Long-term survival and safety of elranatamab in patients with relapsed or refractory multiple myeloma: Update from the MagnetisMM-3 study
Targeting B-cell maturation antigen (BCMA) on myeloma cells has led to improved clinical benefit in heavily pretreated patients with relapsed or refractory multiple myeloma (RRMM), including those patients with triple-class exposed disease.1 Elranatamab, a humanized BCMA-CD3 bispecific antibody, was approved for the treatment of patients with RRMM based on the results from the registrational MagnetisMM-3 study, which enrolled patients previously treated with at least one immunomodulatory drug, one proteasome inhibitor, and one anti-CD38 monoclonal antibody.2, 3 Among BCMA-naive patients (n = 123), treatment with elranatamab led to an objective response rate (ORR) of 61.0%, with 35.0% of patients achieving a complete response (CR) or better.4 After a descriptive median follow-up of 14.7 months (data cutoff approximately 14 months after the last patient's initial dose), the median progression-free survival (PFS) and overall survival (OS) had not been reached, making it difficult to contextualize the survival outcomes in the treatment landscape for RRMM.4 Here, we report updated results, including Kaplan–Meier estimates of PFS and OS after a longer follow-up.
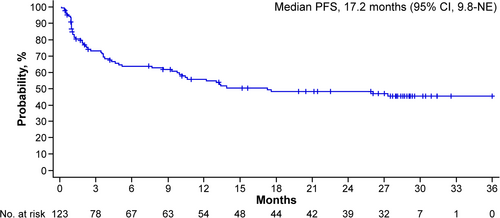
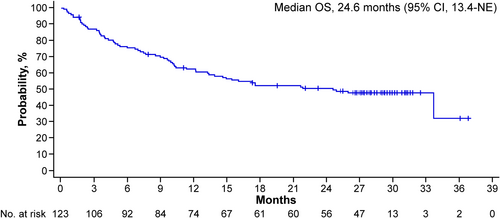
As of the data cutoff of March 26, 2024 (approximately 26 months after the last patient's initial dose), with a median follow-up of 28.4 months (95% confidence interval [CI], 28.0–29.0; estimated by reverse Kaplan–Meier), the ORR was 61.0%, with 37.4% of patients achieving CR or better. Among responders, the median duration of response was not yet reached, and the probability of maintaining response at 24 months was 66.9% (95% CI, 54.4–76.7). The median PFS was 17.2 months (95% CI, 9.8–not-estimable [NE]) (Figure 1) and the median OS was 24.6 months (95% CI, 13.4–NE) (Figure 2).
No new safety signals emerged with longer follow-up. Hematologic adverse events in ≥25% (any grade, maximum grade 3/4) of the patient population included neutropenia (49.6%, 49.6%), anemia (48.8%, 37.4%), thrombocytopenia (31.7%, 23.6%), and lymphopenia (26.8%, 25.2%). Other adverse events of interest observed in patients included infections (70.7%; 42.3% maximum grade 3/4; 6.5% grade 5), cytokine release syndrome (57.7%; all grade ≤2), and immune effector cell–associated neurotoxicity (4.9%; all grade ≤2).
Elranatamab monotherapy continues to improve survival outcomes without new safety signals. Despite the refractory patient population with a high percentage of patients with poor prognostic features in MagnetisMM-3, the median PFS of 17.2 months and the median OS of 24.6 months observed with additional follow-up are promising among bispecific antibodies currently approved for RRMM. In an analysis of MajesTEC-1 with a median follow-up of 23 months, BCMA-naive patients treated with teclistamab had a median PFS of 11.3 months (95% CI, 8.8–16.4) and a median OS of 21.9 months (95% CI, 15.1–NE).5 In the MonumenTAL-1 trial, talquetamab, a bispecific antibody against CD3 and G-protein-coupled receptor, class C, group 5, member D (GPRC5D) showed a median PFS of 7.5 and 11.9 (61% censored) months in the once-weekly and once every 2 weeks dosing cohorts after a median follow-up of 14.9 and 8.6 months, respectively.6 The observed safety profile with elranatamab is consistent with that of teclistamab as well as other BCMA-targeted bispecific antibodies.5, 7-10
In conclusion, extended follow-up from the ongoing phase 2 MagnetisMM-3 trial of elranatamab in BCMA-naive patients with heavily pretreated RRMM demonstrated sustained clinical efficacy and no new safety signals.
