Prognostic impact and clinical value of low levels of flow cytometric MRD at the end of induction in childhood B-lineage acute lymphoblastic leukemia: Results of study ALL-MB 2015
Monitoring of minimal residual disease (MRD) using multicolor flow cytometry (MFC-MRD) or PCR (PCR-MRD) is currently the most powerful tool for assessing response to treatment in childhood B-cell precursor acute lymphoblastic leukemia (BCP-ALL).3 Both methods are typically used at the end of induction (EOI) to identify patients with slow leukemia elimination.1, 3, 4 Traditionally, PCR-MRD is considered more sensitive compared to MFC-MRD, as the reliable positivity threshold is at least one log lower (10−5 vs. 0.01%).2, 5, 6 Recent improvements in the MFC-MRD method now also allow a higher sensitivity with a cut-off value of 0.001%.7, 8 The aim of the present study was to investigate whether MFC-MRD positivity at EOI at particularly low levels (between 0.001% and 0.01%) could provide additional clinically valuable information in children with BCP-ALL treated with a reduced intensity protocol.
Between December 2014 and December 2021, 4925 consecutive pediatric patients (aged 1–18 years) with Ph-negative BCP-ALL in Russia and Belarus were enrolled in the ALL-MB 2015 Moscow-Berlin study (NCT03390387). Patients were categorized into risk groups if they met the criteria listed in Supporting Information S2: Table 1, and then assigned to treatment groups (TG), as shown in Supporting Information S2: Table 2. The therapy courses are shown in Supporting Information S2: Figure 1. Briefly, all patients in the TG A, B, C, D1-2, 1221-SR, and 1221-IR received induction therapy consisting of dexamethasone, vincristine, PEG asparaginase, daunorubicin, and triple intrathecal therapy, followed by three cycles of consolidation (six cycles for TG C) and maintenance therapy (Supporting Information S2: Table 3). Patients who did not achieve remission at EOI (day 36 of therapy) were finally stratified into TG E (Supporting Information S2: Table 2).
The MFC-MRD monitoring study was initially conducted on patients treated in facilities linked to the MFC laboratories of the Moscow-Berlin Group Flow Network.9 In March 2020, the study was extended to all Russian patients who routinely submitted their samples to the central laboratories. The bone marrow samples were taken at the EOI, as previously determined to be the most meaningful time point for the Moscow-Berlin Group protocols.10 The characteristics of the MFC-MRD study patients are listed in Supporting Information S2: Table 4.
MFC-MRD was assessed in two Russian laboratories using a well-harmonized approach.9 Both laboratories used the same MFC method, which is described in detail in the Supporting Information. Both laboratories had participated in the AIEOP-BFM-QA system11 and in intra-group performance tests.12 The high degree of harmonization and the sufficient cellularity of the BM samples at the EOI of the protocols of the Moscow-Berlin Group13 made it possible to define an MRD negativity of <0.001%.
-
MFC-MRD negativity—below 0.001%;
-
Low MFC-MRD—from 0.001% to less than 0.01%;
-
Intermediate/high MFC-MRD—MFC-MRD 0.01% or higher.
Event-free survival (EFS) and cumulative incidence of relapse (CIR) with standard errors (SE) were used as the main statistical points for outcome analysis. Details of the statistics used are described in the Supporting Information.
During the study period, a total of 1381 patients who had achieved remission at EOI were enrolled in the MFC-MRD study (276 were studied in Ekaterinburg and 1105 in Moscow). Of these, 757 children met the SR criteria, 554 met the ImR criteria, and 70 had hyperleukocytosis (WBC ≥ 100 × 109/L). With a median follow-up period of 4.8 years, the following results were achieved: the 5-year EFS ± SE was 87.2 ± 1.4% and the CIR ± SE was 11.0 ± 2.3%. The outcome in the study group did not differ from that of the other 3331 patients with ALL-MB 2015 with comparable characteristics who were not included in the study (Supporting Information S2: Figure 2).
