Prophylactic Versus Reactive (PvR) Feeding in Patients Undergoing (Chemo)radiation in Head and Neck Cancers: Results of a Phase III Randomized Controlled Trial
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
Optimal feeding strategy (Prophylactic vs. Reactive) during Radiation Therapy (RT) for Head and Neck Cancers (HNC) remains to be established.
Methods
This phase III trial randomized (1:1) patients of HNC to prophylactic [nasogastric tube (NGT) placed at least 1 day before RT starting] versus reactive arm (NGT when required). The primary endpoint was the incidence of significant weight loss at 6 months. Secondary endpoints were weight changes, tube duration, dependence, and compliance.
Results
Due to high denial rates, the trial was prematurely closed. Eighty-four patients (40-prophylactic, 44-reactive) were analyzed intention-to-treat. Seventeen patients (39%) required a reactive tube insertion. The proportion of patients with significant weight loss at 6 months in the prophylactic versus reactive arms was 13 (32.5%) versus 10 (15.9%), p = 0.44. The median tube duration was 56 days (IQR: 16.5–91) versus 47 days (IQR: 24–59), p = 0.255.
Conclusion
Our limited analysis found that a reactive NGT should be considered for most HNC patients undergoing RT.
Trial Registration
Clinical trials registry of India: CTRI/2020/01/023049
1 Introduction
Radiation therapy (RT) is integral to managing head and neck cancers (HNC), with an estimated 74% of patients undergoing RT at some point during their treatment, either upfront or in the salvage setting. The importance of optimizing supportive care measures, such as the timing of enteral nutrition, to enhance treatment tolerance and outcomes in resource-limited settings has been emphasized [1, 2]. Nutritional concerns in this group of patients are attributable to both the tumor location and stage, as well as treatment-related factors. These include symptoms such as dysphagia, trismus, odynophagia, dysgeusia, aspiration, mucositis, dermatitis, and worsening of swallowing function [3, 4]. About 50%–60% of patients can lose more than 10% of their baseline body weight during treatment [5]. Poor nutritional status is associated with a higher risk of infections, treatment breaks, prolonged overall treatment time (OTT), poorer survival outcomes, and a deteriorated acute and long-term quality of life (QoL) [6, 7].
The feeding tube placement may follow a prophylactic or a reactive approach. In the prophylactic feeding method, intervention (placement of feeding tube) is done before radiotherapy starts. In a reactive approach, a feeding tube is placed during radiation treatment if indicated (significant weight loss, Grade 2–3 mucositis, dysphagia, dysfunctional swallowing, etc.). Enteral nutrition can be established with a percutaneous endoscopic gastrostomy (PEG) or nasogastric tube (NGT). A prophylactic approach may be associated with reduced treatment breaks; however, it can cause decreased physiological swallowing effort and tube dependency. A reactive approach can avoid unnecessary tube placement in about 40%–50% of patients, reducing the incidence of tube dependency [8].
The optimal type of enteral nutrition in head and neck cancer patients undergoing RT is still debatable. Literature shows an equipoise between modalities and empirical adherence to the prophylactic approach. A few randomized studies point towards the benefit of a prophylactic approach [9-11]. These trials have mainly looked upon patients with advanced stage disease, and most have received a combination of chemoradiotherapy, some of which had enrolled only advanced disease patients [11]. Results of systematic review/meta-analysis have also shown that prophylactic PEG insertion may be associated with a lesser incidence of critical weight loss and improved QoL and may help reduce the rate of treatment interruption and hospital admissions [12, 13]. However, the reliability of these results was questionable owing to a small number in the studies. Further, well-designed randomized trials were necessitated to confirm the beneficial effects of a prophylactic approach. We designed this study to determine the nutritional, survival, and QoL benefits of a prophylactic versus reactive feeding tube approach, along with assessing parameters such as tube duration, tube dependence, and swallowing function.
2 Materials and Methods
2.1 Design
This was a Phase III, superiority design, single center, open-label, randomized controlled trial comparing prophylactic versus reactive enteral nutrition in patients undergoing RT for HNC from a tertiary care center in India between July 2020 and July 2023. The Institutional Ethics Committee approved the study, which was conducted per the Good Practice Guidelines and the Declaration of Helsinki. The study was prospectively registered with the clinical trials registry.