ACKNOWLEDGMENTS
We wish to thank the patients who participated in the trial and medical staff of participating centers. Medical writing support was provided by William Clafshenkel, PharmD, PhD, of Nucleus Global and was funded by Pfizer.
AUTHOR CONTRIBUTIONS
All authors were involved in the trial conception/design or the acquisition, analysis, or interpretation of data. All authors contributed to the drafting of the manuscript and approved the final version.
CONFLICT OF INTEREST STATEMENT
Michael H. Tomasson: consulting or advisory roles for Janssen. Shinsuke Iida: honoraria from Bristol Myers Squibb, Celgene, Janssen, Pfizer, Sanofi, and Takeda; consulting or advisory roles for Janssen, Sanofi, and Takeda; and research funding from AbbVie, Amgen, Bristol Myers Squibb, Caelum Biosciences, Celgene, Daiichi Sankyo, Janssen, Ono, Otsuka, Sanofi, and Takeda. Ruben Niesvizky: consulting or advisory roles for Bristol Myers Squibb, GSK, Janssen, Karyopharm Therapeutics, and Takeda and research funding from Bristol Myers Squibb, GSK, Janssen, Karyopharm Therapeutics, and Takeda. Mohamad Mohty: honoraria from Amgen, Celgene; consulting or advisory roles for Adaptive Biotechnologies, GSK, Jazz Pharmaceuticals, MaaT Pharma, Novartis, Sanofi, and Xenikos; personal fees from Amgen, Astellas, Bristol Myers Squibb, Celgene, Pfizer, Stemline-Menarini and Takeda; and speakers bureau roles for Janssen, Jazz Pharmaceuticals, and Sanofi. Nizar J. Bahlis: honoraria from AbbVie, Amgen, Celgene, Genentech/Roche, GSK, Janssen, Karyopharm Therapeutics, Sanofi, and Takeda; consulting or advisory roles for Amgen, Celgene, Janssen, Karyopharm Therapeutics, Pfizer, Sanofi, and Takeda; personal fees from AbbVie, Amgen, Celgene, Genentech/Roche, GSK, Janssen, Karyopharm Therapeutics, Sanofi, and Takeda; and patents, royalties, and/or other intellectual property interests with Celgene and Janssen. Joaquin Martinez-Lopez: consulting or advisory roles for Bristol Myers Squibb, Janssen, and Novartis; research funding from Astellas and Bristol Myers Squibb; and speakers bureau roles for Bristol Myers Squibb, Janssen-Cilag, and Roche. Paula Rodriguez-Otero: consulting or advisory roles for AbbVie, Bristol Myers Squibb, GSK, Janssen, Pfizer, and Sanofi; personal fees from AbbVie, Celgene, GSK, H3 Biomedicine, Janssen, Pfizer, and Sanofi; travel and accommodations expenses paid by Pfizer; and speakers bureau roles for Bristol Myers Squibb, GSK, Janssen, and Sanofi. Alexander M. Lesokhin: honoraria from iTeos Therapeutics, Janssen, Legend Biotech, Pfizer, Sanofi, and Trillium Therapeutics; consulting or advisory roles for Pfizer, Trillium Therapeutics, and Arcellx; personal fees from iTeos Therapeutics, Janssen, Legend Biotech, Pfizer, Sanofi, and Trillium Therapeutics; research funding from Bristol Myers Squibb, Genentech, Janssen, Pfizer, Sanofi, and Trillium Therapeutics; and patents, royalties and/or other intellectual property interests with Serametrix. Andrea Viqueira, Eric Leip, Umberto Conte, and Sharon T. Sullivan: employment and stock ownership at Pfizer. Guenther Koehne: has no conflicts of interest.
ETHICS STATEMENT
This study was conducted in accordance with the International Council for Harmonisation guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. The study protocol was approved by local or independent institutional review boards or ethics committees at participating sites.
FUNDING
This study was funded by Pfizer.
Open Research
DATA AVAILABILITY STATEMENT
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified patient data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.