At EOI, the MFC-MRD was ≥0.01% in 220 of 1381 patients (15.9%). Among them, 13 children were highly MFC-MRD positive (≥1%) and were therefore considered induction failures.14 207 of the remaining 1368 patients (15.0%) had an MFC-MRD ≥ 0.01% but below 1%; their 5-year EFS ± SE was 71.5 ± 4.6% and CIR ± SE was 26.4 ± 4.6%. In contrast, 1161 children with MFC-MRD < 0.01% had a significantly better outcome: 5-year EFS ± SE 91.2 ± 1.3% and CIR ± SE 7.1 ± 1.2%, p < 0.001 for both (Figure 1A).
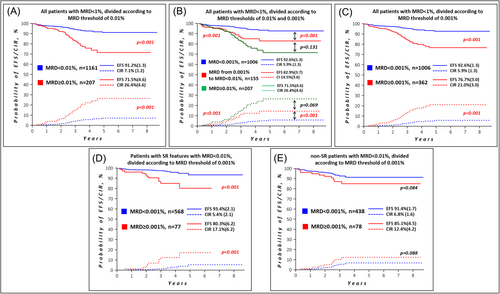
Of the 1161 patients with MFC-MRD < 0.01%, 155 (11.2% of the total study group) had low MFC-MRD (0.001% to less than 0.01%). Table 1 provides brief patient characteristics compared to 1006 children with MFC-MRD < 0.001% (negative) and 207 patients with MFC-MRD ≥ 0.01% but <1%.
| Group 1: MFC-MRD < 0.001% | pG1-G2a | Group 2: MFC-MRD from 0.001% to <0.01% | pG2-G3a | Group 3: MFC-MRD ≥ 0.01% | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Total | 1006 | 100 | 155 | 100 | 207 | 100 | ||
| Sex | ||||||||
| Male | 545 | 54.2 | 0.504 | 89 | 57.4 | 0.668 | 113 | 54.6 |
| Female | 461 | 45.8 | 66 | 42.6 | 94 | 45.4 | ||
| Age | ||||||||
| <10 y.o. | 829 | 82.4 | 0.182 | 135 | 87.1 | 0.111 | 166 | 80.2 |
| ≥10 y.o. | 177 | 17.6 | 20 | 12.9 | 41 | 19.8 | ||
| Initial WBC count | ||||||||
| <50 × 109/L | 857 | 85.2 | 0.081 | 123 | 79.4 | 0.254 | 175 | 84.5 |
| ≥50 × 109/L | 149 | 14.8 | 32 | 20.6 | 32 | 15.5 | ||
| Glucocorticoid responseb | ||||||||
| Good | 956 | 95.0 | 0.932 | 146 | 94.2 | 0.180 | 192 | 92.8 |
| Poor | 16 | 1.6 | 2 | 1.3 | 9 | 4.3 | ||
| ND | 34 | 3.4 | 7 | 4.5 | 6 | 2.9 | ||
| ALL-MB 2008 risk groups | ||||||||
| SRG | 568 | 56.5 | 0.286 | 77 | 49.7 | 0.530 | 106 | 51.2 |
| ImRG | 394 | 39.1 | 70 | 45.2 | 85 | 41.1 | ||
| HRG | 44 | 4.4 | 8 | 5.1 | 16 | 7.7 | ||
| Day 15 bone marrow response (by cytomorphology)c | ||||||||
| M1 | 876 | 87.1 | 0.771 | 138 | 89.0 | <0.001 | 135 | 65.2 |
| M2 | 79 | 7.8 | 13 | 8.4 | 42 | 20.3 | ||
| M3 | 13 | 1.3 | 1 | 0.7 | 22 | 10.6 | ||
| ND | 38 | 3.8 | 3 | 1.9 | 8 | 3.9 | ||
| t(12;21)(p13;q22)/ETV6::RUNX1 | ||||||||
| Present | 278 | 27.6 | 0.080 | 32 | 20.7 | 0.254 | 32 | 15.5 |
| Absent | 725 | 72.1 | 123 | 79.3 | 175 | 84.5 | ||
| ND | 3 | 0.3 | 0 | 0 | 0 | 0 | ||
| CNS leukemia (CNS3 status) | ||||||||
| Present | 23 | 2.3 | 0.075 | 8 | 5.2 | 0.407 | 6 | 2.9 |
| Absent | 976 | 97.0 | 147 | 94.8 | 201 | 97.1 | ||
| ND | 7 | 0.7 | 0 | 0 | 0 | 0 | ||
- Abbreviation: ND, no data.
