AHNS Series: Do you know your guidelines? Optimizing outcomes in reoperative parathyroid surgery: Definitive multidisciplinary joint consensus guidelines of the American Head and Neck Society and the British Association of Endocrine and Thyroid Surgeons
Abstract
Background
Revision parathyroid is challenging due to possible diagnostic uncertainty as well as the technical challenges it can present.
Methods
A multidisciplinary panel of distinguished experts from the American Head and Neck Society (AHNS) Endocrine Section, the British Association of Endocrine and Thyroid Surgeons (BAETS), and other invited experts have reviewed this topic with the purpose of making recommendations based on current best evidence. The literature was also reviewed on May 12, 2017. PubMed (1946-2017), Cochrane SR (2005-2017), CT databases (1997-2017), and Web of Science (1945-2017) were searched with the following strategy: revision and reoperative parathyroidectomy to ensure completeness.
Results
Guideline recommendations were made in 3 domains: preoperative evaluation, surgical management, and alternatives to surgery. Eleven guideline recommendations are proposed.
Conclusion
Reoperative parathyroid surgery is best avoided if possible. Our literature search and subsequent recommendations found that these cases are best managed by experienced surgeons using precision preoperative localization, intraoperative parathyroid hormone (PTH), and the team approach.
1 INTRODUCTION
We reviewed the evaluation and management of patients undergoing reoperative surgery for primary hyperparathyroidism (HPT). A multidisciplinary panel of distinguished experts from the American Head and Neck Society (AHNS) Endocrine Section, the British Association of Endocrine and Thyroid Surgeons (BAETS), and other invited experts have reviewed this topic with the purpose of making recommendations based on current best evidence. This endeavor included 8 current or past presidents of the American Thyroid Association (ATA), American Association of Clinical Endocrinologists, American Academy of Otolaryngology Head and Neck Surgery (AAO-HNS), International Association of Endocrine Surgeons, British Association of Endocrine and Thyroid Surgeons (BAETS), American Association of Endocrine Surgeons, European Society of Endocrine Surgeons, and the AHNS. Consensus recommendations for the diagnosis and management of reoperative procedures for patients with hyperparathyroidism are presented.
Reoperative parathyroid surgery can be associated with significant risks. It may be required in as many as 10% of operated patients with primary hyperparathyroidism.1 Reoperative parathyroid surgery is a complex exercise that requires specialized skills and understanding to successfully optimize patient care. We offer this statement of consensus guidelines in a spirit of interspecialty and international collaboration to improve the care of patients with persistent and recurrent hyperparathyroidism after previous surgery. We feel the blend of expertise of our author panel substantially strengthens the work and ultimately our recommendations. We recognize that some of the recommendations are made with an overarching general/universal purpose and that local parochial issues (such as, for example, what form of imaging may be available) will vary by region and individual center.
This consensus statement from the AHNS and BAETS focuses on comprehensive management. The document includes preoperative diagnostic and intraoperative anatomic surgical maneuvers and technical information. The recommendations of the AHNS, the largest surgical organization in the United States, focused on thyroid cancer treatment, as well as the BAETS, the United Kingdom's endocrine surgical organization, are intended to help guide surgeons in the clinical decision-making process when managing patients requiring reoperative parathyroid surgery. It is our hope that this document improves the quality and reduces variation in management of such patients and facilitates further communication and research for these patients who require parathyroid surgical procedures.
Evidence-based support is drawn from the literature, authors' expert opinion, as well as recent existing AAO-HNS, AHNS, and ATA guidelines. The literature was also reviewed on May 12, 2017, (PubMed 1946-2017; Cochrane SR 2005-2017; CT databases 1997-2017; and Web of Science 1945-2017) and were searched with the following strategy: revision and reoperative parathyroidectomy to ensure completeness. References were classified by the Oxford Centre for Evidence-Based Medicine System.2 The manuscript was generated by members of the author panel and then distributed to governing counsels of both organizations for further iterative feedback and ultimate organizational approval.
2 SECTION 1: PREOPERATIVE EVALUATION
2.1 Recommendation 1a
The first step in the evaluation of the reoperative procedures for patients with hyperparathyroidism must include complete biochemical confirmation of primary HPT with hypercalcemia, relative or absolute hyperparathormonemia, and preferably hypercalciuria before reoperation. Level of evidence: 1 to 3, level of recommendation: strong.
