Evaluation of the R2-ISS in real-world patients with newly diagnosed multiple myeloma: A nationwide cohort study by the Korean Multiple Myeloma Working Party (KMM 2202)
Multiple myeloma (MM) is a plasma cell neoplasm with significant variability in survival outcomes due to its complex and heterogeneous nature. Therefore, the development of an accurate prognostic model is crucial in clinical practice to guide treatment decisions, predict disease outcomes, and inform patients.
Recently, the European Myeloma Network (EMN) developed and validated the second revision of the International Staging System (R2-ISS) using a large number of patients with newly diagnosed MM.1 The R2-ISS incorporates several risk factors, including the ISS, serum lactate dehydrogenase (LDH), del(17p), t(4;14), and 1q gain/amplification. Each risk feature is assigned a specific value (ISS II: 1; ISS III: 1.5; del[17p]: 1; elevated LDH: 1; t[4;14]: 1; and 1q gain/amplification: 0.5 points), and patients are classified into four risk groups based on the cumulative score: low (R2-ISS I, 0 points), low-intermediate (R2-ISS II, 0.5–1 points), intermediate-high (R2-ISS III, 1.5–2.5 points), and high (R2-ISS IV, 3–5 points). The R2-ISS has demonstrated enhanced discriminative capability, particularly for patients with intermediate risk, compared to the original Revised ISS (R-ISS).
Yet, it is important to note that the development and validation of the R2-ISS were primarily based on patients enrolled in clinical trials, which often exclude individuals with comorbidities and poor performance status. Thus, the objective of our study (KMM 2202) is to validate the R2-ISS in an independent cohort of patients from real-world clinical practice and compare its performance to that of the R-ISS.
We collected data from newly diagnosed MM patients treated between January 2010 and July 2019 at 13 hospitals participating in the Korean Multiple Myeloma Working Party in South Korea. All patients received either an immunomodulatory drug (IMiD) or a proteasome inhibitor (PI) as part of their first-line treatment. Only the patients with complete information on both R2-ISS and R-ISS were included in the study. In situ hybridization fluorescence in situ hybridization (iFISH) studies were performed on sorted or immunologically recognized plasma cells, following the specific iFISH methods of each institution. The study received approval from the institutional review boards of all participating institutions.
Kaplan–Meier method was used to estimate survival rates and the log-rank test was employed to compare survival curves. The performance of R2-ISS was evaluated by comparing it with R-ISS using the concordance index (Harrell C-statistic) and Akaike's information criterion (AIC).2, 3 The C-index measures the predictive ability of the model, with higher values indicating better discriminative power and a value of 1 representing perfect discrimination. The AIC provides a relative measure of model quality, where smaller values indicate a better-fitting model.
A total of 572 newly diagnosed MM patients who received IMiDs and/or PIs as part of their first-line treatment and had complete information on R2-ISS and R-ISS were included in the study. The baseline characteristics of the patients are summarized in Table 1. The median follow-up duration was 113.0 months (95% confidence interval [CI]: 109.0–116.0). The median progression-free survival (PFS) was 23.8 months (95% CI: 20.4–26.7), and the median overall survival (OS) was 62.6 months (95% CI: 55.91–70.5) (Supporting Information S1: Figure 1). A comparison of the baseline characteristics between our cohort and the EMN cohort (n = 2226) is summarized in Supporting Information S1: Table 1.
| Characteristics | Total patients (n = 572) |
|---|---|
| Age at diagnosis (years) | 63 (36–88) |
| <65 | 327 (57.2%) |
| ≥65 | 245 (42.8%) |
| Sex | |
| Female | 251 (43.9%) |
| Male | 321 (56.1%) |
| ECOG PS | |
| 0–1 | 399 (69.8%) |
| ≥2 | 163 (28.5%) |
| Not available | 10 (1.7%) |
| ISS | |
| I | 111 (19.4%) |
| II | 231 (40.4%) |
| III | 230 (40.2%) |
| R-ISS | |
| I | 78 (13.6%) |
| II | 389 (68.0%) |
| III | 105 (18.4%) |
| R2-ISS | |
| I | 69 (12.1%) |
| II | 163 (28.5%) |
| III | 283 (49.5%) |
| IV | 57 (10.0%) |
| LDH | |
| Normal | 421 (73.6%) |
| >UNL | 151 (26.4%) |
| Serum creatinine | |
| ≤2 mg/dL | 446 (78.0%) |
| >2 mg/dL | 126 (22.0%) |
| Del(17p) | |
| No | 533 (93.2%) |
| Yes | 39 (6.8%) |
| t(4;14) | |
| No | 491 (85.8%) |
| Yes | 81 (14.2%) |
| t(4;16) | |
| No | 536 (93.7%) |
| Yes | 32 (5.6%) |
| Not available | 4 (0.7%) |
| 1q gain or amplification | |
| No | 455 (79.5%) |
| Yes | 117 (20.5%) |
| First-line treatment | |
| IMiDs | 254 (44.4%) |
| PIs | 241 (42.1%) |
| IMiD-PIs | 77 (13.5%) |
| Upfront ASCT | |
| No | 334 (58.4%) |
| Yes | 238 (41.6%) |
- Note: Data are median (range) or n (%).
