Limited stage mantle cell lymphoma: A real-world study of primary treatment and prognosis in Sweden 2006–2018
Radiotherapy (RT) is an alternative to chemoimmunotherapy (CIT) in early-stage mantle cell lymphoma (MCL) as associated with activity and lower toxicity compared to CIT.1-3 However, little is known how to stratify patients in relation to prognostic factors such as MCL International Prognostic Index (MIPI) and high-risk biology.4-7 Here, we present overall (OS) and progression-free survival (PFS) in relation to prognostic factors and given treatment in a population-based cohort of patients diagnosed with stage I–II MCL in Sweden 2006–2018.
The study included all patients diagnosed with MCL 2006–2018 in the Swedish Lymphoma Register (SLR).8 Early-stage MCL was defined as nodal or extra-nodal stage I or II disease, based on radiology with computer or positron emission tomography (PET) scan, peripheral blood count, and bone marrow examination. Patients were followed up to April 20, 2022. Patient characteristics, treatment, response, and data on documented relapse or progression proved by either radiology and/or biopsy were retrieved from SLR and supplementary medical records review. Data for calculation of Charlson comorbidity index (CCI) and survival data were retrieved from the National Patient Register and the Swedish Population Register respectively.9 Treatment was categorized as CIT, curative (≥24 Gy) or non-curative (<24 Gy) RT, watch and wait, or as other/missing. CIT followed by RT was grouped with CIT. High-risk biology was defined as blastoid histology, Ki67 ≥ 30%, or p53 overexpression (OE). Comparison of variables between subgroups was performed by Student's t-test, Mann–Whitney's test, or chi-square test. The Kaplan–Meier estimator was used for calculation of PFS and OS from end of first treatment if not otherwise specified until date of relapse or progression (PD) (PFS) or end of FU (OS + PFS). Hazard ratios (HRs) were estimated with Cox regression in univariable models by age, sex, ECOG, MIPI, stage, elevated lactate dehydrogenase (LDH), and RT ≥ 24 Gy and by multivariable models including variables with significant HRs (p < 0.05) in univariable analysis. Stata SE 16.1 was used for all analysis. The study was approved by the Regional Board of the Ethical Committee in Lund, Sweden (2018/739).
In total, 1412 MCL patients were identified, of which 173 (13%) fulfilled criteria for stage I–II disease. Out of stage I-II, PET-scan was used for staging in 8% and 22 (13%) patients had extra-nodal disease. Data on high-risk biology was available in 66 (64%) patients, of whom 30 (45%) had at least one high-risk biology marker. Stage I–II patients had lower MIPI, less frequently B symptoms, and elevated LDH compared to stage III–IV (Supporting Information S1: Table 1). Stage I (n = 72) patients were of lower age and blastoid MCL was less frequent compared to stage II (n = 101), but similar in B symptoms, CCI, and MIPI (Table 1).
| Stage I | Stage II | Total | p* | |
|---|---|---|---|---|
| N (%) | 72 (42) | 101 (58) | 173 (100) | |
| Age, median (range) | 69 (22–88) | 73 (47–93) | 71 | 0.138 |
| MIPI mean | 6.07 | 6.17 | 6.13 | 0.332 |
| B Symptoms (col%) | 8 (11) | 16 (16) | 24 (14) | 0.647 |
| CCI 0-1 (col%) | 50 (69) | 63 (62) | 113 (65) | - |
| CCI 2+ (col%) | 22 (31) | 38 (38) | 60 (35) | 0.336 |
| Available data on any high-risk biology marker (col%)a | 29 (40) | 34 (34) | 63 (36) | - |
| Any positive high-risk biology marker | 10 | 20 | 30 | |
|
4 | 10 | 14 | 0.471 |
|
2 | 0 | 2 | 0.234 |
|
8 | 14 | 22 | 0.018 |
| Primary treatment | n (col%) | n (col%) | n (col%) | |
| CITb | 29 (40) | 77 (76) | 106 (61) | 0.000* |
|
9 13) | 26 (26) | 35 (20) | |
|
9 (13) | 19 (19) | 28 (16) | |
|
5 (7) | 14 (1) | 19 (11) | |
|
2 (3) | 9 (9) | 11 (6) | |
|
2 (3) | 3 (3) | 5 (3) | |
|
2 (3) | 6 (6) | 8 (5) | |
| Single RT | 35 (49) | 13 (13) | 48 (28) | |
|
28 (39) | 9 (9) | 37 (21) | 0.000* |
|
7 (6) | 4(4) | 11 (6) | |
| No treatment (WaW) | 4 (6) | 4 (4) | 8 (5) | |
| Other/missingc | 4 (6) | 7 (7) | 11 (6) | |
| N (%) | 72 (42) | 101 (58) | 173 (100) |
- Note: p* indicates p-value of comparisons between stages I and II by Student's t-test (MIPI m), Mann–Whitney (age, median), or chi-square test (categorical variables).
