Monoclonal gammopathy of undetermined significance with multiple paraproteins: A population-based screening study
Abstract
Monoclonal gammopathy of undetermined significance (MGUS) is the precursor of multiple myeloma (MM) and related disorders. MGUS is characterized by asymptomatic paraproteinemia. In some cases, multiple paraproteins can be identified but the clinical implications of this phenomenon are poorly understood. In this study, we aim to inform the approach to this challenging MGUS subgroup by utilizing data from iStopMM, a population-based screening study and randomized trial of follow-up strategies. In total, 75,422 Icelanders over the age of 40 were screened for MGUS with 3389 (4.4%) having at least one paraprotein of whom 303 (9%) had multiple paraproteins. IgM paraproteins were more common in those with multiple paraproteins (49% vs. 27% of paraproteins, p < 0.001), and IgM and non-IgM paraproteins frequently co-occurred (60% of cases). Two-thirds of these participants were randomized to active follow-up where only 31% of multiple paraproteins were persistent. Paraprotein concentrations were mostly independent, and although progression events were few, the progression rate was similar between those with multiple paraproteins and a single paraprotein. In a next-generation flow cytometry (NGF) sub-study, two phenotypically distinct aberrant plasma cell populations could be identified in some with multiple paraproteins. The findings suggest that multiple paraproteins often reflect independent ongoing disease processes that should be monitored independently but otherwise treated similarly to other MGUS cases. Specifically, the findings highlight the need for independent monitoring of IgM and non-IgM paraproteins in these individuals. The study provides novel insights into the management of this understudied MGUS subset.
INTRODUCTION
It was Jan Gösta Waldenström who made the first clear distinction between monoclonal and polyclonal gammopathies in a Harvey lecture in 1961.1 Dr. Waldenström had been studying patients with narrow bands of hypergammaglobulinemia on serum protein electrophoresis (SPEP) or monoclonal gammopathy. He noted that many of those with monoclonal gammopathy had multiple myeloma (MM), a malignancy of bone marrow plasma cells, and the eponymous syndrome of Waldenström macroglobulinemia (WM),2 but some were asymptomatic. Although first believed to be benign, we now know that this phenomenon, later termed monoclonal gammopathy of undetermined significance (MGUS), is the precursor of MM and related disorders, including WM and other lymphoproliferative disorders.3-6 MGUS is characterized by the presence of monoclonal immunoglobulins (paraproteins), detectable on SPEP, in the absence of MM or related disorders. The condition is common in the general population with a prevalence of 4.2% in individuals of Scandinavian descent above the age of 50 years7, 8 and about twice as more common in Black Americans9 and individuals of African descent from Ghana.10 The risk of progression of MGUS to overt malignancy has been estimated at 1%–1.5%,5, 7, 8 but several laboratory markers can be used to identify those with a higher risk of progression including paraprotein concentration and isotype as well as an abnormal free light chain (FLC) ratio.11
After the detection of paraproteins on SPEP, immunofixation electrophoresis (IFE) is commonly used to confirm the presence of paraproteins and to determine the isotype of the paraprotein. The most common isotype is IgG, which is associated with a lower risk of progression,11 followed by IgM and IgA and rarely IgE or IgD.7, 10 Furthermore, the paraprotein isotype can indicate different etiologies with IgG and IgA usually being produced by malignant plasma cells that can develop into MM4, 6 and IgM usually being produced by malignant mature B lymphocytes in WM or other lymphoproliferative disorders.5
In some cases, more than one paraprotein is observed in the same individual. These are usually reported as biclonal gammopathies,12 but triclonal gammopathies have also been reported.13 Here, we propose the term multiple paraproteins for these conditions. The proportion of those with MGUS who have multiple paraproteins differs between studies. This could be due to differences in the age and ethnicity of study participants but also due to differences in the sensitivity of the methods used and the reporting standards. Screening studies indicate that 3%–5% of White individuals with MGUS have multiple paraproteins7, 14 and 2% of individuals with MGUS of African descent in Ghana.10 Utilizing more sensitive mass spectrometry-based diagnostics in a high-risk cohort from the United States, the prevalence of MGUS overall rose from 6% to 41%, of whom 21% had multiple paraproteins, compared with 3% using SPEP and IFE. Most of these additional paraproteins had very low concentration, were transient in about half of cases, and were most commonly of IgM isotype.15
The clinical relevance of the presence of multiple paraproteins in individuals with MGUS is unclear.16 In a single-center study at the Mayo Clinic, a tertiary care referral center in the United States, 393 individuals with biclonal MGUS were followed over a median time of 5.9 years and were observed to have a similar risk of progression as individuals with a single paraprotein.12 Even so, MGUS with multiple paraproteins presents a challenge since it is not clear how to risk stratify or monitor these individuals who have paraproteins of differing concentration and isotype. Furthermore, it is unclear how to approach MGUS when both IgM and non-IgM paraproteins are present. Current guidelines do not provide any recommendations on how to approach this population with MGUS,11 with some calling for more data on the subject to provide clinical guidance.16 We were therefore motivated to examine this understudied group in a large, screened population-based cohort with MGUS. Here, we describe the prevalence, isotype patterns, and clinical outcomes of MGUS with multiple paraproteins.
