Interleukin 18 causes hepatic ischemia/reperfusion injury by suppressing anti-inflammatory cytokine expression in mice
Abstract
Hepatic ischemia/reperfusion injury is a clinically important problem. While the mechanisms of the initial event and subsequent neutrophil-dependent injury are somewhat understood, little is known about the regulation of endogenous hepatoprotective effects on this injury. Interleukin 12 (IL-12) plays a role in the induction of this injury, but involvement of interleukin 18 (IL-18) has not been clarified. Using a murine model of partial hepatic ischemia and subsequent reperfusion, the aim of the current study was to determine whether IL-18 is up-regulated during hepatic ischemia/reperfusion and to determine the role of endogenous IL-18 in the development and regulation of inflammatory hepatic ischemia/reperfusion injury. Hepatic IL-18 expression was up-regulated from 1 to 8 hours after reperfusion. Hepatic ischemia/reperfusion induced nuclear factor-κB (NF-κB) and activator protein 1 (AP-1) activation, as defined by electrophoretic mobility shift assay, and caused significant increases in liver neutrophil recruitment, apoptosis, hepatocellular injury, and liver edema as defined by liver myeloperoxidase content, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate biotin nick end-labeling (TUNEL) staining, serum aminotransferase levels, and liver wet-to-dry weight ratios. In mice treated with neutralizing antibody to IL-18, ischemia/reperfusion-induced increases in CXC chemokine expression, activation of NF-κB and AP-1, and apoptosis were greatly reduced. Furthermore, under blockade of IL-18, anti-inflammatory cytokines such as IL-4 and IL-10 were greatly up-regulated. Signal transducer and activator of transcription 6 (STAT6) was significantly activated under blockade of IL-18. These conditions also caused significant reduction in liver neutrophil sequestration and liver injury. In conclusion, the data suggest that IL-18 is required for facilitating neutrophil-dependent hepatic ischemia/reperfusion injury through suppressing anti-inflammatory cytokine expression. (HEPATOLOGY 2004;39:699–710.)
Interruption of hepatic inflow during hepatectomy (Pringle maneuver) and vascular reconstruction of hepatic transplantation causes hepatic ischemia.1, 2 An acute inflammation associated with this adverse event induces significant organ damage/dysfunction.3 Experimental studies have revealed that after reperfusion, transcriptional factors such as nuclear factor-κB (NF-κB) are activated, and Kupffer cells release reactive oxygen species and proinflammatory cytokines, including tumor necrosis factor-α (TNF-α).4-6 TNF-α plays a major role in the subsequent inflammatory cascade, which promotes organ dysfunction through expression of ELR-positive CXC chemokines, including macrophage inflammatory protein-2 (MIP-2) and adhesion molecules.7, 8 These mediators facilitate neutrophil sequestration in ischemic liver and subsequent neutrophil-dependent organ dysfunction by releasing reactive oxygen species and protease.9
Although a number of investigations have reported that TNF-α aggravates inflammatory injury, some reports demonstrated that blocking interventions for TNF-α have been mostly unsuccessful in human clinical trials.10 These results suggest that other mediators may participate in the development of this inflammatory tissue injury. One of the authors previously demonstrated that interleukin 12 (IL-12) is expressed in the liver during hepatic ischemia and early reperfusion, and is required for induction of TNF-α.11 IL-12 is well known to cooperate with interleukin 18 (IL-18) in giving rise to inflammation12; IL-18 is another possible candidate. IL-18 was first identified as a factor capable of inducing interferon γ (IFN-γ) production from Th1 and natural killer cells in mice with endotoxin shock.13, 14 In addition to being an inducer of IFN-γ, IL-18 stimulates gene expression and synthesis of TNF-α, interleukin 1, Fas ligand, and several chemokines.15 IL-18 stimulation activates the DNA-binding activity of both transcriptional factors, NF-κB and activator protein 1 (AP-1).16, 17 More recently, IL-18 independent of IL-12 has been reported to have the potential to produce Th2 cytokines and induce Th2 cells in an interleukin-4-dependent manner.15 We hypothesized that IL-18 plays another important role in hepatic ischemia/reperfusion injury, but the precise mechanism of IL-18 participating in this injury has not been defined.
In the present study, we sought to determine the role of endogenous IL-18 in the development and regulation of inflammatory hepatic ischemia/reperfusion injury. We investigated expression of pro- and anti-inflammatory cytokines, such as interleukin 4 (IL-4) and interleukin 10 (IL-10), activation of transcriptional factors, neutrophil recruitment, and hepatocellular injury. The data suggest that IL-18 plays an important role in the hepatic inflammatory response to ischemia/reperfusion.
