Epidemiology and risk factors for hepatitis C in Alaska Natives
Abstract
Large cohorts of persons infected with hepatitis C virus (HCV) that include patients with multiple risk exposures and behaviors have been rarely reported. We herein describe a population-based cohort of 759 Alaska Natives (AN) with HCV who were recruited into a long-term follow-up study. History of injection drug use (IDU) was reported by 60.1% and blood transfusion by 14.0%. The most common genotype was 1a (42.0%), followed by 1b (20.3%), 2b (14.7%), 3a (14.3%), and 2a (7.8%). By multivariable analysis, risk exposures (blood transfusion vs. other; P < 0.01; odds ratio [OR], 2.87; 95% confidence interval [CI], 1.51–5.45) and year of infection (P < 0.01; OR, 3.47; 95% CI, 1.34–8.96) were significantly associated with HCV RNA-positivity. Having an RNA concentration ≥2 million copies/mL was associated with male gender (OR, 1.94) and genotype (P < 0.01 overall; 1a vs. 3a: OR, 1.92; 2b vs. 3a: OR, 3.17) by multivariable analysis. In conclusion, the two principal risk exposures for AN infected with HCV (IDU and blood transfusion) are the same as the overall U.S. population. Persons with a history of blood transfusion were more likely to be HCV RNA positive than those without such history. Higher RNA levels found in males may explain the more severe disease previously reported in this group. (HEPATOLOGY 2004;39:325–332.)
Hepatitis C virus (HCV) is a significant cause of chronic liver disease in the United States and throughout the world.1 Data from the third National Health and Nutrition Examination Survey (NHANES III) indicated that an estimated 2.7 million persons have chronic HCV infection in the United States, and that chronic infection seems to be more common among African Americans and as common among Mexican Americans as among white persons.2 However, little is known about the risk factors and prevalence of infection among Alaska Natives (ANs) and American Indians (AIs), because no seroprevalence or outcome studies have been conducted in these populations.
There are a limited number of published studies available that examine the epidemiologic factors, risk factors, and long-term outcome of HCV infection in a general population.3 Several studies describe outcomes for persons who contracted HCV through contaminated blood products.4-8 Other studies evaluated outcomes among HCV-infected persons who were referred to specialty medical centers for treatment,9, 10 and one large study described only persons who were injection drug users (IDU).11 The mortality and morbidity resulting from complications of HCV infection varies according to which patient group is evaluated. Thus, the available literature provides a confusing and sometimes contradictory picture of the expected long-term impact of HCV for outcomes such as the incidences of cirrhosis, end-stage liver disease, and hepatocellular carcinoma and the need for transplant. In addition, limited information has been published regarding the risk exposures and behaviors associated with infection as well as outcome in a population-based cohort with chronic HCV.
We have attempted to enroll into a long-term follow-up study all ANs and AIs living in Alaska who are infected with HCV to improve patient care and to evaluate prognostic features. This is possible because of an integrated system of health care delivery to AN/AI persons living in Alaska. Since 1989, the Alaska Native Medical Center (ANMC) and Arctic Investigations Program of the Centers for Disease Control and Prevention (CDC) has offered HCV testing at no cost to all facilities in Alaska serving AN and AI populations. More than 1,200 persons positive for anti-HCV have been identified, and clinical care for these individuals is coordinated by a statewide hepatology program. In 1994, ANMC and the University of Washington, Seattle, began a population-based longitudinal study to follow up persons infected with HCV to determine the disease outcome over time and to study the risk factors and virologic parameters that are associated with disease progression. This study is currently ongoing, and data on the outcome of HCV infection in this cohort will be analyzed and presented at a later date. This paper describes for the first time the epidemiologic factors, risk factors, genotype distribution of HCV, and factors associated with HCV RNA positivity and RNA concentration among ANs.
Abbreviations
HCV, hepatitis C virus; BT, blood transfusion; IDU, injecting drug use; Anti-HCV, antibody to hepatitis C virus; ETOH, alcohol; AN, Alaska natives; AI, American Indians; ANMC, Alaska Native Medical Center.
Methods
Health Care System and HCV Testing.