- Abbreviations: ASCT, autologous stem cell transplantation; ECOG PS, Eastern Cooperative Oncology Group performance status; IMiDs, immunomodulatory drug; ISS, International Staging System; LDH, lactate dehydrogenase; PI, proteasome inhibitor; R-ISS, Revised International Staging System; R2-ISS, Second revision of the International Staging System; UNL, upper normal limit.
There was a significant difference in both PFS and OS among the R-ISS groups. The median PFS for R-ISS stages I, II, and III were 37.5 months (95% CI: 30.3–50.7), 22.5 months (95% CI: 19.2–26.4), and 17.0 months (95% CI: 13.6–22.8), respectively (p < 0.001) (Figure 1A). The median OS for R-ISS stages I, II, and III were 117.6 months (95% CI: 86.5–not available [NA]) for R-ISS stage I, 61.2 months (95% CI: 52.3–69.1) for R-ISS stage II, and 41.3 months (95% CI: 30.1–57.4) for R-ISS stage III (p < 0.001) (Figure 1B). Similarly, significant differences were observed in both PFS and OS among the R2-ISS groups. The median PFS for R2-ISS stages I, II, III, and IV were 43.8 months (95% CI: 31.2–59.3), 28.8 months (95% CI: 23.0–35.1), 19.4 months (95% CI: 16.5–24.8), and 14.5 months (95% CI: 8.1–18.2), respectively (p < 0.001) (Figure 1C). Similarly, the median OS for R2-ISS stages I, II, III, and IV were 117.6 months (95% CI: 98.5–NA), 82.8 months (95% CI: 67.3–125.9), 49.1 months (95% CI: 40.4–60.9), and 26.1 months (95% CI: 14.9–49.9), respectively (p < 0.001) (Figure 1D).
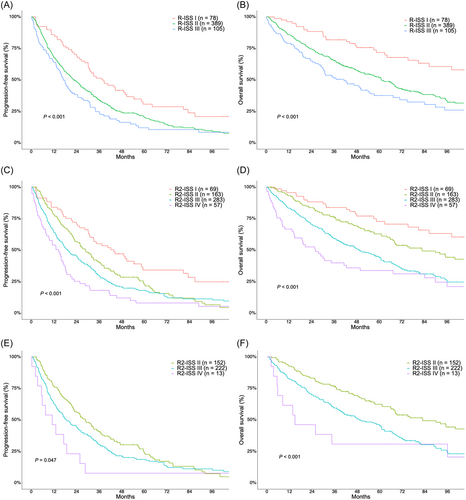
Redistribution of R-ISS I, II, and III patients according to the R2-ISS is summarized in Supporting Information S1: Table 2. Significant differences in both PFS and OS were observed among the R2-ISS stages within the R-ISS II patients. The median PFS for R2-ISS stages I, II, III, and IV were 42.2 months (95% CI: NA), 28.8 months (95% CI: 23.0–36.0), 15.1 months (95% CI: 15.1–24.9), and 11.1 months (95% CI: 5.4–NA), respectively (p = 0.047) (Figure 1E). Median OS was not reached in R2-ISS I, 82.8 months (95% CI: 67.3–125.9) in R2-ISS II, 49.6 months (95% CI: 40.2–62.4) in R2-ISS III, and 14.9 months (95% CI: 5.7–NA) in R2-ISS IV patients (p < 0.001) (Figure 1F). The survival outcomes according to R2-ISS in R-ISS I and III patients are shown in Supporting Information S1: Figure 2.
The R2-ISS demonstrated significantly higher discriminatory power compared to R-ISS for both PFS and OS. The C-index values for R2-ISS were 0.589 (95% CI: 0.560–0.616) for PFS and 0.613 (95% CI: 0.580–0.644) for OS, whereas the C-index values for R-ISS were 0.556 (95% CI: 0.532–0.581) for PFS and 0.567 (95% CI: 0.539–0.594) for OS (p = 0.012 for PFS; p = 0.003 for OS) (Supporting Information S1: Table 3). Additionally, the R2-ISS provided a better fit for the data compared to R-ISS, as indicated by lower AIC values (4984.23 vs. 4996.04 for PFS; 3709.2 vs. 3727.2 for OS).