Abbreviations
NF-κB, nuclear factor-κB; TNF-α, tumor necrosis factor-α; MIP-2, macrophage inflammatory protein-2; IL, interleukin; IFN-γ, interferon γ; AP-1, activator protein-1; IgG, immunoglobulin G; MPO, myeloperoxidase; EDTA, ethylenediaminetetraacetic acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; MCP-1, monocyte chemotactic protein-1; IP-10, interferon-γ inducible protein-10; ELISA, enzyme-linked immunosorbent assay; EMSA, electrophoretic mobility shift assay; PCR, polymerase chain reaction; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate biotin nick end-labeling; mRNA, messenger RNA; STAT6, signal transducer and activator of transcription 6.
Materials and Methods
Hepatic Ischemia/Reperfusion Injury Model and Reagent.
Male C57BL/6 mice (Japan SLC, Inc., Hamamatsu, Japan) weighing 22 to 28 g were used in all experiments. This project was approved by the Chiba University Animal Care and Use Committee and was in compliance with National Institutes of Health guidelines. The model of partial hepatic ischemia and reperfusion employed was prepared as described previously4 Briefly, mice were anesthetized with sodium pentobarbital (60 mg/kg administered intraperitoneally). A midline laparotomy was performed and an atraumatic clip was used to interrupt the arterial and the portal venous blood supply to the left lateral and median lobes of the liver. Sham control mice underwent the same procedure, but without vascular occlusion. Mice were sacrificed after the indicated periods of reperfusion, and liver tissue (from the ischemic lobe) and blood samples were taken for analysis. For IL-18 neutralization studies, just before partial hepatic ischemia, mice received either sterile preimmune immunoglobulin G (IgG) or monoclonal anti–IL-18 antibody (25 μg per body; Medical & Biological Laboratories Co., Ltd., Nagoya, Japan) via the lateral tail vein, and the clip was removed, thus initiating hepatic reperfusion. This dose was adopted with reference to the study of Leung et al.18
Myeloperoxidase Assay.
Liver myeloperoxidase (MPO) content was assessed by methods described elsewhere4 Briefly, liver tissue (50 mg) was homogenized in 2 mL of homogenization buffer (3.4 mmol KH2HPO4, 16 mmol Na2HPO4, pH 7.4). After centrifugation for 20 minutes at 10,000g, 10 volumes of resuspension buffer (43.2 mmol KH2HPO4, 6.5 mmol Na2HPO4, 10 mmol ethylenediaminetetraacetic acid (EDTA), 0.5% hexadecyltrimethylammonium, pH 6.0) was added to the pellet and the samples were sonicated for 10 seconds. After heating for 2 hours at 60°C, the supernatant was reacted with 3,3′,3,5′-tetramethylbenzidine (Sigma Chemical Co., St. Louis, MO) and read at 655 nm.
Liver Edema.
The extent of liver edema was measured by tissue wet-to-dry weight ratios. After dissection, liver samples were weighed and then placed in a drying oven at 55°C until a constant weight was obtained. In this determination, liver edema is represented by an increase in the wet-to-dry weight ratio.
Blood Analysis.
Blood was obtained by cardiac puncture at the time of sacrifice. Serum samples were analyzed for alanine aminotransferase (ALT) and aspartate aminotransferase (AST) as indices of hepatocellular injury, using a diagnostic kit (WAKO Pure Chemical Industries, Osaka, Japan). Serum was also analyzed for IL-18, monocyte chemotactic protein-1 (MCP-1), interferon-γ inducible protein-10 (IP-10), TNF-α, MIP-2, IL-4, and IL-10 by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Medical & Biological Laboratories Co., Ltd.; BioSource International, Camarillo, CA; R&D Systems, Inc., Minneapolis, MN).
ELISA Quantification of Tissue Cytokines.
Quantitative assessment of IL-18, MCP-1, IP-10, TNF-α, MIP-2, IL-4, and IL-10 production in the liver was made by methods adapted from Villavedra et al.19 Briefly, liver tissue was homogenized in 10 volumes of homogenization buffer (10 mmol EDTA, 2 mmol phenylmethylsulfonyl fluoride, 0.1 mg/mL soybean trypsin inhibitor, 1.0 mg/mL bovine serum albumin, 0.02% sodium azide, and 0.2 μL/mL protease inhibitor cocktail [1000x stock; 1 mg/mL leupeptin, 1 mg/mL aprotinin, and 1 mg/mL pepstatin]). After incubating for 2 hours at 4°C, the homogenate was centrifuged at 12,500g for 10 minutes. Supernatant was removed and centrifuged again to obtain clear lysate. Total protein concentration of each sample was measured by a bicinchoninic assay kit (Pierce Chemical Co. Rockford, IL), and samples were dispensed for ELISA assays. Cytokine concentration was calculated per milligram total protein.
Electrophoretic Mobility Shift Assay (EMSA).