Alaska Natives are comprised of three main ethnic groups: Eskimo, Aleut, and Indian. Alaska natives receive their health care at no charge through an integrated health care system of primary community health practitioners in small villages, regional hospitals, and clinics located in medium-sized communities and at the ANMC, a referral hospital with specialty care and sophisticated diagnostic facilities located in Anchorage. It has been estimated that at least 90% of ANs receive most of their health care via this system. Since July 1990, blood donors used in this population have been screened for HCV infection using an ELISA.
Since July 1990, ANMC and Artic Investigations Program has offered at no charge testing for antibody to HCV (anti-HCV) for ANs from anywhere in Alaska. All anti-HCV tests from hospitals and clinics in Anchorage, Fairbanks, Sitka, Juneau, and Bethel are carried out at ANMC. These areas account for 79% (93,882 persons) of the AN population.12 Sera for HCV testing also are received intermittently at ANMC from the other areas of the state. The ANMC Viral Hepatitis Program staff conducts hepatology clinics 3 days a week in Anchorage, and one to three times per year in Fairbanks, Juneau, Sitka, Ketchikan, Barrow, and Bethel. Patients are referred from all areas in Alaska to ANMC for liver biopsies and evaluation for treatment, according to NIH Consensus Conference Recommendations.13
HCV Registry, Recruitment Efforts, and Enrollment.
In 1992, the ANMC Viral Hepatitis Program began a registry of ANs with HCV to improve patient care and tracking. All persons positive for anti-HCV and who have a positive confirmatory test (either recombinant immunoblot assay [RIBA] or polymerase chain reaction [PCR] for HCV RNA) are enrolled in this registry. In 1994, a formal study of HCV outcomes was begun by recruiting patients from this registry. The Alaska Area Native Investigational Review Board, the Indian Health Service National, and three regional AN health boards approved this study. All persons identified to be anti-HCV positive, or referred to one of the hepatology clinics, are invited by letter or phone call to participate in the long-term study. Those enrolled in the long-term follow-up study provide written and oral informed consent. Since 1997, patients not responding to the letters have had a note placed in their medical charts asking that they be referred to one of the hepatology clinics. Patients hospitalized at ANMC who are identified as anti-HCV positive are visited in the hospital and are invited to participate in the study.
On enrollment, a history, physical examination, and laboratory evaluations are carried out for each participant. Participants complete an in-person standard interview administered by one of the study nurses to obtain demographic data and information about risk exposures and behaviors associated with HCV infection. The two principal risk exposures that we asked patients were a history of intravenous drug use (IDU) and history of blood transfusion or blood products exposure before July 1992. Other behaviors of interest included household member history of HCV, history of tattooing, history of intranasal cocaine use, >10 lifetime sexual partners, and a sexual partner with a history of IDU. For purposes of analysis, persons with IDU were assigned to that risk exposure category. Persons who were not intravenous drug users but had received a blood transfusion were assigned to the blood transfusion risk exposure category. Persons without a history of IDU and who had not received a blood transfusion were assigned to the “other” category. Individual medical records of participants are reviewed for relevant medical history, including history of liver disease, previous liver biopsy, and alcohol use.
Retrospective Testing of Stored Sera and Estimation of Date of Infection.
The Alaska Area Native Health Services has a serum bank containing more than 600,000 sera collected from previous studies over the past 30 years that is maintained by the CDC Arctic Investigations Program in Anchorage, Alaska, and many HCV long-term study enrollees have sera stored here. When participants are enrolled in the study for HCV follow-up, their consent also is obtained for anti-HCV testing of stored sera. Available sera are tested in chronologic order to identify the earliest anti-HCV-positive date. The estimated date of infection is determined using the historical risk factor data and the results from tests of stored sera. The estimated date of infection is taken as the date of first intravenous drug use (IDU) or blood transfusion exposure (before July 1992) correlated with anti-HCV testing of stored sera specimen. For those persons without a history of IDU or blood transfusion, the estimated date of infection is taken as the date midway between the date of the last anti-HCV-negative sera result and the first anti-HCV-positive result.
Laboratory Testing.