- Abbreviations: BR, rituximab-bendamustine; CCI, Charlson comorbidity index; CIT, chemoimmunotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; MIPI, mantle cell lymphoma international prognostic index; OE p53, overexpression of p53; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; RT, radiotherapy.
- a Due to limited data on high-risk biology markers and risk of selection bias, data is not presented with percentage of all cases.
- b Chemoimmunotherapy included rituximab in 92 (87%) patients, 27 (93%) of stage I and 65 (84%) of cases with stage II.
- c Other/missing including no treatment of unknown reason (n = 4)/missing data (n = 7).
Of all 173 stage I–II patients, 106 (68%) patients received CIT, most frequently rituximab (R) with bendamustine (BR) (20%), the Nordic MCL2 protocol10 (16%), and R-CHOP (11%). 48 (28%) patients received single RT, among whom 37 (21% of all stage I/II) ≥24 Gy. Combinatory CIT with RT was delivered in 12 (11% of all CIT) patients (Table 1). Patients receiving RT ≥ 24 Gy were younger, had lower MIPI (mean MIPI 5.79 vs. 6.17, p < 0.001), and less high-risk biology compared to CIT (Supporting Information S1: Table 2). There was no difference in CCI among patients receiving RT ≥ 24 Gy and CIT. Stage I more often received RT ≥ 24 Gy, 28 (39%) of 72 patients compared to 9 (9%) of 101 with stage II (p < 0.001). Stage II received more frequently CIT compared to stage I, 77 (76%) of 101 patients compared to 29 (40%) of 72 patients (p < 0.001).
After primary treatment, 100 (75%) of 132 evaluated patients (76%) were in complete remission (CR), 25 (19%) in partial remission (PR), and 7 (5%) had stable (SD) or progressive disease (PD). There was no significant difference in CR rate between stage I and II patients or after CIT and RT ≥ 24 Gy (Supporting Information S1: Table 3).
Of the 11 (6%) patients treated with RT <24 Gy, 7 of 8 evaluated patients had CR. As demonstrated in Table 1, these patients were older and presented with inferior performance status and more comorbidities. Due to the small number of patients, further analysis was not performed.
Second-line treatment was administered in 70 (40%) patients. Of these, 46 (74%) received CIT with BR (n = 15, 33%), R-CHOP/cytarabine (including Nordic MCL2) (n = 9, 19%), or chlorambucil (n = 7, 15%). Seventeen patients (24%) received RT as second line, of whom 10 (59%) had RT as primary treatment. Data on recurrence, site was not available.
At a median FU-time of 3.98 (interquartile range [IQR]: 1.35–6.81) years of the entire cohort, median OS was 9.6 (95% confidence interval [CI]: 6.60–NR) years in stage I–II and 4.7 (95% CI 0.68–5.17) years in stage III–IV (Supporting Information S1: Figure 1).
At a median FU of 5.78 (IQR 2.73–8.46) years from end of first treatment in stage I–II, 5-y-OS was 69% (95% CI: 60–0.76) in stage I–II. 5-y-OS in stage I was 86% (95% CI: 66–94) after RT ≥ 24 Gy and 67% (95% CI: 64–94) after CIT. In stage II, 5-y-OS was 78% (95% CI: 36–94) after RT ≥ 24 Gy and 62% (95% CI: 50–72) after CIT (Figure 1). Age, MIPI, ECOG 2-4, and RT ≥ 24 Gy were significantly associated with OS in univariable analysis. In multivariable analysis, MIPI and age were associated with OS but neither stage nor treatment with RT ≥ 24 Gy versus CIT (Supporting Information S1: Table 4).
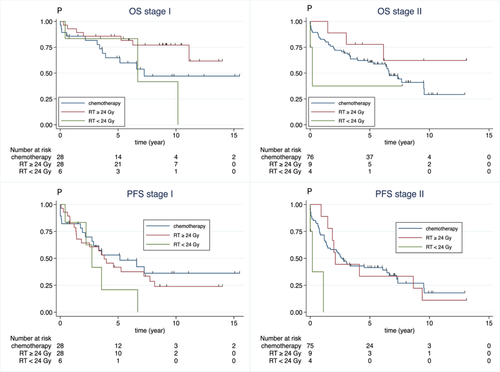
Overall (OS) and progression-free survival (PFS) in patients with stage I (left) and stage II (right) MCL stratified by primary treatment with CIT (blue), single RT ≥ 24 Gy (red) calculated by Kaplan–Meier estimates from end of primary treatment until progressive disease or relapse (PFS), death or last FU, whichever came first (OS + PFS). Median FU (year) of all patients were 5.78 (interquartile range [IQR]: 2.73–8.46) years, for stage I 6.49 (IQR: 0.24–13.8) and stage II 5.15 (IQR: 0.18–12.41) years, respectively. *N with data on end of treatment = 140 (81%). CIT, chemoimmunotherapy; FU, follow-up; OS, overall survival; PFS, progression-free survival; RT, radiotherapy.