Nuclear extracts of liver tissue were prepared according to the method of Deryckere and Gannon.20 Protein concentrations were determined by bicinchoninic acid assay with trichloroacetic acid precipitation, using bovine serum albumin as a reference standard (Pierce Chemical Co.). Double-stranded NF-κB, AP-1, and signal transducer and activator of transcription 6 (STAT6) consensus oligonucleotide (Promega, Madison, WI) was end-labeled with [γ-32P] ATP (110 TBq/mmol, Amersham, Arlington Heights, IL). Binding reactions containing equal amounts of protein (20 μg) and 35 fmol (≈50,000 cpm, Cherenkov counting) of oligonucleotide were performed for 30 minutes in binding buffer (4% glycerol, 1 mmol MgCl2, 0.5 mmol EDTA, pH 8.0, 0.5 mmol dithiothreitol, 50 mmol NaCl, 10 mmol Tris, pH 7.6, and 50 μg/mL poly [dI•dC]; Pharmacia, Piscataway, NJ). Reaction volumes were held constant to 15 μL. Reaction products were separated in a 4% polyacrylimide gel and analyzed by autoradiography. Autoradiographs were digitized, and activation of STAT6 was quantitated with image analysis software (National Institutes of Health image 1.60, Bethesda, MD).
Real-Time Quantitative Polymerase Chain Reaction (PCR).
Total RNA from liver tissue was extracted using RNeasy Mini Kit (Qiagen Inc., Valencia, CA). An aliquot of 1 μg of total RNA was reverse transcribed to complementary DNA using a Ready-To-Go T-Primed First-Strand Kit (Amersham Pharmacia Biotech Inc., Piscataway, NJ). Real-time PCR was performed using a LightCycler rapid thermal cycler system (Roche Diagnostics Ltd., Lewes, UK) according to the manufacturer's instructions. Reactions were performed in a 20 μL volume with 0.5 μmol primers and MgCl2 concentration optimized between 2 and 5 μmol. Nucleotides, Taq DNA polymerase, and buffer were included in the LightCycler-DNA Master SYBR Green I mix (Roche Diagnostics Ltd.). A typical protocol took approximately 50 minutes to complete and included a 10-minute denaturation followed by 40 cycles with a 95°C denaturation for 15 seconds, 59°C annealing for 10 seconds, and 72°C extension for 15 seconds. Detection of the fluorescent product was carried out at the end of the 72°C extension period. To confirm amplification specificity, the PCR products from each primer pair were subjected to melting curve analysis and subsequent agarose gel electrophoresis. The quantification data were analyzed with the LightCycler analysis software as described by Morrison et al.21 Primers used in PCR reactions are shown in Table 1.
| Gene | Primer Sequence | |
|---|---|---|
| β-actin | Sense: | 5′-GTGGGCCGCTCTAGGCACCA-3′ |
| Antisense: | 5′-CGGTTGGCCTTAGGGTTCAGGGGGG-3′ | |
| MIP-2 | Sense: | 5′-GAACAAAGGCAAGGCTAACTGA-3′ |
| Antisense: | 5′-AACATAACAACATCTGGGCAAT-3′ | |
| IP-10 | Sense: | 5′-CCCGGGAATTCATACCATGAACCCAAGTGCTGCC-3′ |
| Antisense: | 5′-GTCACGATGAATTCCTTAAGGAGCCCTTTTAGACCT-3′ | |
| MCP-1 | Sense: | 5′-CTCACCTGCTGCTACTCATTC-3′ |
| Antisense: | 5′-GCATGAGGTGGTTGTGAAAAA-3′ | |
| IL-4 | Sense: | 5′-CCTCACAGCAACGAAGAACA-3′ |
| Antisense: | 5′-TGCTCTTTAGGCTTTCCAGG-3′ | |
| IL-10 | Sense: | 5′-ATAACTGCACCCACTTCCCA-3′ |
| Antisense: | 5′-GGTCTTCAGCTTCTCACCCA-3′ | |
| TNF-α | Sense: | 5′-AGCCCACGTAGCAAACCACCAA-3′ |
| Antisense: | 5′-ACACCCATTCCCTTCACAGAGCAAT-3′ |
Analysis of Apoptosis and Histological Examination.
In order to examine sensitive quantification of apoptotic hepatocytes, we used the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate biotin nick end-labeling (TUNEL) method, which labels the characteristic DNA double-strand breaks.22 The assay was carried out utilizing an apoptosis in situ detection kit (Wako Pure Chemical Co. Ltd., Tokyo, Japan) according to the manufacturer's instructions. Comparative staining with hematoxylin and eosin was also performed.
Statistical Analysis.
All data are expressed as mean ± SEM. Data were analyzed with a 1-way analysis of variance, and individual group means were then compared with a Student-Newman-Keuls test. Differences were considered significant when P values were less than .05.
Results
Expression of IL-18 During Hepatic Ischemia/Reperfusion.
To determine whether IL-18 was up-regulated during hepatic ischemia/reperfusion, we assessed serum levels of IL-18 by ELISA. Sham-operated controls had detectable expression of serum levels of IL-18 (Fig. 1A). At the end of 90 minutes of hepatic ischemia just before reperfusion, serum IL-18 expression did not increase relative to sham-operated controls. However, after reperfusion, serum expression of IL-18 significantly increased 1 hour after reperfusion—with maximal production at 4 hours of reperfusion—and remained elevated up to 24 hours of reperfusion (Fig. 1A). To examine actual hepatic expression of IL-18, we assessed hepatic protein levels of IL-18 by ELISA. Hepatic IL-18 expression significantly increased 1 hour after reperfusion and remained elevated up to 8 hours of reperfusion, but returned to baseline levels 24 hours after reperfusion (Fig. 1B).