Testing for anti-HCV is performed by enzyme-linked immunosorbent assay using commercial assays (Abbott Laboratories, Abbott Park, IL) at ANMC. Persons with an anti-HCV antibody-positive specimen have confirmatory testing by RIBA or PCR. If PCR was the first test performed and the result was negative for HCV RNA, then a RIBA test was performed. If RIBA was the first confirmatory test performed and it was positive, PCR was then performed. Testing for HCV RNA and HCV genotype was performed at the University of Washington. HCV viral RNA levels were determined by the branched DNA assay version 2.0 (Bayer Corporation, Tarrytown, NY) and by quantitative reverse transcription PCR. The limit of detection of the branched DNA assay is 200,000 genome equivalents/mL (g equivalents/mL). These results were converted to international units (IU/mL) using the manufacturer's recommended conversion factor of 5.8 g equivalents/mL to 1 IU/mL. For samples negative below this limit, an endpoint dilution assay using Roche Amplicor (Roche Diagnostic Systems, Branchburg, NY) was used to characterize further low-level viremia (limit, 100 copies/mL). HCV genotype was performed by restriction fragment length polymorphism analysis of the 5′ noncoding region as previously described.14 All participants are screened for antibody to hepatitis B core antigen, and those who are positive also are tested for hepatitis B surface antigen and antibody to hepatitis B surface antigen.
Statistical Analysis.
Minimum prevalence estimates were calculated as of June 30, 2002, using population denominators estimated from the 2000 U.S. census data. Comparisons of proportions were made using the χ2 test or logistic regression in univariable analyses. Continuous variables were compared between groups by use of the Wilcoxon rank sum test. Comparisons of genotype distribution were made with a likelihood ratio test using the software StatXact 4.0 (Cytel Software Corporation, Cambridge, MA). Exact P values or Monte Carlo P values for the likelihood ratio test were reported. Multivariable analyses of the proportion of participants who were RNA positive and with RNA concentration levels ≥ 2 million were conducted by use of logistic regression. Variables with a univariable P value less than 0.25 were considered in the multivariable models. Forward selection was used to evaluate main effects. Variables were considered confounders and remained in the model if their exclusion changed the value of the coefficient(s) of interest by more than 15%. We tested for statistical significance of two-way interactions after selection of main effects. P values were considered statistically significant at the 0.05 level.
Results
Participant Demographics.
A total of 981 ANs and AIs were found to be anti-HCV-positive and had a positive confirmatory test between July 1992 and June 2002. The minimum estimated prevalence of HCV in the AN population was found to be 0.82%, with the highest prevalence, 2.63%, found in those aged 40 to 59 years (Table 1). Of these, 759 (77.4%) are enrolled in the study. The mean age of the enrollees was 39.7 years (range, 1–82 years). Of those enrolled, 45.7% were male and 67.2% resided in an urban area (Anchorage, Fairbanks, or Juneau). No significant differences in age, gender, and residence (urban, rural) were seen between those persons who were enrolled and those not enrolled.
| Characteristic | Minimum Prevalence Estimate % of Population (number) [95% confidence interval] |
|---|---|
| Age (yrs) | |
| <20 | 0.02% (9) [0.00–0.03] |
| 20-39 | 0.81% (271) [0.72–0.92] |
| 40-59 | 2.63% (644) [2.43–2.84] |
| 60+ | 0.62% (57) [0.47–0.80] |
| Gender | |
| Female | 0.88% (528) [0.81–0.96] |
| Male | 0.75% (453) [0.69–0.83] |
| Residence | |
| Urban | 1.70% (645) [1.57–1.83] |
| Rural | 0.41% (335)* [0.37–0.87] |
| All | 0.82% (981) [0.77–0.87] |
- * One person with unknown residence.
Distribution of Risk Factors.