2.2 Inclusion and Exclusion Criteria
All newly diagnosed nonmetastatic T1–T4 oropharyngeal, laryngeal, hypopharyngeal, or oral cavity cancers with any N stage, planned for definitive or adjuvant RT or chemoradiotherapy with a Karnofsky Performance status ≥ 70 were eligible and screened for the study. Patients were excluded if they had baseline grade ≥ 3 dysphagia (CTCAE v5.0), aspiration, had undergone previous head and neck surgery affecting swallowing, had a prior history of head and neck radiation, or were treated with neoadjuvant chemotherapy (were at high risk of developing malnutrition prior to treatment).
2.3 Study Endpoints
The study's primary endpoint was to evaluate the impact of prophylactic enteral tube placement compared to the reactive tube (P-PEG/NGT vs. R-PEG/NGT) on the risk of significant weight loss (10% of baseline body weight) at 6 months after RT completion. Secondary endpoints included the evaluation of the relative variation of weight changes over 24 months, duration of tube use (defined as the duration between the date of tube insertion to the date of tube removal), incidence of tube dependence (defined as duration of tube use more than 12 months post treatment), compliance to therapy, RT treatment breaks, and QoL parameters. All patients during treatment were reviewed every week in clinic or more frequently if required. Compliance to therapy was assessed through clinical documentation of treatment adherence, including attendance records, medication logs, and completion of the planned treatment within the expected OTT. No questionnaires were administered. Progression-free survival (PFS) was calculated as the time interval between the date of randomization and documented clinic-radiological progression or death. Overall survival (OS) was calculated from the date of randomization until death from any cause.
2.4 Sample Size Estimation
Our assumptions were based on a retrospective study conducted at our institute. Based on previously published institutional data, 30% of patients required reactive PEG/NGT insertion during radiotherapy to the head and neck region. Assuming 30% of patients have significant weight loss at 6 months in the control group, we hypothesized that prophylactic enteral tube placement would be associated with a 10% absolute decrease in this proportion (20% versus 30% of patients with significant weight loss) [14]. With a two-sided alpha of 5%, power of 80%, and accounting for 10% attrition, the target accrual was 580 patients (290 in each arm) (chi-squared test) to be accrued over 5 years. The sample size calculation was performed using East v6 Cytel software.
2.5 Randomization and Interventions
All suitable patients after accrual in the study were randomized (1:1) between the two arms. Randomization was balanced using a dynamic allocation procedure based on a minimization program controlling for the stratification factors with a random factor set at 0.8, centrally performed using a computer program ensuring concealment for allocation of the next patient. Stratification was based on site (oropharynx 70%, larynx and hypopharynx 15%, oral cavity 15%), treatment modality {RT only 30%, Chemoradiation (CTRT) 70%}, treatment setting (adjuvant RT/CTRT 40%, definitive RT/CTRT 60%), node-positive/node-negative (50% each), and RT technique {conventional 60%, Intensity Modulated Radiation therapy (IMRT) 40%}.
2.6 Study Arms
All patients underwent standard preradiation baseline dietary evaluation and counseling. Nutritional assessment was conducted by a registered dietitian using structured hospital-based protocols. Data collected included anthropometric parameters (height, weight, BMI, percent weight loss, usual and dry weight), route and texture of diet (e.g., oral, pureed), dietary intake compliance (%), and prescribed nutrition goals (total calories and protein per day). A prophylactic NGT was inserted at least 1 day before RT started in the prophylactic arm. Patients randomized to the control arm underwent treatment as per institutional protocol. The indications for a reactive tube insertion in this group were ≥ 10% of baseline weight loss since the commencement of therapy, ≥ Grade 3 mucositis (CTCAE Vr 5), dysphagia, aspiration, or dyselectrolytemia, and poor/decreased oral intake: not meeting the recommended daily calorie intake.
2.7 Treatment Protocol
A decision regarding the treatment plan was made after a multidisciplinary meeting. Patients were treated with both conventional and IMRT techniques [15-17]. Conventional techniques involve using anterolateral wedged pairs or bilateral portals based on target volume with patient-specific tissue compensators. Phased treatment was carried out with shrinking fields after 46 Gy to maintain cord constraints. The RT volumes for conformal treatment encompassed the grossly involved regions with elective nodal irradiation in the definitive setting and the post-op bed and draining nodal station in the adjuvant setting. A total 70 Gy equivalent (2 Gy per fraction) was administered in the definitive setting and up to 60–64 Gy equivalent (2 Gy per fraction) in the adjuvant setting. Planning complied with the ICRU 83 recommendations, and QUANTEC dose constraints were met in IMRT planning. Concurrent platinum-based chemotherapy (weekly or three weekly) was used if the patient was considered fit for the same by the medical oncologist and as per institutional guidelines.
