transdifferentiate to duct cells16 and to hepatocytes (as demonstrated in this study), and thus have multipotential differentiation capacity. We previously reported that transdifferentiating acinar cells express the transcription factors Pdx-1 and Ptf-1/p48,16 the best available markers of pancreatic protodifferentiated progenitor cells.45 Hypothetically, the multipotential nature of acinar cells also may suggest the capacity to differentiate to insulin-producing ß-cells. The acinar cell line AR42-J indeed has been shown to transdifferentiate to the ß-cell phenotype under certain culture conditions46, 47 and to hepatocytes under other conditions.14 If conditions can be found that direct endocrine differentiation from normal acinar cells, large numbers of potential precursor cells may become available for allogeneic islet cell transplantation.48
The AR42J cell line initially was derived from an acinar exocrine tumor in the pancreas of a carcinogen-treated rat.49 It exhibits an amphicrine phenotype, which is the combination of exocrine and endocrine characteristics,50 a phenotype that is not normally found in the pancreas. Are these transformed cells immortalized multipotent pancreatic stem cells, partially dedifferentiated cells that have reacquired fetal characteristics, or does their phenotypic plasticity reflect a transdifferentiation potential that also may be present in mature, untransformed acinar cells? Our study indicates that the latter possibility may be true. Our present study reports that exocrine acinar cells derived from normal adult rat pancreas can transdifferentiate to hepatocyte-like cells in primary culture. It emphasizes the importance of transdifferentiation as a possible mechanism for the generation of multipotent progenitor cells and tissue regeneration.
Acknowledgements
The authors thank Danny Pipeleers (Free University of Brussels, Belgium) for logistic and general support and Emmy De Blay and Ann Nuyts for expert technical assistance.