A risk exposure and behavior history was obtained for 750 (98.8%) study participants. Overall, 451 (60.1%) reported a history of IDU and 105 (14.0%) had a blood transfusion as their risk exposure. Other behaviors of interest were reported in 20.3% (n = 152); these included 39 (25.7%) with a history of tattooing, 105 (69.1%) who used intranasal cocaine, 95 (62.5%) who had >10 lifetime sexual partners, 22 (14.5%) who had lived with a household member with HCV, and 46 (30.3%) who had a sexual partner with a history of IDU. Forty-two participants (5.6%) did not report any risk exposures or behaviors of interest. Females did not report any risk exposures or behaviors of interest (7.9%) more often than males (2.9%; P < 0.01). There was no difference in the distribution of risk exposures or other behaviors of interest between urban and rural residents. Twenty-five enrollees (3.3 %) were known to be human immunodeficiency virus positive. Twenty-two percent (n = 167) of enrollees were positive for antibody to hepatitis B core antigen, of whom 11 (1.4%) were coinfected with hepatitis B virus (hepatitis B surface antigen positive).
Estimated Date of HCV Infection.
The year of HCV infection was estimated for 611 (80.5%) of the 759 participants using historical risk factor data and the results from anti-HCV tests on stored sera. The median estimated length of HCV infection at consent was 13.0 years (range, 0–49 years; Fig. 1). There was no difference in length of infection by residence; however, for males, the median length of infection (16.0 years) was higher than for females (11.0 years; P < 0.01). The median length of infection was higher for blood transfusion recipients (16.0 years) and those with a history of IDU (15.0 years) than for those without these two risk factors (7.0 years). The median age at the estimated date of infection was 24.0 years (range, 0–65 years). For those with a blood transfusion as their risk exposure, the median age at infection was 27.0 years (range, 0–65 years) compared with the median age for IDU of 22.0 years (range, 8–57; P < 0.01). There was no difference in median age at estimated date of infection by gender or residence.
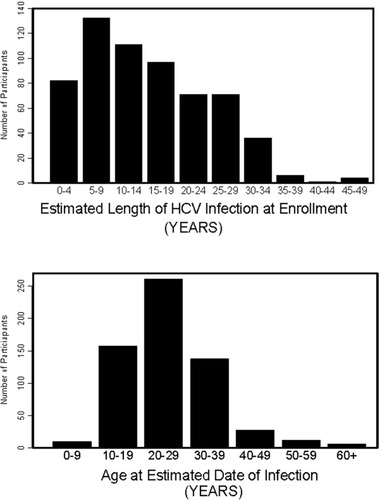
The estimated length of HCV infection in Alaska Natives at enrollment and the age of participants at their estimated date of infection.
HCV Genotype.
Genotype was determined within 1 year after enrollment for 498 (97.1%) of the 513 HCV RNA-positive participants. For 15 persons, HCV RNA was isolated, but we were unable to determine the genotype. The HCV genotype distribution by age group found in the AN population is shown in Table 2. The percentage of persons with HCV genotype 1 increased along with age group (<40 years [58%], 40–50 years [67%], ≥50 years [70%]; P = 0.03), and the percentage with HCV genotype 3 decreased with age group (P < 0.01; Table 2). We found no association between genotype and gender, residence (rural or urban), age at estimated date of HCV acquisition (<20 years, 20–39 years, ≥40 years), risk exposure (IDU, blood transfusion, or neither), and estimated decade of HCV infection (before 1970, 1970s, 1980s, after 1989).
| Genotype | Age Category at Enrollment | |||
|---|---|---|---|---|
| 0–<40 years % of age group (n) | 40–<50 years % of age group (n) | >50 years % of age group (n) | All Ages % (n) | |
| 1a | 39.9% (89) | 43.0% (90) | 45.4% (30) | 42.0% (209) |
| 1b | 17.0% (38) | 23.0% (48) | 22.7% (15) | 20.3% (101) |
| 1a/1b | 0.9% (2) | 0.5% (1) | 1.5% (1) | 1.0% (4) |
| 2a | 9.9% (22) | 4.8% (10) | 10.6% (7) | 7.8% (39) |
| 2b | 13.5% (30) | 16.3% (34) | 15.2% (10) | 14.7% (73) |
| 3a | 18.8% (42) | 12.4% (26) | 4.6% (3) | 14.3% (71) |
| Total | (223) | (209) | (66) | (498) |
HCV RNA Testing.