METHODS
Study cohort
Study data were acquired from the Iceland screens, treats, or prevent multiple myeloma study (iStopMM), a population-based screening study for MGUS and randomized trial of follow-up strategies in Iceland. In 2016, all residents of Iceland born in 1975 and earlier were offered participation and 80,759 (54% of the underlying population) provided informed consent for participation in the study. In total, 75,422 of these (93%) were then screened for MGUS using SPEP and FLC assay with additional IFE if signs of paraproteins on SPEP. Those who had MGUS were randomized to three follow-up arms: arm 1 was not contacted and continued care in the Icelandic healthcare system as if they had never been screened while arms 2 and 3 were called into a study clinical center where they were followed according to current guidelines11 or according to a more intensive follow-up strategy including bone marrow sampling and imaging for all, respectively. The study design and recruitment have been described elsewhere.17
The study cohort included all participants in iStopMM who had at least one paraprotein confirmed on IFE at screening. Those who had more than one paraprotein at the time of screening were considered as having multiple paraproteins. Kappa or lambda bands without a corresponding heavy chain were not considered paraproteins.
Screening
After acquiring informed consent, serum samples were collected alongside and in addition to blood sampling being performed in the Icelandic healthcare system. Additionally, an active sample drive was initiated after 3 years of passive sampling. All samples were processed, aliquoted, and marked with unidentifiable study numbers at the laboratory at Landspítali–The National University Hospital of Iceland (NUHI). Samples were then shipped to The Binding Site laboratory in Birmingham, UK, where all samples were screened by SPEP by capillary zone electrophoresis (CZE; Helena Laboratories) and FLC assay (Freelite® assay on an Optilite® turbidimeter; The Binding Site; part of Thermo-Fisher Scientific). IFE (Helena Laboratories) was then performed on samples with clear or suspected paraproteins or an abnormal FLC ratio. CZE and IFE were assessed independently by at least two experienced observers.
Study data
Baseline characteristics, including SPEP, IFE, and FLC assay results, were acquired from all in the study cohort. Data on age and sex were acquired from Registers Iceland, a complete record of all residents of Iceland who each have a unique national identification number. Paraprotein number, isotypes, and paraprotein concentration were acquired from the screening sample.
Follow-up data including repeat SPEP and IFE were collected from a central laboratory database at the NUHI clinical laboratory, regardless of study arm, including SPEP and IFE done at the study clinic but also those done in clinical practice. Progression to more active disease was acquired from those in arms 2 and 3 who had at least one study clinic visit.
Statistical analysis
The prevalence of multiple paraproteins was calculated based on the number of individuals with at least one paraprotein and a 95% confidence interval (CI) calculated using nonparametric bootstrapping with 1000 iterations. Baseline characteristics were compared between those with multiple paraproteins and a single paraprotein using Student's t-test and chi-squared tests. We compared individuals with multiple paraproteins to those with a single paraprotein by whether the detected paraproteins could be quantifiable (i.e., were detectable on SPEP and not just IFE) or not, using the paraprotein with the highest concentration for each individual. Clinical outcomes, MM, WM, SMM, amyloidosis, other lymphoproliferative diseases (LP), and having a normal SPEP and IFE during follow-up, were compared between those with multiple paraproteins and a single paraprotein using logistic regression models adjusting for age and sex.