The biochemical diagnosis of persistent and recurrent primary hyperparathyroidism is the same as for the initial diagnosis of primary HPT, specifically, hypercalcemia in the presence of a high or inappropriately unsuppressed parathyroid hormone (PTH) level.1, 3, 4
Persistent HPT is defined as hypercalcemia within 6 months of the initial parathyroidectomy, whereas recurrent HPT is defined as hypercalcemia occurring 6 months or more after parathyroidectomy. Persistent HPT is typically due to inadequate surgery, whereas recurrent HPT is usually due to a lack of appreciation for the underlying disease process (either sporadic or genetic; eg, multiple endocrine neoplasia type-1)5, 6 Recurrent HPT in multiple endocrine neoplasia type-1 is an expected outcome after adequate primary initially successful surgery. Patients with recurrent HPT are more likely to have a family history of hyperparathyroidism and/or have had their initial parathyroid surgery performed earlier in life.
Patients who have had parathyroid surgery have typically met one or more of the generally accepted guidelines for surgery. In these patients, indications for surgery have not changed except for the fact that they have had unsuccessful parathyroid surgery and now have new increased risks associated with the reoperative procedure. In those patients who underwent parathyroid surgery, but did not meet any specific guidelines, the decision to consider another parathyroid operation is not straightforward. Asymptomatic patients with primary hyperparathyroidism have been followed for up to a decade without overt evidence of clinically significant disease progression.1
The threshold for reoperative surgery should be higher than for first time surgery; therefore, a quantitative assessment of disease severity is required. Basic assessment of the degree of hypercalcemia (such as a serum calcium >3.00 mmol/L [12.0 mg/dL]) may be complemented by evaluation of kidney function (especially the glomerular filtration rate), renal imaging (abdominal ultrasound or CT for nephrolithiasis/nephrocalcinosis), somatic symptoms (musculoskeletal aches and pains), assessment of bone health (with dual-energy X-ray absorptiometry), and an assessment of cardiovascular risk. Ultimately, thorough and more aggressive imaging than for the initial operation is the basis of successful parathyroid reoperation.7, 8
The overarching cost-benefit analysis of reoperative parathyroid surgery blends the stable issues of disease risk/morbidity and the now increased surgical risk of the more difficult scarred surgical field. The decision to move forward with surgery must be nuanced and shared with the patient and their endocrinologist.
2.2 Recommendation 1b
The diagnosis of HPT must be secured through exclusion of nonparathyroid causes of hypercalcemia and/or hyperparathormonemia. Level of evidence: 2C, level of recommendation: moderate.
Several factors may influence biochemical results and lead to confusion in patient assessment. Vitamin D deficiency and calcium deficiency, particularly if severe, may mask primary HPT, as such patients may present with a normal calcium level. In addition, the 24-hour urine calcium excretion may be low in severely calcium-deficient individuals and the biochemical picture can even mimic secondary hyperparathyroidism. Correction of any documented deficiencies and repeat testing after replacement therapy may reveal the true diagnosis of primary HPT. It must be noted that patients who are vitamin D deficient and are hypercalcemic at the outset should be repleted cautiously; otherwise the hypercalcemia can be exacerbated.
The use of thiazide diuretics and lithium, which may raise PTH and cause mild hypercalcemia, may mimic the diagnosis of primary HPT. These medications should be discontinued when possible and the biochemical evaluation repeated after 6 weeks. Nonparathyroid-mediated hypercalcemia (ie, lymphoma, granulomatous diseases, hyperthyroidism, metastatic bone disease, multiple myeloma, vitamin D toxicity, and milk-alkali syndrome) are distinguished from primary HPT by an appropriately suppressed PTH paired with the elevated calcium. The PTH-related peptide can be assayed when PTH is suppressed in the presence of hypercalcemia. Familial hypocalciuric hypercalcemia must be excluded with a 24-hour urine collection and estimation of the calcium: creatinine clearance ratio. This can be done based on low urinary calcium: creatinine excretion ratio of <0.01. If below 0.01, a mutational analysis of the calcium-sensing receptor and possibly G alpha 11 and AP2S1 may be pursued. Conversely, a renal leak of calcium (hypercalciuria) can result in a compensatory raise in PTH and would cause persistent hyperparathormonemia even after successful surgery for hyperparathyroidism.9
2.3 Recommendation 2
Detailed review of past surgical and pathologic data is essential to optimize surgical success. Level of evidence: 3A-B, level of recommendation: strong.
When a patient is referred for consideration of further parathyroid surgery, it is essential that the multidisciplinary team gathers key patient data. The increased risk of a second exploration through a scarred field requires additional scrutiny to be certain of the diagnosis and to decide whether additional surgery is justified.10 The prior operative notes and pathology reports typically provide important information (although some interpretation may be required), and should be retrieved along with the imaging reports and images obtained before the original operation. This data can be transferred to a surgical planning worksheet (see Figure 1).