HCV RNA testing using a branched DNA assay or reverse transcription PCR was positive for 513 (73.2%) of the 701 who were tested within 1 year of enrollment (Table 3). The percentage of participants who were RNA positive did not differ by the year enrolled into the long-term study. Males were more likely to be RNA positive than females (odds ratio [OR], 1.51; 95% confidence interval [CI], 1.07–2.12; Table 3). Urban and rural enrollees had similar proportions of persons who were RNA positive. Of human immunodeficiency virus positive patients and hepatitis B virus carriers, 80% (20/25) and 50% (5/10), respectively, were RNA positive. Persons who had a blood transfusion as their risk factor were more likely to be RNA positive than those with a history of IDU as their risk or those with no risk exposure history (OR, 2.66; 95% CI, 1.44–4.89). No significant difference was found in the proportion who were RNA positive between those with IDU as a risk factor and those with neither IDU nor blood transfusion. Those who acquired their HCV infection at age 40 years or older tended to be more likely to be RNA positive than those infected when younger than age 40 years (OR, 2.19; 95% CI, 0.90–5.34). The RNA -positivity rate differed by estimated decade of infection (P < 0.01). This was the result of a higher frequency among those infected with HCV before 1970 compared with those infected after 1969 (OR, 3.83; 95% CI, 1.49–9.80). In a multivariable analysis that considered gender, age at infection (<20 years, 20–29 years, 30–39 years, ≥40 years), residence, risk factor, and decade of infection, only blood transfusion as a risk factor (vs. all others, P < 0.01; OR, 2.87; 95% CI, 1.51–5.45) and decade of infection (<1970 vs. after 1969, P < 0.01; OR, 3.47; 95% CI, 1.34–8.96) were independent factors associated with HCV RNA positivity. Controlling for age at infection (P = 0.58), the odds ratio for blood transfusion as a risk exposure for RNA positivity is 2.63 (95% CI, 1.37–5.04) and for decade of infection is 3.56 (95% CI, 1.34–9.47).
| Characteristic | % Hepatitis C Virus RNA+ | n | P Value | Odds Ratio [95% Confidence Interval] |
|---|---|---|---|---|
| Gender | ||||
| Male | 77.5 | 381 | 0.02 | 1.51 [1.07–2.12] |
| Female | 69.6 | 320 | ||
| Location | ||||
| Rural | 76.1 | 230 | 0.22 | 1.25 [0.87–1.80] |
| Urban | 71.7 | 471 | ||
| Risk factor | ||||
| Blood transfusion | 86.7 | 98 | <0.01* | 2.66 [1.44–4.89]† |
| Intravenous drug user | 69.5 | 423 | ||
| All others | 75.0 | 172 | ||
| Age at estimated infection date (yrs) | ||||
| <20 | 75.2 | 157 | 0.16‡ | 2.19 [0.90–5.34]§ |
| 20-29 | 68.8 | 250 | ||
| 30-39 | 72.2 | 126 | ||
| 40+ | 84.6 | 39 | ||
| Decade of estimated hepatitis C virus infection | ||||
| Before 1970 | 90.2 | 51 | <0.01∥ | 3.83 [1.49–9.81]** |
| 1970s | 76.0 | 146 | ||
| 1980s | 67.1 | 210 | ||
| After 1989 | 70.3 | 165 |
- Abbreviations: RT-PCR, reverse-transcription polymerase chain reaction; PCR, polymerase chain reaction.
- * P value comparing percent RT-PCR-positive rate for all three groups was <0.01. P value comparing percent PCR-positive for intravenous drug users vs. others was 0.18. P value comparing percent PCR-positive for blood transfusion participants versus all others combined was <0.01.
- † Odds of a participant being PCR positive if their risk factor is a blood transfusion versus the odds for all other groups combined.
- ‡ P value comparing percent RT-PCR-positive for all four age groups.
- § Odds of a participant being PCR positive if they were more than 40 years of age at estimated date of infection compared with if they were younger than 40 years of age.
- ∥ P value comparing percent PCR-positive rate for all four groups was <0.01. P value comparing percent PCR positive for 1970s, 1980s, and after 1989 was 0.19. P value comparing percent PCR positive before 1970 and after 1970 was <0.01.
- ** Odds of a participant being PCR positive if they were estimated to have been infected before 1970 compared with after 1969.