- a Patients' distributions were compared with two-sided chi-square test.
- b Poor glucocorticoid response: blast count in peripheral blood ≥1000 cells/µL on day 8.
- c M1 bone marrow status was defined as leukemia cells <5%, M2 as leukemia cells 5%–25%, and M3 as leukemia cells ≥25%.
The treatment outcomes of 155 children with low MFC-MRD values were worse than those of 1006 children with MFC-MRD < 0.001% (Figure 1B): 5-year EFS ± SE 82.9 ± 3.7% versus 92.6 ± 1.3% and CIR ± SE 14.5 ± 3.6% versus 5.9 ± 1.3% (p < 0.001 for both). The relapse incidence in patients with various MFC-MRD levels is shown in Supporting Information S2: Table 5. The outcome of all 362 children with MFC-MRD ≥ 0.001% compared to the MFC-MRD-negative group (n = 1006) is shown in Figure 1C.
In the ALL-MB Group protocols, the MFC-MRD for EOI is generally used in the context of initially clinically defined risk groups.10 Of the 645 patients classified as SR who had an MFC-MRD < 0.01%, 77 had an MFC-MRD ≥ 0.001%. Compared to 568 children with an MFC-MRD < 0.001% (Figure 1D), they had a worse EFS (80.3 ± 6.2% vs. 93.4 ± 2.1%) and CIR (17.1 ± 6.2% vs. 5.4 ± 2.1%). In the non-SR group, 516 children had an MFC-MRD < 0.01%. MFC-MRD ≥ 0.001% was found in 78 of them. Only insignificant differences in EFS were seen between these 78 children and the other 438 children (Figure 1E). Low MFC-MRD values therefore only appear to have potential clinical significance in patients with SR features. The distribution of patients in the SR group according to the different thresholds for MFC-MRD at EOI is shown in Supporting Information S2: Table 6 (top panel). As shown, clinical application of the MFC-MRD threshold of 0.001% in SR patients would increase the number of MFC-MRD slow responders 5.5-fold (183 instead of 33 patients) compared to our conventional threshold of 0.1%. At the same time, the group of patients with a rapid response is reduced by 20.9%, with only an insignificant improvement of EFS (91.8 ± 1.6% to 93.4 ± 2.1%) and CIR (6.8 ± 1.5% to 5.4 ± 2.1%). A similar trend was observed in non-SR patients (Supporting Information S2: Table 6, bottom panel).
Various studies have shown that it is technically feasible to approach the MFC sensitivity of 0.001%.7, 8, 15 Finally, the EuroFlow consortium has demonstrated that high sensitivity of MFC-MRD detection in BCP-ALL patients can be achieved in routine practice if certain standardization procedures are performed and a sufficient number of cells are examined.8 In contrast, it is also clear that standardizing the detection of low MRD values (below 0.01%) by MFC is challenging.11, 16
In the current study, we show that the application of the highly-sensitive well-harmonized MFC-MRD method9 allows the identification of a considerable proportion of children with low MFC-MRD values (from 0.001% to less than 0.01%). Although we differ in part from the EuroFlow antibody set and approach,8 our methodology is similar in its main features and is also similar to the approaches of other groups in this field.5 Using the 0.001% threshold to define MRD positivity would increase the proportion of MFC MRD-positive patients to 362/1368 (26.5%), that is, 1.75-fold. This significant increase in MFC-MRD-positive patients correlates with previously published data describing the EOI as the time point with a large number of patients with MRD positivity just below the commonly used 0.01% threshold of the MFC-MRD.2, 6 Moreover, the reproducibility of our data is also based on the lower intensity of the treatment regimen. As previously shown,13 in ALL-MB group protocols, sufficient numbers of BM cells can be assessed at EOI in virtually all patients, as induction is relatively mild and BM regeneration is more active than in more intensive protocols.