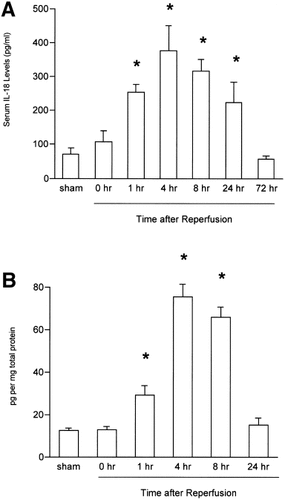
(A) Serum IL-18 expression during hepatic ischemia/reperfusion. (B)Hepatic IL-18 expression during hepatic ischemia/reperfusion. Samples obtained from sham control mice and mice undergoing ischemia for 90 minutes and then reperfusion were analyzed by ELISA. Data represent the mean ± SEM. *P < .05 versus sham-operated controls. For all groups, n = 5–7.
Role of Endogenous IL-18 in the Activation of NF-κB and AP-1.
In order to determine whether endogenous IL-18 regulates the activation of transcriptional factors NF-κB and AP-1, induced by hepatic ischemia/reperfusion, we performed EMSA. After reperfusion, nuclear translocation of NF-κB in mice with preimmune IgG increased 1 hour after reperfusion. NF-κB remained activated for 4 hours after reperfusion and was slightly decreased after 8 hours (Fig. 2A). Nuclear translocation of AP-1 slightly increased after 1 hour, and AP-1 was significantly activated 4 to 8 hours after reperfusion (Fig. 2B). Under neutralization of IL-18, activation of NF-κB was decreased relative to treatment with preimmune IgG. Under blockade of IL-18, activation of AP-1 was decreased relative to mice treated with preimmune IgG 1 to 4 hours after reperfusion. Activation of AP-1 under blockade of IL-18 was slightly increased but was still attenuated relative to positive control with preimmune IgG 8 hours after reperfusion (Fig. 2B).
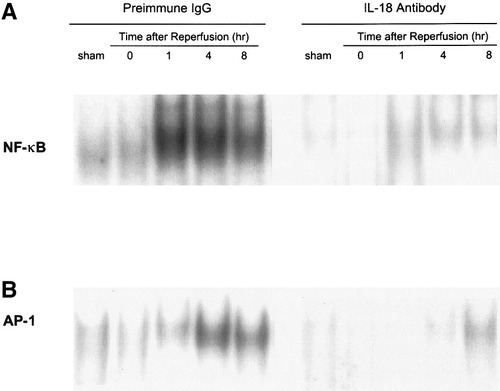
Effects of anti–IL-18 antibody on (A) NF-κB and (B) AP-1 activation in whole-liver homogenates during hepatic ischemia/reperfusion. Nuclear extracts from liver tissue were subjected to EMSA. (A and B) Results are representative of 4 separate time-course experiments. Mice that underwent sham operation or ischemia for 90 minutes were treated with preimmune IgG or anti–IL-18 antibody.
Endogenous IL-18 Is Required for Liver Apoptosis.
Since IL-18 signaling is known to induce hepatocyte apoptosis,15 histological examination by the TUNEL method was performed to investigate whether endogenous IL-18 was necessary for hepatocyte apoptosis. TUNEL-positive apoptotic cells were found 4 hours after reperfusion. In mice treated with anti–IL-18 antibody, a significant reduction in TUNEL-positive apoptotic cells was seen relative to preimmune IgG treatment mice (Fig. 3).
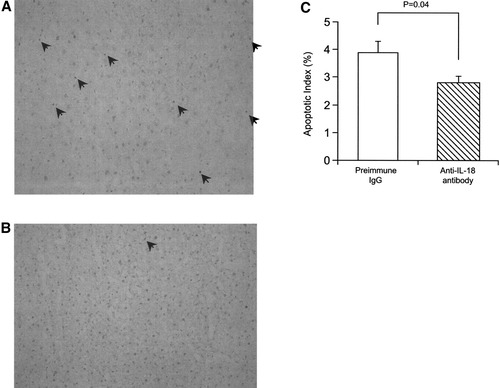
Effects of anti–IL-18 antibody on hepatic ischemia/reperfusion-induced apoptosis. TUNEL method was performed, using liver tissue harvested after ischemia and 4 hours of reperfusion in mice treated with (A) preimmune IgG or (B) anti–IL-18 antibody. Apoptotic cells are indicated with the arrow. (C) Apoptotic index. Data represent the mean ± SEM. For all groups, n = 5.
Role of Endogenous IL-18 in Hepatic Injury.