During treatment, patients were reviewed regularly every week by treating radiation oncologists. Body weight and weekly acute toxicity were recorded. A weekly review with a speech-swallow therapist and dietitian was done for all patients, along with laboratory investigations that included a complete blood count and biochemistry. QoL was planned to be assessed using the EORTC QLQ-C30 and QLQ-H&N35 questionnaires at predefined time points; however, due to incomplete data collection, QoL analysis was not performed.
2.8 Follow-Up
After treatment completion, patients were followed up as per institutional protocol. The first follow-up was scheduled for 2–3 months after treatment conclusion and then every 3 months. Parameters, including weight and laboratory investigations, were recorded at each follow-up (every 3 months) until 2 years after treatment completion. A dietician and speech swallowing therapist review was also done at each follow-up.
2.9 Statistical Analysis
Clinicopathological and demographic variables were summarized using descriptive statistics and analyzed using central tendency and dispersion measures. Time-to-event outcomes (PFS and OS) were calculated using the product limit method of Kaplan–Meier and reported as point estimates with corresponding 95% confidence intervals (CI). The statistical plan included univariate analysis of prognostic variables using the log-rank test. All analyses were conducted on an intention-to-treat (ITT) basis, including all randomized patients in their assigned groups, regardless of whether the intervention was received. Analysis was performed using Statistical Package for Social Services (SPSS) Version 24.0 (IBM Corp., Armonk, IL., IL., U.S.A) and R version 4.0.2 [18].
3 Results
We sought permission from the Institutional Review Board (IRB) due to the high rate of patient denial for trial participation and poor accrual, which was granted, and the trial was prematurely closed in September 2023.
3.1 Demographics
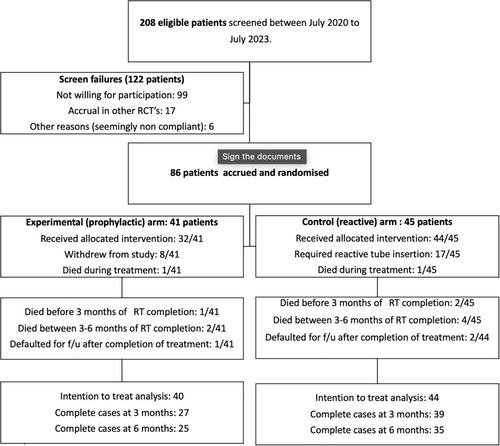
Between July 2020 and July 2023, 208 patients were screened, and 86 patients were enrolled in the study (Figure 1). One hundred and twenty-two patients were screened and failed the study, of which 99 patients were unwilling to participate, 17 were accrued in similar studies, and six were referred out for treatment/were seemingly noncompliant. Two of the 86 patients died during RT; hence, with IRB approval, we analyzed 84 patients on an ITT basis. Forty patients (48%) were randomized to the experimental/study arm (prophylactic) and 44 (52%) to the control arm (reactive). The majority of patients were male (n = 75, 89.3%). The median age at presentation was 51 years (IQR: 41–61). Seventy-seven (91.6%) had a significant addiction history (smoking/tobacco chewing/alcohol intake). The most common primary site was the oral cavity (n = 54, 64.3%), followed by the oropharynx (n = 17, 20.2%) (Table 1).