The clinical relevance of these low MFC-MRD values still seems controversial. In the current study, children with detectable MFC-MRD values at EOI below 0.01% had an intermediate prognosis between fast and slow responders. Similar results were obtained in previous studies in which low MRD values were measured using various molecular techniques.17, 18 In contrast, setting 0.001% as the threshold for MFC-MRD positivity reduced the differences in EFS and CIR between the poor-risk and better-risk groups, mainly due to the higher proportion of MFC-MRD-positive patients with relatively good outcomes.
In the current study, the difference in outcome between MFC-MRD-negative patients and patients with very low MRD levels was limited to patients with SR features. However, for SR patients in the protocols of the “Moscow-Berlin” Group, the most clinically informative threshold is two logarithms higher than 0.001%19 and remained the strongest discriminator (Supporting Information S2: Figure 3). Possible use of later time points to better understand the outcomes of children with low MFC-MRD at the time of EOI seems not to be effective, as we know that even the assessment of MFC-MRD at the end of consolidation does not provide additional relevant information to the EOI data20 for patients treated in the current Moscow-Berlin Group protocols.
In summary, low-level MFC-MRD positivity (below 0.01%) on EOI identifies a substantial group of children with B-lineage ALL with intermediate prognosis. However, for clinical purposes, higher sensitivity currently offers no clear advantage. If the clinical relevance of the more precise measurement methodology is so borderline, one would also have to ask what the consequences are for patients who are classified as higher risk on the basis of the new definition. If patients are identified as higher risk according to new criteria, the consequence is usually an immediate intensification of therapy. For a considerable proportion of the patients affected, however, this would ultimately only mean an intensification of treatment without a substantial clinical benefit.
ACKNOWLEDGMENTS
The authors thank all doctors, nurses, and laboratory personnel in participating institutions, who were involved in patients diagnostics, management, and monitoring. We also thank “Podari Zhizn” charitable foundation for their support of centralized flow cytometric MRD monitoring.
AUTHOR CONTRIBUTIONS
Alexander Popov: Concept and design of the study, MFC-MRD data, data analysis and interpretation, writing the article. Guenter Henze: Concept and design of the study, data analysis and interpretation, writing the article. Julia Roumiantseva: Database handling, data analysis and interpretation, writing the article. Oleg Budanov: Database handling, statistics. Ekaterina Mikhailova: MFC-MRD data. Polina Lavrova: MFC-MRD data. Tatiana Verzhbitskaya: MFC-MRD data. Zhan Permikin: MFC-MRD data. Grigory Tsaur: Data analysis and interpretation. Svetlana Lagoyko: Database handling, data analysis. Liudmila Zharikova: Database handling, data analysis. Natalia Miakova: Patients data. Dmitry Litvinov: Patients data. Larisa Fechina: Concept and design of the study, general supervising, writing the article. Galina Novichkova: Concept and design of the study, general supervising, writing the article. Alexander Karachunskiy: Concept and design of the study, data analysis and interpretation, general supervising, writing the article. All authors have read and approved the final version of manuscript.
CONFLICT OF INTEREST STATEMENT
The authors have no relevant financial or non-financial interests to disclose.
CONSENT
Informed consent for the collection and investigation of samples was obtained from patients' parents or legal guardians.
ETHICS STATEMENT
This study was performed in line with the principles of the Declaration of Helsinki. The study was approved by the Ethics Committee of the Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology.
FUNDING
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.