To assess whether endogenous IL-18 was required for hepatocellular injury and liver edema, serum levels of ALT and AST were measured and liver wet-to-dry weight ratios were determined up to 24 hours of reperfusion. Ischemia/reperfusion induced a significant increase in serum ALT and AST levels relative to sham-operated controls (P < .001). Administration of anti–IL-18 antibody reduced ischemia/reperfusion-induced serum ALT and AST levels 4 hours after reperfusion (Fig. 4A and B). Anti–IL-18 antibody reduced serum ALT and AST levels relative to positive control 8 to 24 hours after reperfusion (Fig. 4A and B). Significant liver edema was induced by hepatic ischemia/reperfusion up to 24 hours after reperfusion. Administration of anti–IL-18 antibody reduced liver wet-to-dry weight ratios 4 to 24 hours after reperfusion (Fig. 4C).
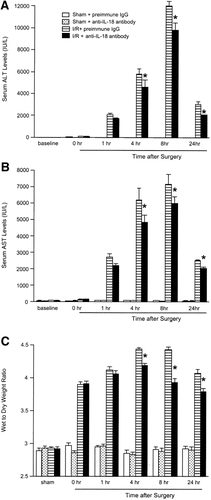
Effects of anti–IL-18 antibody on hepatocellular injury and liver edema induced by hepatic ischemia/reperfusion. Serum levels of (A) ALT and (B) AST were determined as an index of hepatocellular injury. (C) Liver edema was determined by tissue wet-to-dry weight ratio. Mice that underwent sham operation (Sham) or ischemia for 90 minutes (I/R) were treated with either preimmune IgG or anti–IL-18 antibody. *P < .05 versus treatment with preimmune IgG. For all groups, n = 7–10.
Endogenous IL-18 Is Required for Liver Neutrophil Recruitment.
To assess whether blockade of IL-18 was related to the reduction in liver neutrophil accumulation, we measured MPO content and examined histology. Administration of anti–IL-18 antibody did not lead to any significant alterations in liver MPO content after 4 hours of reperfusion (P = .053), but significantly reduced MPO content 8 to 24 hours after reperfusion (Fig. 5A). These results were consistent with histological findings that neutrophil recruitment decreased and that hepatic necrosis was reduced relative to mice treated with preimmune IgG 8 hours after reperfusion (Fig. 5B and C).
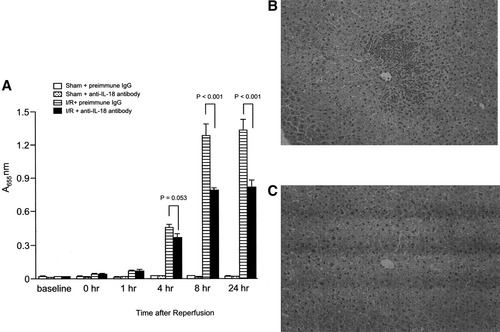
Effects of anti–IL-18 antibody on liver neutrophil recruitment after hepatic ischemia/reperfusion. (A) Liver neutrophil recruitment was analyzed for MPO content. Mice that underwent sham operation (Sham) or ischemia for 90 minutes (I/R) were treated with either preimmune IgG or anti–IL-18 antibody. (B) Abundant neutrophil accumulation and high degrees of necrosis were seen 8 hours after reperfusion. Mice that underwent ischemia for 90 minutes were treated with preimmune IgG. (C) Neutrophil accumulation and degree of necrosis were reduced 8 hours after reperfusion. Mice that underwent ischemia for 90 minutes were treated with anti-IL-18 antibody. Original magnification ×200. Data are mean ± SEM. For all groups, n = 7–10.
Effects of Endogenous IL-18 on Expression of Hepatic CXC Chemokines and CC Chemokines.
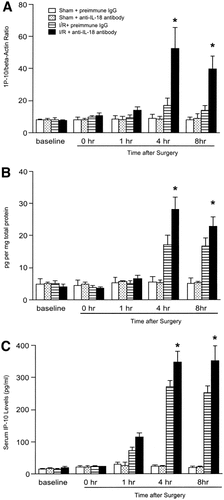
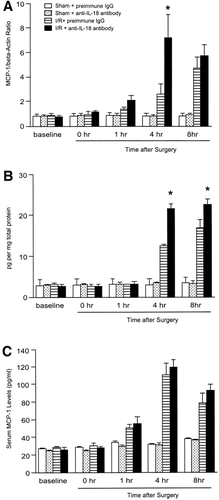
To determine whether endogenous IL-18 induces proinflammatory mediator expression, messenger RNA (mRNA) expression and hepatic and serum levels of TNF-α, MIP-2, MCP-1, and IP-10 were assessed. Administration of anti–IL-18 antibody did not lead to significant reduction in mRNA expression, nor did hepatic protein and serum levels of TNF-α for abrogated hepatic ischemia/reperfusion-induced increase up to 8 hours after reperfusion (Fig. 6). Administration of anti–IL-18 antibody did not reduce mRNA expression of MIP-2 until 4 hours after reperfusion. However, 8 hours after reperfusion, mRNA expression of MIP-2 (Fig. 7A) was significantly reduced in mice treated with anti–IL-18 antibody. This finding was consistent with the result that hepatic protein and serum levels of MIP-2 in mice treated with anti–IL-18 antibody were significantly reduced relative to mice treated with preimmune IgG 8 hours after reperfusion (Fig. 7). Hepatic ischemia/reperfusion increased mRNA expression of MCP-1 and IP-10. Administration of anti–IL-18 antibody significantly increased MCP-1 mRNA expression 4 hours after reperfusion, but did not significantly increase MCP-1 mRNA expression 8 hours after reperfusion (Fig. 8). Hepatic protein levels of MCP-1, but not serum levels of MCP-1, were significantly increased 4 to 8 hours after reperfusion (Fig. 8). Anti–IL-18 antibody administration significantly up-regulated ischemia/reperfusion-induced mRNA expression as well as hepatic and serum levels of IP-10 at 4 to 8 hours of reperfusion (Fig. 9).