HCV RNA Concentration.
HCV RNA concentration was determined for 492 (95.9%) of the 513 RNA-positive participants. The median RNA concentration was 3.84 million copies/mL (662,069 IU/mL) and 61% (299/492) of participants had a concentration ≥2 million (344,828 IU/mL; Table 4). Males were more likely to have an RNA concentration ≥2 million genome equivalents than females (OR, 2.18; 95% CI, 1.51–3.17). No differences were observed in the proportion of participants with an RNA concentration ≥2 million by residence (urban or rural), risk factor, or age when infected. However, RNA levels increased along with the estimated duration of HCV infection. For each 5-year increase in the duration of infection, the odds of an RNA level ≥2 million copies/mL increased by 1.16 times (95% CI, 1.04–1.29). The percentage of participants with RNA levels ≥2 million varied significantly by genotype. RNA concentrations ≥2 million were seen most commonly among persons infected with genotypes 1a (66%; 132/200) and 2b (79%; 55/70). A multivariable analysis that considered gender, residence, risk factor, age at infection, duration of infection, and genotype found that gender (males vs. females, OR, 1.94; 95% CI, 1.24–3.03; P < 0.01) and genotype (P < 0.01 overall; 1a vs. 3a: OR, 1.92; 95% CI, 1.02–3.64; 2b vs. 3a: OR, 3.17; 95% CI, 1.36–7.39) were independently associated with having an RNA concentration level ≥2 million genome equivalents. Although in this model the duration of HCV infection was not predictive of RNA level (P = 0.12), we adjusted for this variable as a confounder in the final model.
| Characteristic | Median Concentration | % ≥2 Million | P Value Odds Ratio [95% Confidence Interval]* |
|---|---|---|---|
| Gender | |||
| Male | 5.87 | 70% (169/241) | <0.01 |
| Female | 2.21 | 52% (130/251) | 2.18 † [1.51–3.17] |
| Location | |||
| Rural | 4.10 | 60% (102/170) | 0.80 |
| Urban | 3.79 | 61% (197/322) | 0.95 † [0.65–1.39] |
| Risk exposure or behavior | |||
| Intravenous drug user | 4.17 | 65% (184/284) | 0.11 |
| Blood transfusion | 3.86 | 58% (46/80) | |
| All others | 3.04 | 54% (67/123) | |
| Age (yrs) at estimated infection date | |||
| <20 | 5.64 | 65% (75/115) | 0.15 |
| 20-29 | 4.08 | 64% (106/166) | 0.93† [0.84–1.03] |
| 30-39 | 2.94 | 56% (49/87) | |
| 40+ | 3.21 | 52% (15/29) | |
| Estimated length of infection (yrs) | |||
| 0-5 | 1.45 | 47% (23/49) | <0.01 |
| 6-14 | 3.31 | 59% (82/140) | 1.16‡ [1.04–1.29] |
| 15-25 | 4.12 | 65% (77/119) | |
| 25+ | 8.28 | 71% (63/89) | |
| Genotype | |||
| 1a | 4.07 | 66% (132/200) | <0.01§ |
| 1b | 3.47 | 57% (57/100) | 2.06 † [1.17–3.63]∥ |
| 2a | 3.26 | 51% (19/37) | 3.90 † [1.84–8.23]** |
| 2b | 10.49 | 79% (55/70) | |
| 3a | 1.90 | 48% (32/66) |
- * P values refer to comparison of proportions.
- † Odds ratio for a 5-year increase in age at estimated infection date.
- ‡ Odds ratio for a 5-year increase in estimated length of infection.
- § P value comparing percent for all five groups.
- ∥ Odds ratio and confidence interval for genotype 1a versus 3a.
- ** Odds ratio and confidence interval for genotype 2b versus 3a.