Next-generation flow cytometry of bone marrow
A subset of iStopMM participants were included in a flow cytometry sub-study. This included all participants with SMM and MM when possible and a conveniency sample of participants with MGUS. Bone marrow samples were acquired in EDTA tubes and transferred to a nearby study flow cytometry laboratory where next-generation flow cytometry (NGF) using the standardized MM-MRD EuroFlow® panel and protocol was performed. Antibody kits (Cytognos) and drop-in antibodies of CD138-BV421 (BD Biosciences) and CD27-BV510 (BioLegend) were used as described elsewhere.18, 19
For this study, we reviewed the available NGF results for participants with multiple paraproteins to identify cases with multiple or absent phenotypically abnormal plasma cell populations in the bone marrow.
RESULTS
A total of 3389 (4.4%) participants had at least one paraprotein. Of those, 303 had multiple paraproteins at screening (9.0%, 95% confidence interval [CI]: 8.1%–10.1%). Those who had multiple paraproteins were significantly older (72.0 vs. 68.9 years, p < 0.001) than those with a single paraprotein. IgM paraproteins were more common among those with multiple paraproteins (49% vs. 27% of all paraproteins, p < 0.001), while IgG was less common (48% vs. 63%; p < 0.001). However, IgA paraproteins, as a portion of total paraproteins, were the same for those with multiple paraproteins and a single paraprotein (12% vs. 13%, p = 0.63). There was no difference in the concentration of paraproteins (0.24 and 0.25 g/dL respectively; p = 0.41) (Table 1).
| Multiple paraproteins | Single paraprotein | Total | OR (95% CI) | p | |
|---|---|---|---|---|---|
| n | 303 | 3086 | 3389 | ||
| Median age (range) | 73 (43–100) | 69 (41–98) | 70 (41–100) | <0.001 | |
| % male | 58% | 53% | 54% | 0.12 | |
| Isotypes | |||||
| IgA | 83 (12%) | 402 (13%) | 485 (13%) | 0.50 | |
| IgG | 334 (48%) | 1957 (63%) | 2291 (61%) | <0.001 | |
| IgM | 275 (40%) | 727 (23%) | 1002 (27%) | <0.001 | |
| Median highest paraprotein (range) | 0.24 (0.02–6.3) | 0.25 (0.05–3.0) | 0.24 (0.02–6.3) | 0.41 | |
| No quantifiable paraprotein | 536 (17%) | 43 (14%) | 579 (17%) | 0.18 | |
| Outcomes | |||||
| Available | 167 (55%) | 1893 (61%) | 2060 (61%) | ||
| Multiple myeloma | 1 (0.6%) | 36 (1.9%) | 37 (1.1%) | 0.31 (0.04–2.25) | 0.22 |
| Smoldering multiple myeloma | 21 (12.6%) | 186 (9.8%) | 207 (6.1%) | 1.32 (0.84–2.07) | 0.23 |
| Waldenström macroglobulinemia | 4 (2.4%) | 9 (0.5%) | 13 (0.4%) | 4.78 (1.47–15.6) | 0.009a |
| Amyloidosis | 1 (0.6%) | 1 (0.1%) | 2 (0.1%) | 12.9 (0.80–207) | 0.07 |
| Other lymphoproliferative diseases | 0 (0%) | 15 (0.8%) | 15 (0.4%) | - | |
- Abbreviation: OR, odds ratio.
- a This was no longer significant when limiting the analysis to those with at least one IgM paraprotein.