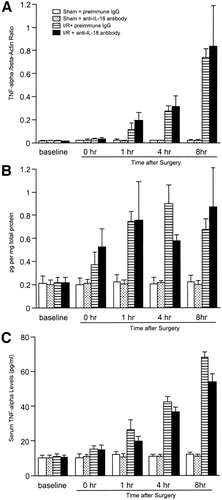
Effects of anti–IL-18 antibody on (A) hepatic mRNA expression, (B) hepatic protein expression, and (C) serum levels of TNF-α. Liver RNA extracts were analyzed by real-time PCR. Protein expression was analyzed by ELISA. Mice that underwent sham operation (Sham) or ischemia for 90 minutes (I/R) were treated with either preimmune IgG or anti–IL-18 antibody. Data represent the mean ± SEM. For all groups, n = 5–7.
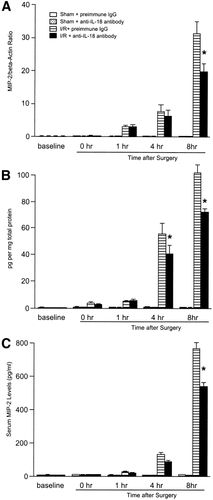
Effects of anti–IL-18 antibody on (A) hepatic mRNA expression, (B) hepatic protein expression, and (C) serum levels of MIP-2. Liver RNA extracts were analyzed by real-time PCR. Protein expression was analyzed by ELISA. Mice that underwent sham operation (Sham) or ischemia for 90 minutes (I/R) were treated with either preimmune IgG or anti–IL-18 antibody. Data represent the mean ± SEM. *P < 0.05 versus treatment with preimmune IgG. For all groups, n = 5–7.
Effects of anti–IL-18 antibody on (A) hepatic mRNA expression,(B) protein expression, and (C) serum levels of MCP-1. Liver RNA extracts were analyzed by real-time PCR. Protein expression was analyzed by ELISA. Mice that underwent sham operation (Sham) or ischemia for 90 minutes (I/R) were treated with either preimmune IgG or anti–IL-18 antibody. Data represent the mean ± SEM. *P < .05 versus treatment with preimmune IgG. For all groups, n = 5–7.
Effects of anti–IL-18 antibody on (A) hepatic mRNA expression, (B) hepatic protein expression, and (C) serum levels of IP-10. Liver RNA extracts were analyzed by real-time PCR. Protein expression was analyzed by ELISA. Mice that underwent sham operation (Sham) or ischemia for 90 minutes (I/R) were treated with either preimmune IgG or anti–IL-18 antibody. Data represent the mean ± SEM. *P < .05 versus treatment with preimmune IgG. For all groups, n = 5–7.
Endogenous IL-18 Suppresses Expression of Anti-inflammatory Cytokines (IL-4 and IL-10).
Since IL-18 is known to be Th1 cytokine, we determined whether the administration of anti–IL-18 antibody could increase anti-inflammatory cytokine expression. Both mRNA and hepatic protein expression of IL-4 were slightly increased 4 hours after reperfusion in mice treated with preimmune IgG (Fig. 10). In mice receiving anti–IL-18 antibody, hepatic mRNA and protein expression of IL-4 were significantly increased 1 hour after reperfusion. This increase was dramatic 4 and 8 hours after reperfusion (Fig. 10). This finding was consistent with the result that serum levels of IL-4 were significantly increased 4 to 8 hours after reperfusion (Fig. 10). Hepatic expression of mRNA and protein levels of IL-10 were increased 4 to 8 hours after reperfusion in mice treated with preimmune IgG (Fig. 11). In mice treated with anti–IL-18 antibody, mRNA and hepatic and serum protein expression of IL-10 were significantly increased 1 hour after reperfusion, and they remained elevated up to 8 hours after reperfusion (Fig. 11).