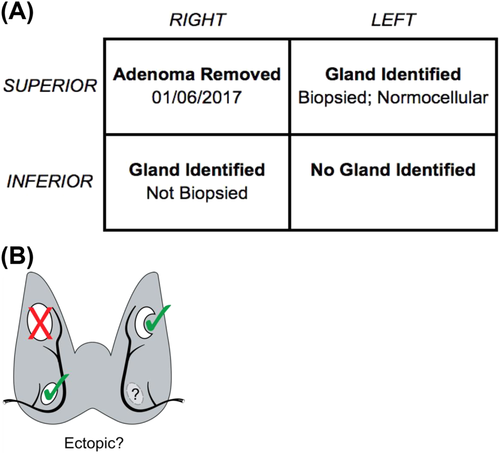
Once a decision to operate has been made, the likelihood of single or multiple gland pathology and the likely location of the (missed) lesion(s) need to be determined. If none or only normal parathyroid gland(s) were excised at the first operation, it is likely that the patient still retains a solitary adenoma.11 Therefore, a forensic analysis of the operation notes, the pathology report, and biochemistry results both intraoperatively and perioperatively is fundamental. Particular attention should be paid to which glands were identified, which were removed, and what the pathology was on each excised specimen. From this one might derive the most likely site (quadrant) for the lesion, increase the likelihood of success, and reduce the likelihood of hypoparathyroidism that might result from revision exploration. This analysis also anticipates where scarring is most likely to be encountered.12, 13 The possibility of parathyromatosis should also be considered if there was any suggestion of capsule rupture during the previous surgery. It should be possible to establish from the pathology and imaging reports whether the tissue removed was parathyroid and ascertain its weight and histological characteristics.
2.3.1 Common anatomic explanations for surgical failure
As a matter of definition, persistent or recurrent hyperparathyroidism requiring reoperation means that the patient must harbor one or more hyperfunctioning parathyroid glands. These may be situated within the central compartment in “normal” or “near normal” locations or they may be in ectopic positions. Knowledge of the normal anatomic positions, known embryological variants, and parathyroid migration patterns is requisite in the preoperative planning.
Failed parathyroid operations often have identifiable anatomic or technical reasons (ie, a low lying superior gland). Missed adenomas are usually in “normal” positions, although their size over time may promote additional descent into the central neck compartment.14 A large superior gland will often sink into a paraesophageal location posterior to the recurrent laryngeal nerve. Surgeon reluctance at the initial operation to manipulate the nerve is a common cause of failure to identify such an adenoma. A missed lower gland is often associated with an undescended thymus, and missed upper glands are more typically retroesophageal or retropharyngeal.15 The clinical statement is true: “if you are looking for a missed upper gland look low, if looking for a missed lower gland look high.”
The true incidence of ectopic and supernumerary glands in the neck is on the order of 1% with autopsy series suggesting a higher frequency than encountered clinically.16 Failure to identify glands in characteristic anatomic position(s) is a more common occurrence than the presence of a supernumerary gland. True intrathyroidal parathyroids are also rare; they are more commonly closely attached to nodular thyroid gland capsule than truly intrathyroidal.17 An unappreciated intrathoracic gland may be within the thymus, the posterior mediastinum, or the aortopulmonary window.18 Unless heralded by imaging, ectopic gland detection before the first reexploration is uncommon because most primary operations focus upon suspected abnormal glands within the neck.
Unappreciated multiple gland disease (whether from a “double adenoma” or “hyperplasia”) constitutes a different problem from frank failure to localize the offending adenoma at the initial operation. Unsuccessful control of 4-gland disease, present in some 10% of patients, is not typically associated with difficulty in locating the glands, but rather with misinterpreting the character of abnormal glands found in usual locations. If the original surgeon used intraoperative PTH, they may have been misled by a reassuring drop in PTH when an insufficient parathyroidectomy had been performed. In such cases, more extensive parathyroid resection combined with a plan for cryopreservation and autotransplantation may have been indicated.
2.4 Recommendation 3
Assessment of the patient's current health status and previous surgical complications, if any, must be made before planning for reoperative surgery. Level of evidence: 1-3, level of recommendation: moderate.
Key factors to be considered include known anesthetic risk(s), technical difficulty of additional surgery in a scarred area, and previous complications, such as vocal cord palsy. Finally, these factors need to be brought to bear on the decision on whether the degree of hyperparathyroidism merits reoperation at the present time.19, 20
One must be mindful of interim events that may have occurred that place the patient at greater risk. There may have been progression of existing medical comorbidities from the time of the first procedure. Additional medical conditions may have developed. The persistent or recurrent hyperparathyroidism may have exacerbated or accelerated end organ damage.