At screening, the most common isotype pattern was IgG and IgM (40%), followed by multiple IgG (22%), multiple IgM (16%), and IgG and IgA (16%). IgA and IgM and multiple IgA patterns were rarer. Of those with multiple paraproteins, 76% had two paraproteins, 20% had three paraproteins, and 4% had four identifiable paraproteins. There was one case of six paraproteins (IgG and IgM pattern). Age and sex were not associated with different isotype patterns or numbers of paraproteins (Table 2). Having at least one IgM paraprotein was associated with having a higher number of paraproteins (2.2 vs. 2.4 paraproteins, p = 0.001).
| IgG–IgM | IgG only | IgM only | IgG–IgA | IgA–IgM | IgA only | Total | |
|---|---|---|---|---|---|---|---|
| n | 120 (40%) | 67 (22%) | 49 (16%) | 48 (16%) | 12 (4%) | 6 (2%) | 302 |
| Median age (range) | 73 (44–100) | 74 (47–95) | 74 (44–87) | 71 (43–96) | 71 (48–83) | 73 (57–81) | 73 (43–100) |
| % male | 56% | 43% | 59% | 79% | 50% | 83% | 58% |
| n paraproteins | |||||||
| 2 | 79 (65.8%) | 61 (91.0%) | 38 (77.6%) | 38 (79.2%) | 11 (91.7%) | 4 (66.7%) | 231 (76%) |
| 3 | 34 (28.3%) | 6 (9.0%) | 8 (16.3%) | 9 (18.8%) | 1 (8.3%) | 1 (16.7%) | 59 (20%) |
| 4 | 6 (5.0%) | 0 (0%) | 3 (6.1%) | 1 (2.1%) | 0 (0%) | 1 (16.7%) | 11 (4%) |
| 6 | 1 (0.8%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.3%) |
| n quantifiable paraproteins | |||||||
| 0 | 22 (18%) | 7 (10%) | 8 (16%) | 3 (6%) | 3 (25%) | 0 (0%) | 43 (14%) |
| 1 | 54 (45%) | 26 (39%) | 27 (55%) | 20 (42%) | 5 (42%) | 2 (33%) | 134 (44%) |
| 2 | 39 (33%) | 33 (49%) | 13 (27%) | 22 (46%) | 4 (33%) | 4 (67%) | 115 (38%) |
| 3 | 5 (4%) | 1 (2%) | 1 (2%) | 3 (6%) | 0 (0%) | 0 (0%) | 10 (3%) |
Overall, 83% of all detected paraproteins could be quantified on SPEP and the rest was only observable on IFE (limit of detection: ~0.01 g/dL). Analyzing each paraprotein independently, there was no statistically significant difference in the number of quantifiable paraproteins for those with multiple paraproteins or a single paraprotein (86% vs. 83%; p = 0.18). Multiple paraproteins could be quantified in 43% of those with multiple paraproteins. Individuals with higher number of paraproteins and at least one IgM paraprotein were found to have more nonquantifiable paraproteins (p < 0.001 and p = 0.001 respectively). There was no difference in the paraprotein concentration between those with multiple and a single quantifiable paraprotein (mean concentration 0.36 and 0.35 g/dL respectively, p = 0.78). In those cases where both paraproteins could be quantifiable, their concentrations were slightly, but statistically, significantly related (r2 = 0.08; 95% CI: 0.002–0.3).
During a median follow-up of 3.6 years, 31% of those who had multiple paraproteins at baseline had multiple detectable paraproteins during follow-up. Quantifiable paraproteins and higher concentrations were more likely to be persistent (p < 0.001 and p < 0.001, respectively) but there was no difference between paraprotein isotypes (p = 0.97). When multiple paraproteins could be quantified at screening, multiple paraproteins were more likely to be observed during follow-up as compared to those with a single and no quantifiable paraproteins (49% vs. 19% vs. 17% respectively, p < 0.001) (Table 3). There was only one individual (0.6%) with multiple paraproteins who developed MM compared to 36 (1.9%) of those with a single paraprotein, but the difference was not statistically significant (p = 0.22). There was no difference in the risk of WM after limiting the analysis to those with IgM paraproteins (4% vs. 2%; p = 0.54). There were no cases of other lymphoproliferative diseases (i.e., CLL and NHL) in those with multiple paraproteins compared to 15 (0.8%) in those with a single paraprotein (Table 1).