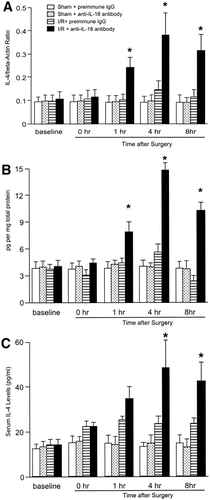
Effects of anti–IL-18 antibody (Ab) on (A) hepatic mRNA expression, (B) hepatic protein expression , and (C) serum levels of IL-4. Liver RNA extracts were analyzed by real-time PCR. Protein expression was analyzed by ELISA. Mice that underwent sham operation (Sham) or ischemia for 90 minutes (I/R) were treated with either preimmune IgG or anti–IL-18 antibody. Data represent the mean ± SEM. *P < .05 versus treatment with preimmune IgG. For all groups, n = 5–7.
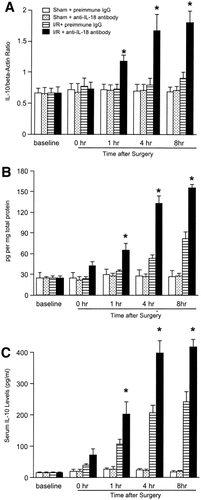
Effects of anti–IL-18 antibody (Ab) on (A) hepatic mRNA expression, (B) hepatic protein expression, and (C) serum levels of IL-10. Liver RNA extracts were analyzed by real-time PCR. Protein expression was analyzed by ELISA. Mice that underwent sham operation (Sham) or ischemia for 90 minutes (I/R) were treated with either preimmune IgG or anti–IL-18 antibody. Data represent the mean ± SEM. *P < .05 versus treatment with preimmune IgG. For all groups, n = 5–7.
Increased Anti-Inflammatory Cytokine Stimulates Activation of STAT6.
Since one of us previously demonstrated that IL-4 reduced hepatic ischemia/reperfusion injury by activation of STAT623 we performed EMSA to determine whether STAT6 was activated under condition of blockade of IL-18. After hepatic reperfusion, nuclear translocation of STAT6 was not increased in mice with preimmune IgG administration. On the contrary, nuclear translocation of STAT6 was increased 4 hours after reperfusion and associated with maximal IL-4 expression in mice under blockade of IL-18 (Fig. 12).
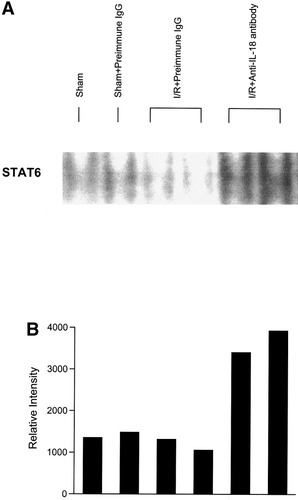
Effects of anti–IL-18 antibody on hepatic STAT6 activation. Nuclear extracts from liver tissue were subjected to EMSA. (A) Results are representative after 4 hours of reperfusion. Preimmune IgG or anti–IL-18 antibody was injected intravenously just before ischemia. (B) Autoradiographs were digitized, and activation of STAT6 was quantitated by image analysis.
Discussion
To date, IL-18 has been known for its ability to stimulate IFN-γ production from Th1 and NK cells during reactions to infection.14-17 The function of IL-18 in infectious inflammatory injury is known to occur mainly in cooperation with IL-12.12 However, few investigations have been accomplished regarding the role of IL-18 during noninfectious acute inflammatory reactions such as those that occur in hepatic ischemia/reperfusion. In this study, we provide evidence that IL-18 may play another role in hepatic ischemia/reperfusion injury. As shown by tissue ELISA, hepatic ischemia induced IL-18 expression 1 hour after reperfusion, up to 8 hours after reperfusion. IL-18 is known to be produced from Kupffer cells, macrophages, and antigen-presenting cells in the liver.13, 15 Since Kupffer cells are known to be activated shortly after reperfusion,5, 24 it is probable that Kupffer cells mainly produce IL-18 in the initial phase.
NF-κB and AP-1 are rapidly activated transcriptional factors that regulate the expression of inflammatory mediators such as TNF-α.4, 25 IL-18 has been reported to activate the DNA binding activity of NF-κB and AP-1.12, 15, 16 Our attempts to investigate whether neutralization of IL-18 suppresses activation of NF-κB and AP-1 in Kupffer cells have been limited because of difficulty in isolating enough Kupffer cells for sufficient nuclear extraction as a result of hypoperfused hepatic sinusoids caused by capillary plugging. It seems unlikely that the target cells for IL-18 effects in the initial phase are Kupffer cells, since neutralization of IL-18 was not capable of reducing TNF-α expression and the Kupffer-cell-mediated initial phase of liver injury as shown in Fig. 4. One of the authors previously demonstrated that IL-12 is up-regulated during the ischemia period and the initial phase of reperfusion and that IL-12 plays a role in the induction of the hepatic inflammatory response by up-regulating TNF-α expression.11 Lack of the ability to reduce TNF-α expression in the initial phase by anti–IL-18 antibody may be explained by the fact that Kupffer cells are activated shortly after reperfusion and IL-12 is already expressed before IL-18 expression.5, 11 IL-18 has been known to up-regulate expression of Fas ligand of lymphocytes, which binds to Fas ligand of hepatocytes, leading to hepatocyte apoptosis.15 The current study showed that anti–IL-18 antibody reduced hepatocyte apoptosis, suggesting that the target cells for IL-18 effects are, at least in part, lymphocytes.