| Prophylactic group (n = 40) | Reactive group (n = 44) | p | |
|---|---|---|---|
| Gender | |||
| Male | 36 (43%) | 39 (46%) | 0.83 |
| Female | 4 (5%) | 5 (6%) | |
| Significant Addiction (Tobacco/Alcohol) | |||
| Yes | 11 (13%) | 9 (10%) | 0.45 |
| No | 29 (35%) | 35 (42%) | |
| Site | |||
| Oral cavity | 25 (30%) | 29 (34%) | 0.064 |
| Oropharynx | 10 (12%) | 7 (8%) | |
| Larynx | 2 (2%) | 8 (10%) | |
| Hypopharynx | 3 (4%) | 0 | |
| T stage (AJCC 8th edition) | |||
| Tx | 0 | 2 (2%) | 0.55 |
| T0 | 1 (1%) | 0 | |
| T1 | 3 (4%) | 3 (4%) | |
| T2 | 12 (14%) | 9 (11%) | |
| T3 | 11 (13%) | 15 (18%) | |
| T4 | 13 (15%) | 15 (18%) | |
| N stage (AJCC 8th edition) | |||
| N0 | 13 (16%) | 17 (20%) | 0.21 |
| N1 | 7 (8%) | 10 (12%) | |
| N2 | 12 (14%) | 5 (6%) | |
| N3 | 8 (10%) | 12 (14%) | |
| Stage (AJCC 8th edition) | |||
| I | 1 (1%) | 2 (2%) | 0.56 |
| II | 10 (12%) | 6 (7%) | |
| III | 11 (13%) | 12 (14%) | |
| IV | 18 (22%) | 24 (29%) | |
| Histology | |||
| Squamous cell carcinoma | 39 (47%) | 44 (52%) | 0.29 |
| Neuro endocrine tumor | 1 (1%) | 0 | |
| Treatment modality | |||
| Adjuvant | 25 (30%) | 29 (34%) | 0.75 |
| Definitive | 15 (18%) | 15 (18%) | |
| Treatment technique | |||
| Conventional | 29 (35%) | 27 (32%) | 0.28 |
| IMRT | 11 (13%) | 17 (20%) | |
| Concurrent chemotherapy | |||
| Yes | 21 (25%) | 20 (24%) | 0.52 |
| No | 19 (23%) | 24 (28%) | |
| Chemotherapy Regime | |||
| Cisplatin | 12 (14%) | 15 (18%) | 0.59 |
| Docetaxel | 3 (4%) | 2 (2%) | |
| Cisplatin + nimotuzumab | 0 (%) | 1 (1%) | |
| Cisplatin + etoposide | 1 (1%) | 0 | |
| 5-FU + carboplatin | 1 (1%) | 1 (1%) | |
| Nimotuzumab | 4 (5%) | 1 (1%) | |
- Note: The table summarizes the number of patients (and percentages) in each category, with p values indicating the statistical significance of differences between groups.
- Abbreviations: 5-FU, 5 fluorouracil; IMRT, intensity modulated radiation therapy.
3.2 Treatment Details
Fifty-four patients (64%) received radiation in adjuvant and 30 (36%) in a definitive setting. A majority (n = 56, 67%) of patients were treated with conventional bilateral/anterolateral portals on Cobalt-60 treatment units, and 28 patients (33%) with IMRT. About half of the patients (n = 41, 49%) received concurrent chemotherapy, with three weekly cisplatin (at 100 mg/m2) being the most commonly used regimen. The median time to start RT from the surgery date was 41 days (IQR: 36.5–47.25 days), and the treatment package time was 79 days (IQR: 73–89 days).
Seventeen (38.6%) out of 44 patients randomized to the reactive NGT arm required a reactive tube placement, the most common reason being severe oral/oropharyngeal mucositis (n = 12), followed by significant weight loss (n = 5). Weight loss was more pronounced during the second to third week of treatment. The median time in which patients required a reactive tube insertion after RT started was 25 days (IQR: 17–28 days). Of the 41 patients randomized to the prophylactic group, 32 (78%) received the NGT as planned. In 9 patients (22%), the tube was not placed due to patient refusal.
3.3 Primary Endpoint: Nutritional Outcomes
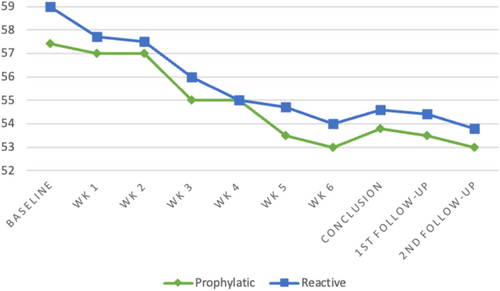
At 6 months post treatment completion, 36 patients each were analyzed in the prophylactic and reactive arms, respectively (Figure 1). The proportion of patients with significant weight loss at this point was 13 (32.5%) versus 10 (15.9%), p = 0.44. The proportion of patients with significant weight loss in the prophylactic versus reactive arms at other time points post-RT were as follows–at conclusion: 13 (33%) versus 7 (16%), p = 0.075; at 3 months: 16 (42.1%) versus 16 (40%), p = 0.85 (Table 2).