| Multiple quantifiable | Single quantifiable | No quantifiable | p | |
|---|---|---|---|---|
| n | 125 | 135 | 43 | |
| Median age (range) | 74 (44–100) | 73 (43–97) | 71 (48–90) | 0.45 |
| % male | 57% | 56% | 67% | 0.39 |
| Mean number of total paraproteins (SD) | 2.3 (0.6) | 2.2 (0.5) | 2.5 (0.7) | 0.004 |
| Nr total paraproteins | ||||
| 2 | 93 (74%) | 113 (84%) | 25 (58%) | |
| 3 | 26 (21%) | 20 (15%) | 14 (33%) | |
| ≥4 | 6 (5%) | 2 (2%) | 4 (9%) | |
| Paraprotein isotypes | ||||
| IgA | 44 (15%) | 33 (11%) | 6 (6%) | 0.02 |
| IgG | 151 (52%) | 137 (46%) | 46 (43%) | 0.14 |
| IgM | 93 (32%) | 126 (43%) | 56 (52%) | <0.001 |
| Paraproteins detected during follow-up | ||||
| n available | 76 (61%) | 83 (61%) | 24 (56%) | |
| None detected | 5 (7%) | 9 (11%) | 5 (21%) | <0.001 |
| Single paraprotein | 34 (45%) | 58 (70%) | 15 (63%) | <0.001 |
| Multiple paraproteins | 37 (49%) | 16 (19%) | 4 (17%) | <0.001 |
NGF results were available for 20 individuals with multiple paraproteins. Of those, 10 had MGUS and 10 had SMM. At least one distinct phenotypically aberrant plasma cell population could be identified in 7 out of 10 MGUS cases and all SMM cases. In four of those 17 cases, a second aberrant plasma cell population could be identified with differing expression of CD27, CD45, CD56, CD81, and CD117 as well as by different cytoplasmic light-chain expression in two cases (Figure 1).
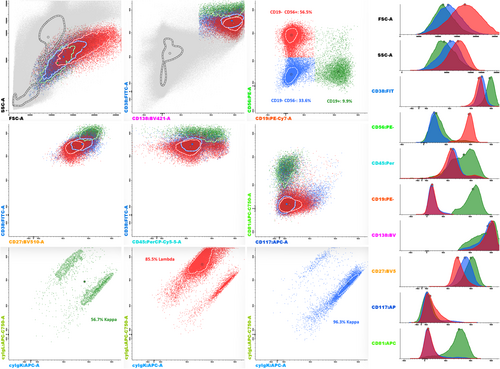
DISCUSSION
This population-based study of 75,000 individuals is the first comprehensive population-based study on individuals with MGUS and multiple paraproteins. We found the prevalence of multiple paraproteins in MGUS to be 9%, and higher with increasing age. This is a higher prevalence than previously reported in studies using conventional diagnostics (SPEP and IFE) (<5%)7, 10, 14 but lower than reported in studies utilizing mass spectrometry-based diagnostics (21%),15 suggesting that the difference may be due to differences in the sensitivity of the techniques or reporting standards. The findings show that a considerable number of those with MGUS have multiple paraproteins, highlighting the need for specific guidance on how to follow these individuals.11, 16 Furthermore, we find that the frequency of more than two paraproteins warrants the use of the term multiple paraproteins to describe this phenomenon instead of separating them as biclonal, triclonal, or similar. Future clinical guidelines of MGUS follow-up should address the care of this group of individuals with MGUS and multiple paraproteins specifically.
Interestingly, a majority of the paraproteins were transient with only a third having persistent multiple paraproteins through follow-up but paraproteins with higher concentrations were less likely to be transient. This indicates that some of the transient paraproteins may have gone below the limit of detection of IFE but some of the paraproteins were likely false positives, a conclusion which is supported by the absence of a clonal plasma cell population in 3 out of 10 available NGF analyses. Transient paraproteins have also been observed to be common overall when using more sensitive mass spectrometry-based diagnostics for MGUS screening,15 suggesting that small transient paraproteins could occur naturally. Very little has been published on transient paraproteins overall and their clinical implications are poorly understood.20, 21 They could be due to measurement error but the appearance of paraproteins could also occur in the wake of infection or other immune system activation with later immune suppression or physiological disappearance during the resolution of that immune response. However, data on this were not available for this study. Further studies, including some that are ongoing as part of iStopMM,22 are needed to inform the approach to transient paraproteins in MGUS overall and in those with multiple paraproteins.