We and others previously demonstrated that hepatic ischemia/reperfusion injurycomprises two phases.8, 26 Neutrophils mainly mediate the injury in the late phase.5, 8, 26 Cursio et al. demonstrated that apoptosis is also associated with hepatic ischemia/reperfusion injury.27 However, Gujral et al. reported that oncotic necrosis seems to be the principal mechanism of cell death for hepatocytes in longer periods of ischemia (60-120 minutes).28 We previously demonstrated that serum ALT levels are maximal 8 hours after reperfusion,8, 29 and the current histological study consistent with Gujral et al.28 showed that hepatic diffuse necrosis is induced concomitant with a significant increase in neutrophil recruitment. The reduced neutrophil accumulation under blockade of IL-18 is strikingly associated with a significant reduction in MIP-2 expression. The reduced expression of MIP-2 was linked with a reduction in the activation of NF-κB and AP-1. In agreement with these data is a recent article demonstrating that IL-18 plays a role in inducing neutrophil-mediated injury.18
The current data demonstrated that IL-10 and IL-4 were up-regulated under blockade of IL-18. It is likely that neutralization of IL-18 may cause lymphocytes to become Th2 cells, which would increase IL-4 and IL-10 expression. We previously demonstrated that IL-10 reduces this injury by suppressing NF-κB activation.4 Bogdan et al. revealed that macrophages are a primary cellular target of IL-10.30 The mechanism of the IL-18 inhibitory effect on IL-10 expression needs to be elucidated, but it seems likely that elevated hepatic production of IL-10 under blockade of IL-18 reduces activation of NF-κB in Kupffer cells and subsequent hepatocyte injury, resulting in a decrease in ELR-positive CXC chemokine(MIP-2) expression. Our previous study demonstrated that IL-4 induces STAT6 activation, which reduces hepatic ischemia/reperfusion injury associated with a decrease in neutrophil sequestration and MIP-2 expression, and the study demonstrated that this effect is completely lost in STAT6 knockout mice.23, 31, 32 More recent studies have suggested that activation of STAT6 may be involved in the suppression of proinflammatory mediator expression in macrophages.33, 34 Thus, the activation of STAT6 seems to be related to a significant reduction in MIP-2 expression, leading to neutrophil recruitment and subsequent hepatic injury. In the current results, significant up-regulation of IL-10 and STAT6 activation appear to profoundly down-regulate MIP-2 expression 8 hours after reperfusion under blockade of IL-18 expression. Taken together, it seems likely that expression of IL-18 suppresses the production of the anti-inflammatory cytokines IL-4 and IL-10, leading to hepatic inflammatory injury. In agreement with these data are recent articles demonstrating that IL-18 plays a role in inducing neutrophil recruitment and tissue ischemia/reperfusion injury.35, 36
The most surprising result was up-regulation of MCP-1 and IP-10 expression. MCP-1 is known to accumulate mononuclear cells in inflammatory sites, and IP-10 is known to accumulate T lymphocytes and induce expression of IFN-γ, which stimulates macrophage function.37, 38 Furthermore, Zwacka et al. have reported that T lymphocytes play a key role in activation of Kupffer cells and neutrophils during hepatic ischemia/reperfusion.39 We previously found by flow cytometric analysis that hepatic ischemia/reperfusion-induced up-regulation of MCP-1 and IP-10 increases accumulation of lymphocytes in the liver (data not shown). The present study showed that reduction in liver injury under blockade of IL-18 seemed to be modest, suggesting that there may be a compensatory effect of MCP-1 and IP-10 to the development of hepatic injury. Furthermore, it has been demonstrated that increased production of TNF-α is associated with neutrophil-dependent liver injury following hepatic ischemia/reperfusion.40 The inability to reduce TNF-α production by neutralizing IL-18 may suggest that TNF-α may also compensate for inflammatory cascade. There still remains concern that up-regulation of IP-10 under blockade of IL-18 might be protective for this injury because Colletti et al. demonstrated that a decrease in the balance of ELR-positive to ELR-negative CXC chemokines has a protective effect on hepatic ischemia/reperfusion injury.41
In conclusion, our data suggest that the function of IL-18 is distinct from that of IL-12, which mainly functions in the late phase of reperfusion by suppressing production of anti-inflammatory cytokines. The present data expand our knowledge of the immunoregulatory properties of IL-18. Our findings are clinically applicable to ischemia/reperfusion injury during hepatectomy and liver transplantation. Modulation of IL-18 expression may enhance endogenous protective effects, leading to a reduction in organ injury.