At end of FU, 58 (33%) of 173 patients with stage I–II were alive without relapse, 72 (43%) patients were alive after relapse/PD and 43 (24%) patients had died from any cause (Supporting Information S1: Figure 1). Median PFS in stage I–II was 3.1 years (95% CI: 2.38–4.53). In stage I, 5-y-PFS was 53% (95% CI: 32–70) after CIT and 42% (95% CI: 23–59) after RT ≥ 24 Gy. In stage II, 5-y-PFS was 41% (95% CI: 30–52) after CIT and 33% (95% CI: 08–62) after RT ≥ 24 Gy. Age and MIPI were associated with PFS in both univariable and multivariable analysis but neither stage nor treatment with RT ≥ 24 Gy versus CIT. (Supporting Information S1: Table 4).
Out of 11 patients with stage I–II receiving low dose RT < 24 Gy, 7 (88%) of 8 evaluated patients achieved CR, 5-y-OS was 75% (95% CI: 31–93) and 5-y-PFS 14% (95% CI: 1–44).
Here, we demonstrate that ECOG PS, age and MIPI are robust prognostic markers for OS and PFS in early-stage MCL in a large population-based cohort of patients treated with RT only or with standard CIT regimens including BR, R-CHOP, or the dose-intensified Nordic MCL2 protocol.
The 5-y-OS stage I-II MCL in our cohort was lower than previously reported, probably related to higher age and more patients receiving low-dose radiation in our study.5
Our results confirm long-term survival with 5-y-OS > 85% after RT in stage I and CIT not being superior to RT in a cohort where all patients received rituximab and by adjustment for age and MIPI.5 The observed lower age in patients receiving RT ≥ 24 Gy compared to CIT may be explained by preference of RT over intensified protocols such as the Nordic MCL2 based on toxicity profile.2 A low risk of local relapse and higher risk of distant relapse after RT compared to CIT could be related to underestimated stage.5, 11 Unfortunately, lack of data on relapse site in our cohort limited such analysis. The nonsuperior PFS after RT ≥ 24 Gy in comparison to CIT indicates that patients are not cured by this strategy. However, the high CR rate and the favorable OS after RT in stage I patients support this strategy, as associated with low toxicity without affecting long-term prognosis. Moreover, the response rate and overall survival after low dose RT < 24 Gy seem to be comparable to RT ≥ 24 Gy, indicating that lower doses of RT could be preferable, that is, in elderly or frail patients. Of note, the small number of patients treated with <24 Gy RT limited further analysis on outcome in relation to prognostic factors and radiation dose. In stage II, the superior unadjusted PFS after CIT compared to curative RT is probably related to microscopic, advanced disease, and supporting the use of CIT.
High-risk biology has been confirmed as a prognostic marker in advanced-stage MCL, and here we demonstrate its presence even in early-stage disease, although the prognostic value could not be fully evaluated due to limited coverage in the registry.12, 13 Being a retrospective analysis, main limitations of this study include lack of intention to treat information, reasons for selected treatment, and patient's quality of life during and after treatment which would be valuable to evaluate. Moreover, data on lymphoma-specific death would have been valuable for the interpretation of the results.
To conclude, these findings support the use of RT as single modality in stage I MCL, preferably ≥24 Gy, as this was associated with long-term OS. Stage II is associated with higher MIPI and inferior outcome which supports the use of CIT. Still, the survival curves do not show a plateau indicating that none of the strategies are curative and future update including evaluation of biological markers and novel agents are needed to improve prognosis in these patients.
AUTHOR CONTRIBUTIONS
Alexandra Albertsson-Lindblad, Sara Ekberg, Ingrid Glimelius, Karin E. Smedby, and Mats Jerkeman designed the study. All coauthors participated in the collection of data. Alexandra Albertsson-Lindblad and Sara Ekberg prepared data and performed analysis. Alexandra Albertsson-Lindblad wrote manuscript, which was critically reviewed by all coauthors.
CONFLICT OF INTEREST STATEMENT
Ingrid Glimelius: Support to the department for educational purposes from Kite-Gilead and Jansen Cilag. Participate in a real-world data collaboration with support to the department from Takeda. Karin Ekströms-Smedby: Real-world data collaboration with Abbvie, Astra Zeneca, Janssen, Roche, BM. The remaining authors have no conflict of interest to report.
ETHICS STATEMENT
The study was approved by the Regional Board of the Ethical Committee in Lund, Sweden (2018/739).
FUNDING
This study was financed by Mrs. Berta Kamprad's Cancer Foundation. The funding agency has no implication with the protocol design, data analysis, or interpretation of the results. Furthermore, the funding agency is not involved in the decision to write, submit, or to publish the research article. All authors have access to the data.
Open Research
DATA AVAILABILITY STATEMENT
The study's data is derived from nationwide registers. Access to this data is subject to national data protection laws, but it can be obtained from the authors with permission from the Swedish Authority for Privacy Protection. The statistical analysis plan can be shared by the corresponding author. Interested researchers can contact the corresponding author for collaborative projects.