Paraproteins of IgM isotype were more common in those with multiple paraproteins, seen in 60% of cases. Furthermore, the presence of IgM paraproteins was associated with higher numbers of paraproteins and more transient paraproteins. IgM and non-IgM paraprotein, which usually indicate two separate underlying disease entities, co-occurred in more than 40% of those with multiple paraproteins, with IgG and IgM being the most common isotype pattern. Interestingly, the paraprotein concentration was not significantly different between those with multiple paraproteins and a single paraprotein and there was only a weak correlation (r2 = 0.08) between the concentration of multiple paraproteins in the same individual. This indicates that the multiple paraproteins are mostly independent, a conclusion that is supported by the available NGF analyses showing two distinct phenotypically aberrant cell populations in the bone marrow of some individuals with multiple paraproteins. The findings therefore indicate that multiple paraproteins often reflect the presence of multiple independent clonal cell populations and that clinicians should monitor the different paraproteins separately. Based on these findings, we suggest that MGUS risk assessments be made using the paraprotein of highest paraprotein concentration and that changes in paraprotein concentrations be monitored separately for each paraprotein.
The clinical outcomes of MGUS with multiple paraproteins were similar to those with a single paraprotein. This is consistent with prior data on individuals with two paraproteins.12 However, it is notable that only one individual with multiple paraproteins progressed to MM, and although there were a considerable number who progressed to WM, there were no observed cases of NHL and other LPs. These observations should be interpreted with caution due to the low number of progression events that makes it impossible to rule out an association of multiple paraproteins and increased or decreased risk of MGUS progression. However, the findings support the conclusion that the different paraproteins represent, at least somewhat, independent disease processes. The findings also highlight the need to consider both MM as well as WM and other LPs as potential progression events when IgM and non-IgM paraproteins co-occur. We, therefore, suggest that, after repeat testing to determine the persistence of multiple paraproteins, those with both IgM and non-IgM paraproteins be followed both as IgM and non-IgM MGUS.
The study has several strengths. First, it is based on a screened cohort. This is unlike most other studies on MGUS where MGUS is diagnosed incidentally during the work-up for other diseases leading to biased selection of individuals with other medical disorders into the MGUS group.23 Second, because follow-up of participants is part of a clinical trial data on follow-up SPEP and IFE results and progression patterns are of high quality and their collection standardized across study participants. Third, this is the first study that we are aware of where NGF data are available for individuals with multiple paraproteins providing insights into the underlying biology of multiple paraproteins. Finally, all screening SPEP and IFE analyses were performed in the same laboratory and reviewed by the same experienced reviewers, limiting operator-dependent factors in screening samples.
There are also important limitations to the study. First, the median follow-up was short and progression events were few, which may have precluded the detection of differences in progression risk. Second, follow-up SPEP and IFE analyses were performed in another laboratory and interlaboratory differences or different reporting guidelines may have contributed to the high rate of transient paraproteins in the study. Third, NGF data were available in a minority of participants and cannot discern the genotype of the different aberrant plasma cell populations. Furthermore, different clonal population may have had the same phenotype making them non-discernible on NGF. Finally, the study population of iStopMM is genetically homogenous and almost exclusively white, potentially limiting the generalizability of the findings to other populations with MGUS.
In conclusion, in this population-based screening study with more than 75,000 participants, we present the first comprehensive study on individuals with MGUS and multiple paraproteins. The findings show that just under 10% of those with MGUS have multiple paraproteins but that most of these are transient. IgM paraprotein is more common among those with multiple paraproteins but other isotypes are also often present and IgM and non-IgM paraproteins co-occur frequently presenting a clinical challenge. Interestingly, our findings indicate that the different paraprotein reflect relatively independent underlying disease processes, but that, during the relatively short study follow-up, progression risk is similar for those with multiple and single paraproteins. More follow-up and future studies are needed to confirm this finding. The findings highlight the need for specific guidance on how to follow individuals with MGUS and multiple paraproteins. We suggest that laboratory reports and clinicians consider the multiple paraproteins separately and follow them independently, being particularly mindful of the potential of the different progression events in individuals with co-occurring IgM and non-IgM paraproteins. The findings provide some clarity to a previously confusing and understudied field of plasma cell disorders but also highlight the lack of attention that this subgroup has in the medical literature. These complex cases are where guidance is most sorely needed. By applying these lessons to clinical practice, the care of individuals with MGUS can be improved and hopefully their overall outcomes.