Discussion
This is the first population-based study to report the anti-HCV prevalence, risk exposures, and behaviors and HCV RNA levels among AN or NA populations. This report is also unique in that we describe a large population-based cohort who has been recruited into long-term follow-up. Identifying and enrolling this cohort was possible because of an integrated health care system for ANs with a centralized laboratory for testing and regional liver clinics. Of the 981 ANs identified with confirmed HCV infection, 759 (77%) were enrolled into the cohort. Some ANs with anti-HCV would not be detected by our case-finding efforts, such as persons receiving care from the private medical sector, persons living in some rural areas where testing is more difficult to obtain, and asymptomatic persons who do not know they are infected. However, we believe this group is small, because only 1,216 ANs positive for anti-HCV have been reported to the state of Alaska as of October 1, 2002, approximately 3% more than we have reported here (Castrodale L, Alaska Department of Health and Social Service, personal communication, 2002).
Similar to other reports from North America and Europe,2, 8, 15-21 the major risk exposure for HCV among ANs was a history of IDU (60%), with a history of blood transfusion before 1992 as the second leading risk exposure (14%). IDU has been reported to account for 45% to 65% of HCV transmission,17-19 but was more common (81%) among persons using a Veterans Administration hospital.14 Blood transfusion has been identified as a risk exposure for 18% to 25% of persons.17-19
The minimum prevalence estimate of anti-HCV among ANs is 0.8%, and 73.2% had HCV infections that were positive for HCV RNA. The proportion of persons with HCV exposure who have HCV RNA in their sera is similar to the 74% reported from NHANES III.2 HCV is more common among urban than in rural ANs. Although 45% of the AN population lives in urban areas, 65% of identified HCV cases were in urban residents. This finding is supported by a 1993 survey of ANs younger than age 30 years living in rural southwest Alaska, where only one person (0.2%) was anti-HCV positive (CDC, unpublished data, 1995). In contrast, hepatitis B is much more prevalent in rural than in urban Alaska.22 Studies among those with a history of IDU residing in Anchorage, Alaska, indicate that there was no difference found in the frequency of injecting drug use among ANs than other ethnic groups.23
Compared with the overall U.S. population in NHANES, we found a lower prevalence of HCV genotype 1a (42% vs. 56.7%) and higher prevalence of types 2a (7.8% vs. 3.5%) and 3a (14.3% vs. 7.4%) among ANs.2 We found a lower prevalence of genotype 1 (62%) and a higher prevalence of genotypes 2 and 3 (38%) compared with a study of 6,807 patients from 10 clinic-based practices in the U.S., 73% and 22%, respectively.24 Persons infected with HCV genotypes 2 and 3 have a higher success rate with antiviral treatments compared with those with genotype 1.13, 25 Thus, a higher proportion of ANs have a greater likelihood of responding to antiviral therapy. The ANTHC Viral Hepatitis Program has been evaluating HCV-infected ANs actively for therapy and offers treatment at no charge to eligible persons who meet the NIH Consensus Conference recommendations for therapy.13
We found that persons whose risk factor was blood transfusion were more likely to be HCV RNA positive than those with IDU as a risk factor or those without these risk factors. Previous studies have shown wide variation in the proportion of persons who are anti-HCV and HCV RNA positive, ranging from 55% to 90%.11, 19, 25-27 These previous studies generally have found that the prevalence of HCV RNA positivity is lower in younger age groups. However, we were not able to show significant differences in the rate of HCV RNA positivity with age. We found higher HCV RNA levels in males compared with females and for persons with genotypes 1b and 2b. In a large U.S. multicenter study, higher levels of HCV RNA also has been found in males compared with females and in persons infected with genotype 1 versus genotypes 2 and 3.24 Male gender has been shown to be a risk factor for the development of fibrosis8, 28; the higher level of HCV RNA found in men could be a factor in this process, yet this remains to be proven.
In conclusion, the prevalence estimates and major risk exposures in this population-based cohort of ANs with HCV are similar to those found in other populations in the United States. Approximately one quarter of the patients in our study cleared HCV infection spontaneously and significant differences in viral clearance were seen between those with a history of blood transfusion compared with those with other risk factors. Significant differences in the distribution of HCV genotypes exist between our population and the general U.S. population. Higher levels of HCV RNA are associated with male gender and genotypes 1a and 2b. We will continue to follow up this cohort to determine risk factors for the development of end-stage liver disease and hepatocellular carcinoma and factors associated with better clinical outcomes from treatment with antiviral drugs.