| Prophylactic (n = 40) | Reactive (n = 44) | p | |
|---|---|---|---|
| Median weight at baseline | 57.4 kg (IQR: 51–64.7) | 59 kg (IQR: 49–69.7) | 0.68 |
| Week 1 | 57.4 Kg (IQR: 51.4–67) | 57.4 Kg (IQR: 51.4–67) | 0.47 |
| Week 2 | 57.2 Kg (IQR: 51.5–65.5) | 57.2 Kg (IQR: 51.5–65.5) | 0.23 |
| Week 3 | 56 Kg (IQR: 51–65) | 56 Kg (IQR: 51–65) | 0.32 |
| Week 4 | 55 Kg (IQR: 50.5–65.6) | 55 Kg (IQR: 50.5–65.6) | 0.39 |
| Week 5 | 54.8 Kg (IQR: 48.5–54.8) | 54.8 Kg (IQR: 48.5–63.4) | 0.25 |
| Week 6 | 55 Kg (IQR: 50–63) | 55 Kg (IQR: 50-63Kg) | 0.04 |
| Median weight at conclusion | 53.8 kg (IQR: 48–60) | 54.6 kg (IQR: 48.2–65) | 0.363 |
| Median weight post-RT 3 months | 53.5 kg (IQR: 46–61) | 54.4 kg (IQR: 46.5–62.6) | 0.724 |
| Median weight post-RT 6 months | 53 kg (IQR: 48–63.6) | 53.8 kg (IQR: 49.2–63.5) | 0.735 |
| Median BMI at baseline | 21.3 kg/m2 (IQR: 18.7–23.5) | 22.3 kg/m2 (IQR: 18.9–25.7) | 0.216 |
| Median BMI at conclusion | 19.8 kg/m2 (IQR: 17.3–22.1) | 20.5 kg/m2 (IQR: 17.4–23) | 0.119 |
| Median BMI post-RT 3 months | 19.4 kg/m2 (IQR: 16.3–22) | 20.25 kg/m2 (IQR: 18–23) | 0.315 |
| Median BMI post-RT 6 months | 19.5 kg/m2 (IQR: 16.7–23.4) | 20.6 kg/m2 (IQR: 18.1–23.2) | 0.280 |
| Significant weight loss at conclusion | 13/40 (33%) | 7/44 (16%) | 0.075 |
| Significant weight loss at 3 months | 16/38 (42%) | 16/40 (40%) | 0.85 |
| Significant weight loss at 6 months | 13/26 (50%) | 10/36 (28%) | 0.448 |
- Abbreviations: BMI, body mass index; RT, radiation therapy.
For the overall cohort, median weight loss from RT starting to conclusion, at 3 months and 6 months, was 3 kgs (IQR 2–6), 4.2 kgs (IQR 2–7), and 4 kgs (IQR 0.05–6.7). The median weight loss at various time points for the prophylactic versus reactive cohort was as follows: at the conclusion of RT—3.3 kg (IQR: 2–7) versus 2.5 kg (IQR: 1.5–4.6), p = 0.36; at 3 months post RT completion—4.2 kg (IQR: 1–7) versus 4.5 kg (IQR: 2.2–7), p = 0.72; at 6 months post RT completion—4.4 kg (IQR 2.5–7) versus 3.3 kg (IQR: 2.25–7), p = 0.73 (Table 2; Figure 2).
3.4 Tube Duration and Tube Dependence
Median tube duration (calculated from the date of completion of RT) in the prophylactic versus reactive arms was 56 days (IQR: 16.5–91) versus 47 days (IQR: 24–59), p = 0.255. Tube dependence beyond 6 months was seen in four patients, 2 in the prophylactic arm and 2 in the reactive arm.