The prognostic value of R2-ISS on OS was explored in different subgroups of patients. The R2-ISS maintained its prognostic significance in young (age <65), elderly (age ≥65), IMiD-treated, PI-treated, and IMiD plus PI-treated patients (Supporting Information S1: Figure 3A–G). Furthermore, we evaluated the prognostic value of R2-ISS on OS in subgroups of patients with a baseline Eastern Cooperative Oncology Group performance status (ECOG PS) of ≥2 and/or serum creatinine >2 mg/dL (n = 242), who are typically considered ineligible for participation in clinical trials. We found a significant difference in median OS according to R2-ISS in these patients (Supporting Information S1: Figure 3H). The performance of R2-ISS in terms of PFS in the same subgroups is shown in Supporting Information S1: Figure 4.
The present study successfully validated the R2-ISS in real-world patients with newly diagnosed MM. Notably, this is the first study to directly compare the performance of R2-ISS with R-ISS, and it demonstrated that R2-ISS significantly enhanced the discriminatory power and model fitness compared to R-ISS. Compared to the original EMN cohort, our real-world study included patients who were generally older and had a higher prevalence of high-risk diseases according to R-ISS or R2-ISS. Additionally, while the EMN cohort primarily consisted of transplant-eligible patients (approximately 80%), 40% of our cohort underwent upfront ASCT, indicating the inclusion of both transplant- and nontransplant-eligible patients. Furthermore, around 40% of the patients in our cohort were considered ineligible for clinical trial participation due to their poor performance status and/or renal impairment. Despite these notable differences from the original EMN cohort, the R2-ISS demonstrated consistent prognostic power in our patients. Moreover, R2-ISS effectively stratified patients with distinct survival outcomes across different age groups (elderly and young patients) and among those treated with PIs, IMiDs, or IMiDs plus PIs. Importantly, R2-ISS maintained its prognostic significance even in patients who were deemed ineligible for clinical trial participation.
While the R-ISS demonstrated prognostic value in our MM population, a majority of patients (68.0%) were classified as R-ISS II, highlighting its limitations as reported in previous studies.4, 5 By utilizing the R2-ISS, we were able to reclassify R-ISS II patients into R2-ISS stage II (39.1%) or III (57.1%), leading to significantly different survival outcomes based on their R2-ISS classification. This reclassification resulted in a substantial improvement in discriminatory power, as indicated by the C-statistics, and enhanced model fit, as assessed by the AIC, compared to the R-ISS. Nevertheless, considering the increased complexity involved in calculating the R2-ISS in comparison with the R-ISS, its applicability in real-world clinical practice warrants further observation.
This study has several limitations to consider. As with any retrospective study, there is a possibility of selection bias that limits the generalizability of the findings. Additionally, similar to the EMN study, our study did not include patients who were treated with newer treatment combinations involving anti-CD38 monoclonal antibodies, or next-generation IMiDs and/or PIs, such as pomalidomide and carfilzomib. Therefore, further studies are needed to validate the performance of the R2-ISS in these patient populations
In summary, the R2-ISS was successfully validated in real-world MM patients who received primary therapy with IMiDs and/or PIs. It showed a significant improvement in discriminatory power and model fitness compared to the R-ISS, mainly by effectively reclassifying R-ISS II patients into different risk groups.
AUTHOR CONTRIBUTIONS
Dok Hyun Yoon contributed to the conceptual design of the study. Dok Hyun Yoon, Kihyun Kim, Sang Eun Yoon, Sung-Hoon Jung, Je-Jung Lee, Joon Ho Moon, Hee Jeong Cho, Ho Sup Lee, Ka-Won Kang, Sung-Yong Kim, Hyeon-Seok Eom, Yeung-Chul Mun, Young Hoon Park, Sung-Soo Yoon, Young Rok Do, Won Sik Lee, and Chang-Ki Min were involved in data acquisition. Dok Hyun Yoon and Hyungwoo Cho were involved in data analysis, interpretation, writing, and editing of the manuscript. Kihyun Kim, Sang Eun Yoon, Sung-Hoon Jung, Je-Jung Lee, Joon Ho Moon, Hee Jeong Cho, Ho Sup Lee, Ka-Won Kang, Sung-Yong Kim, Hyeon-Seok Eom, Yeung-Chul Mun, Young Hoon Park, Sung-Soo Yoon, Young Rok Do, Won Sik Lee, and Chang-Ki Min reviewed the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING
This research received no funding.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.