2.5 Recommendation 4
Preoperative laryngeal assessment is required in all patients before reoperative parathyroid surgery. Level of evidence: 3A, level of recommendation: strong.
It is imperative to assess the reoperative patient for laryngeal complications that might have occurred as a result of the first parathyroidectomy procedure. These may or may not be heralded by a reported change in voice. Both the Scandinavian Endocrine Registry and the British Endocrine Audit data suggest rates of vocal cord paralysis increase if patients are examined routinely as opposed to only if symptomatic; typically, these rates double.21-23 It is essential in all patients that vocal cord assessment by flexible laryngoscopy be performed as part of their initial evaluation. If there is a vocal cord weakness, this presents a major challenge. If reexploration is confined to the paralyzed or paretic side, perhaps the patient would be exposed to little or no additional risk of ill effects upon their voice from the reoperative procedure. If, however, surgery is conducted on the contralateral side, the only remaining mobile cord is placed at risk. The patient is now at an increased risk for a postoperative bilateral vocal cord paresis that could require a tracheotomy. This possibility must be clearly discussed with the patient with full and written informed consent obtained, which significantly impacts on the core decision making for reoperative surgery.24 The mandatory nature of the laryngeal examination is denoted in multiple major recent endocrine multidisciplinary guidelines, including the AAO-HNS, the ATA guidelines, and the AHNS guidelines.23, 25, 26
2.6 Recommendation 5a
Preoperative imaging of reoperative parathyroid surgical patients is mandatory. Level of evidence: 1-3, level of recommendation: strong.
The importance of obtaining any imaging undertaken before first-time surgery for review cannot be understated. It is common that review by a radiologist experienced in parathyroid imaging will identify a likely location of the disease.3 Thus, localization of the target parathyroid lesion is essential before proceeding with reoperative parathyroid surgery.
2.7 Recommendation 5b
Using the imaging modality that is readily available, most reliable, and cost-effective in a given health system is recommended. This will vary depending on location. Level of evidence: 1-3, level of recommendation: moderate.
2.7.1 Ultrasound
After previous parathyroid surgery, a patient with persistent or recurrent hyperparathyroidism should undergo ultrasonography of the neck as the first-line imaging study. Ultrasound is highly operator-dependent, and a candidate parathyroid adenoma may be recognized where it was missed by a prior sonographer. Surgeon-performed ultrasound have been shown to be at least as successful as radiologist-performed ultrasound for parathyroid disease; in any case, experience and motivation are key.27 In addition to high sensitivity (72%-87% in the reoperative setting), ultrasound has the added advantage of revealing nodular thyroid disease, regional anatomic features, including lymphadenopathy, and postoperative changes from the previous parathyroid exploration, all things that can impact on a subsequent exploration.28 If ultrasound findings are concordant with other available first-time imaging studies, then ultrasound localization may be sufficient to enable parathyroid reoperation. However, cross-sectional imaging will usually be desired. To substantiate ultrasound findings if a parathyroid adenoma candidate is identified, ultrasound-guided fine-needle aspiration for PTH washout can be considered.29, 30 Ultrasound-guided PTH is not routinely recommended for first-time parathyroid localization; however, it may be helpful after failed prior parathyroid exploration because of the increased risks of reoperative surgery. If ultrasound scans are nonlocalizing, or if ultrasound findings are equivocal, more imaging studies are indicated.31
2.7.2 Scintigraphy
Ultrasound scans and parathyroid scintigraphy have been shown to play complementary roles; in fact, utilizing both modalities are superior to either imaging method alone.32 Technetium-99m radiolabeled methoxyisobutylisonitrile (known as “MIBI”) is the current standard internationally. Early (about 20-30 minutes) and delayed (2-3 hours) postinjection (dual phase) static planar images are acquired in the typical anterior and oblique views.33 Although the radiotracer “washes out” from normal tissues, it is persistently accumulated in a parathyroid adenoma.34 In addition to planar images, single photon emission CT (SPECT) and or hybrid SPECT/CT can be performed. The SPECT in addition to planar imaging increases sensitivity to 67%, as compared to 42% for planar imaging alone.35 Further hybrid SPECT/CT offers the combination of functional and anatomic imaging to localize a parathyroid adenoma with a sensitivity approaching 97%.36 In all cases, images should extend below the aortic arch to evaluate for an ectopic parathyroid.