3.5 Survival Outcomes
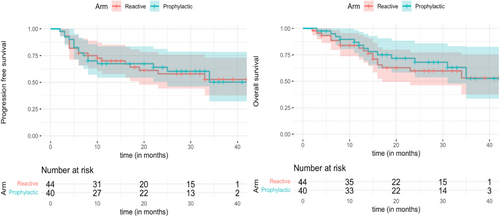
At a median follow-up (surviving patients) of 31 months (IQR 20–36), the 2-year PFS of the entire cohort was 61% (95% CI: 49.2–72.2), and 2-year OS was 63.7% (95% CI: 52.3–75.1), respectively. No significant differences between the prophylactic versus reactive arms were seen in the 2-year PFS: 64% (95% CI: 46–79) versus 57.9% (95% CI: 44–76), p = 0.9; and 2-year OS: 66% (95% CI: 52–84) versus 59% (95% CI: 45–76), p = 0.64 (Figure 3).
3.6 Toxicities and Compliance
The incidence of acute and late toxicities was comparable in both arms (Table 3). Acute dermatitis, mucositis, and xerostomia ≥ Grade 2 were seen in 11 (27.5%), 30 (75%), and 28 (70%) patients in the prophylactic arm and 12 (27.2%), 28 (63.6%) and 29 (65.9%) patients in the reactive arm. Incidence of ≥ Grade 2 dyselectrolytemia (sodium, potassium, magnesium) was seen in 8 (20%) patients in the prophylactic arm and 11 (25%) patients in the reactive arm. Overall, six unplanned hospitalizations occurred (3 prophylactic, three reactive). In the prophylactic arm, two hospitalizations were due to febrile neutropenia and one due to Grade 4 hyponatremia. In the reactive arm, one hospitalization was due to febrile neutropenia, and two were due to toxicities (mucositis). Unplanned treatment break was seen in two patients (1 in each arm), neither of which was due to treatment-related side effects.
| Incidence of ≥ Grade 2 toxicity (CTCAE ver 5.0) | Prophylactic arm (n = 40) | Reactive arm (n = 44) | p |
|---|---|---|---|
| Dermatitis | 11 (27.5%) | 12 (27.2%) | 0.54 |
| Mucositis | 30 (75%) | 28 (63.6%) | 0.34 |
| Xerostomia | 28 (70%) | 29 (65.9%) | 0.47 |
| Nausea | 12 (30%) | 8 (18%) | 0.45 |
| Vomiting | 5 (12.5%) | 6 (13.6%) | 0.34 |
| Neutropenia | 6 (15%) | 7 (15.9%) | 0.42 |
| Fever | 7 (17.5%) | 6 (13.6%) | 0.46 |
| Pain | 21 (52.5%) | 20 (45.5%) | 0.50 |
| Diarrhea | 3 (7.5%) | 4 (9%) | 0.31 |
| Hyponatremia | 8 (20%) | 11 (25%) | 0.37 |
| Hypokalaemia | 4 (10%) | 3 (7%) | 0.20 |
| Hypomagnesemia | 2 (5%) | 2 (4.5%) | 0.33 |
3.7 Subgroup Analysis
Patients undergoing adjuvant RT had a higher probability of significant weight loss (OR: 2.5) although this was not statistically significant, p = 0.06. None of the other factors were statistically significant for impacting significant weight loss at 6 months (Figure 4).

4 Discussion
Although this randomized controlled trial did not meet the planned accrual, there was no significant difference in the weight outcomes at various endpoints between the prophylactic and reactive feeding tube arms.
Three prospective randomized trials have tried to address the issue of prophylactic versus reactive feeding tubes. In 2008, Salas et al. primarily evaluated the changes in QoL in a small group of 39 patients with unresectable HNC and reported a significant posttreatment improvement in QoL at 6 months in the prophylactic feeding group [10]. The Swedish group led by Silander et al. showed a significantly lower weight loss at 2 months and an improved 6-month global QoL with prophylactic tube insertion in 134 patients [9]. Lastly, an Australian study in 2017 published their data that showed similar outcomes between both modalities [11]. This highlights the fact that the problem statement is the same, but the endpoints evaluated have been different, further adding to equipoise in the literature about this important question/issue.
The major difference between these trials and our study was the inclusion of locally advanced cases. Definitive cases formed a majority of the cohort in the other studies. In contrast, patients of all stages and predominantly adjuvant cases form a major part of the current report. We excluded patients who required a persistent feeding tube postoperatively due to a major surgical defect. These reasons can perhaps explain the similar weight outcomes of prophylactic versus reactive arms in our study.