AUTHOR CONTRIBUTIONS
Sæmundur Rögnvaldsson conceived the study and its design with further input from Sigrun Thorsteinsdóttir, Ingigerdur S. Sverrisdottir, Malin Hultcrantz, Jón Þ. Óskarsson, Thorir E. Long, Robert Palmason, and Sigurdur Y. Kristinsson. Data analysis was performed by Sæmundur Rögnvaldsson. Sigurdur Y. Kristinsson supervised the project and the iStopMM study. Sæmundur Rögnvaldsson, Jón Þ. Óskarsson, Sigrun Thorsteinsdóttir, Malin Hultcrantz, Elias Eythorsson, Thorir E. Long, Isleifur Olafsson, Ingunn Thorsteinsdottir, Brynjar Vidarsson, Pall T. Onundarson, Bjarni A. Agnarsson, Margret Sigurdardottir, Asbjorn Jonsson, Brian G. M. Durie, Stephen Harding, Ola Landgren, Thorvardur J. Love, and Sigurdur Y. Kristinsson are part of the iStopMM investigators team and made major contributions to the study conduction. All coauthors had access to and edited the manuscript.
CONFLICT OF INTEREST STATEMENT
Sæmundur Rögnvaldsson: Honoraria for scientific talks: Siemens Healthineers and Johnson & Johnson. Malin Hultcrantz: Amgen, GlaxoSmithKline, Daiichi Sankyo Company–Research grant; Bristol-Meyers Squibb, Curio Science, GlaxoSmithKline, Intellisphere–consultant/advisor. Stephen Harding: Current employment at The BindingsSite Ltd. Ola Landgren: Research funding from NCI/NIH, FDA, LLS, Rising Tide Foundation, MMRF, IMF, Paula and Rodger Riney Foundation, Tow Foundation, Myeloma Solutions Fund, Perelman Family Foundation, Amgen, Celgene, Janssen, Takeda, Glenmark, Seattle Genetics, Karyopharm; Honoraria for scientific talks/participated in advisory boards for: Adaptive, Amgen, Binding Site, BMS, Celgene, Cellectis, Glenmark, Janssen, Juno, Pfizer; Independent Data Monitoring Committees for: Takeda, Merck, Janssen. Sigurdur Y. Kristinsson: Research funding from Amgen and Celgene; Independent Data Monitoring Committee for Jansen.
ETHICS STATEMENT
The study protocol, all information material, biobank, and questionnaires were approved by the Icelandic National Bioethics Committee (Number 16-022, date: 2016-04-26) with approval from the Icelandic Data Protection Agency. Access to national healthcare registries has been approved by the Icelandic Directorate of Health and the Icelandic Cancer Society. The study was preregistered on ClinicalTrials.gov (ClinicaTrials.gov identifier: NCT03327597). All participants in the study provided written informed consent for the study.
FUNDING
The work of this study was funded by research funding from Landspítali–the National University Hospital of Iceland, the Black Swan Research Initiative by International Myeloma Foundation, the European Research Council (grant agreement No 173857), The Leukemia & Lymphoma Society, and The Icelandic Centre for Research (grant agreement No 716677). OL is supported by the Sylvester Comprehensive Cancer Center NCI Core Grant (P30 CA 240139), Riney Family Multiple Myeloma Research Program Fund, Tow Foundation, Myeloma Solutions Fund, and Perelman Family Foundation.
Open Research
DATA AVAILABILITY STATEMENT
Current approvals do not allow for sharing of the underlying study data. However, data may be shared with other investigators pending the review of the iStopMM investigators and the Icelandic Scientific Ethics Committee.