The modality used in our trial for enteral feeding was a NGT. Although insertion of a PEG may be generally more acceptable in patients, in our experience, NGT insertion is associated with lesser overall days of tube feeding dependence, and hence this remains a preferred modality for most of our patients. NGT placement is also associated with a lower financial burden for the patient compared to PEG, as it involves lesser procedural costs and maintenance during its use. A small randomized trial (n = 33) reported by Corry et al. from Australia found no significant differences in overall complication rates, chest infection rates, or in patients' assessment of their overall QoL [19] between the methods of enteral feeding. The cost of a PEG tube was 10 times that of an NGT. The duration of use of PEG tubes was significantly longer, a median of 139 days compared with a median of 66 days for NGT. The UK TUBE study initially screened 75 patients, of whom 17 (23%) were recruited and randomized. Among these, compliance and retention were high, with 16 out of 17 starting and completing their assigned interventions with minimal complication rates for both PEG and NGT [20]. A recently conducted systematic review also reported similar outcomes with PEG and NGT for patients undergoing RT/CTRT for HNC [21]. As with other trials, this study again highlights the major challenge of accrual in randomized trials evaluating prophylactic versus reactive feeding strategies [20]. We utilized NGT as the modality for enteral feeding, which could account for our higher screen failure rate than that reported by others.
Although underpowered, the result of this prospective trial is similar to a retrospective institutional analysis of 526 patients of oral cavity SCC reported from our institute [14]. In this study, there was more significant weight loss in the prophylactic cohort at 6 months (5 versus 3 kg, p = 0.002). Reactive tube insertion was required in 27.6% of patients. A major difference between this study and the current randomized trial was that 81.3% of patients in the prophylactic cohort received a PEG.
One of the weaknesses of the trial was that many patients received RT with conventional technique. We allowed conventional technique in the trial as we wanted to ascertain the benefit of the prophylactic feeding strategy in routine clinical practice across scenarios. With the projected fairly large number of target patient accrual (more than 500), we anticipated that the trial would have been able to answer the question with both conventional and conformal techniques. However, the available results from the limited accrual in this trial as well as other reports suggest that the benefit of prophylactic feeding tube may be further less in the era of IMRT with a significant sparing of Dysphagia Aspiration Related Structures (DARS). Also, weight loss prior to radiation could not be fully captured in patients who underwent upfront surgery, as these patients did not receive a formal nutritional assessment prior to surgery. Consequently, any weight loss occurring between diagnosis, surgery, and radiation initiation was not systematically recorded. Nine patients in the prophylactic NGT group did not receive the intervention due to unwillingness. This nonadherence was addressed by using an ITT analysis, which reflects clinical reality and maintains the integrity of the randomization. Another limitation of this study is the absence of QoL data analysis due to incomplete and insufficient questionnaire responses.
The strength of this study as compared to other studies was that it was designed to answer the question of prophylactic versus reactive for HNC in all scenarios. Although it can be argued that patients may have complete weight recovery at 6 months post RT completion, the results of this study do show that reactive feeding tube insertion is associated with similar weight dynamics even post completion of RT and at 3 months post RT completion (which is the time period when acute effects of RT usually peak).
We conclude that it remains challenging to accrue patients in trials evaluating optimal feeding strategies in head neck cancers. In our limited analysis, we did not find any difference in patients undergoing prophylactic NGT insertion. Perhaps, this strategy should be reserved only for high-risk patients such as patients undergoing major glossectomies and reirradiation.
Author Contributions
Study conceptualization: Sarbani Ghosh Laskar. Study design: Sarbani Ghosh Laskar, Meetakshi Gupta, Shreyasee Karmakar, Samarpita Mohanty. Study conduct: All Authors. Data collection: Meenakshi Jeeva, Faizalam Khan. Statistical analysis: Shwetabh Sinha, Meenakshi Jeeva. Manuscript writing: Meenakshi Jeeva. Manuscript editing: Sarbani Ghosh Laskar, Shwetabh Sinha. Manuscript finalization/approval: All Authors.
Acknowledgments
We would like to acknowledge the experts at the CREDO oncology protocol development workshop who helped design this study.
Ethics Statement
The Institutional Ethics Committee approved the study, which was conducted per the Good Practice Guidelines and the Declaration of Helsinki.
Consent
Informed consent was obtained from all patients who participated in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