2.7.3 CT and MRI
The most useful technique – described as 4D CT – involves an initial unenhanced study followed by 2 or 3 contrast-enhanced acquisitions during arterial and venous phases of contrast medium enhancement through the neck and mediastinum.37 The typical parathyroid gland demonstrates peak enhancement on the arterial phase, followed by a washout of contrast on the delayed phase images, thus helping to differentiate it.38-40 This modality has been shown to be considerably better than both ultrasound and MIBI in localizing parathyroid disease in patients with recurrent or persistent disease after previous surgery.41-43
Dynamic contrast-enhanced MRI may also be used to localize parathyroid adenomas, taking advantage of the hypervascular nature and differential contrast washout from parathyroid adenomas compared with lymph nodes and thyroid tissue.44-46
2.8 Parathyroid venous sampling and parathyroid angiography
Venous sampling may consist simply of a single sample taken from each internal jugular vein under ultrasound guidance to lateralize disease to one side of the neck or 20 to 30 samples obtained from numerous vessels draining the neck and upper thorax via a femoral venous approach under fluoroscopic guidance. The latter more invasive procedure should only be considered when other cross-sectional imaging studies and scintigraphy are unhelpful.47-50 Combining venous sampling with selective parathyroid arteriography has been shown to improve localization when compared to venous sampling alone.50 Its major advantages are the ability to identify parathyroid glands that cannot be otherwise visualized and to demonstrate venous drainage from neck and upper thoracic vessels that may have been disturbed as a consequence of previous central neck surgery. This study is highly reliant on operator expertise and is potentially associated with serious but uncommon complications, including stroke.
3 SECTION 2: SURGICAL MANAGEMENT
3.1 Recommendation 6
Reoperative parathyroid surgery should be performed in the setting of an identified target gland(s) on preoperative imaging studies. Level of evidence: 3A-B, level of recommendation: strong.
The need for a precision operation is imperative in reoperative parathyroid surgery; therefore, having a specific imaged target is essential.51 Focused exploration and excision based on a preoperative image in conjunction with intraoperative PTH is the most effective way to manage the patient who requires reoperative parathyroid surgery. Unguided bilateral exploration of what can be a significantly scarred central neck is not recommended because it is challenging, often results in a second failure to treat hyperparathyroidism, and risks complications, such as hypoparathyroidism, bilateral vocal cord paralysis, as well as tracheal and cervical esophageal injury.
3.2 Recommendation 7
Parathyroid reoperations are challenging and should be undertaken by experienced surgeons. Level of evidence: 1-3, level of recommendation: moderate.
National datasets revealed lower cure rates with reoperative parathyroid surgery 84% compared to 95% for first time surgery. Although cure rates are lower, complication rates are higher with a 6-fold increase in vocal cord paralysis and a doubling of bleeding rate compared to primary surgery.52 Permanent hypoparathyroidism rates of between 10% and 20% are also described.12
Patients should be assessed and managed on a risk benefit basis. Not all patients with recurrent HPT are surgical candidates. Deciding when to operate is complex and multifaceted, balancing a decrease in cure rate with increased complications compared to primary surgery. Discussion by a multidisciplinary team constitutes good clinical practice.
Morbidity, mortality, length of stay, and cost are inversely proportional to surgical volume.53-55 Institutional experience has been shown to influence operative success; with higher volume institutions having more favorable outcomes.56, 57 For primary HPT surgeons, having an annual volume in excess of 50 cases have better outcomes compared with lower volume surgeons but there are no studies examining this question for reoperative surgery.58
3.3 Recommendation 8
Parathyroids should be approached through nondissected planes avoiding, when possible, most heavily scarred regions. Level of evidence: 1-3, level of recommendation: moderate.
In some cases, the patient has cervical scarring from prior thyroid or anterior cervical spine surgery, whereas others have had prior parathyroid surgery. If the first operation is done by an inexperienced parathyroid surgeon who missed a well localized adenoma, reoperation can be rather straightforward because the surgeon failed to go deep enough in the neck for a superior adenoma or inferior enough within the thymus for an inferior parathyroid adenoma.59-61
A number of approaches are available to the reoperative parathyroid surgeon through less-dissected planes, which can make reoperation more likely to be successful and, at the same time, limiting the risks of complications.
3.3.1 Original midline approach
If a patient has undergone a previous minimally invasive or targeted parathyroidectomy on one side and additional glands are localized on the contralateral side, it is perfectly reasonable to go through an original midline approach, especially if the strap muscles on the side in question have not been badly scarred.62, 63 If after dealing with an abnormal gland(s) on the previously unoperated side, the intraoperative PTH level does not fall appropriately, then it may be best to approach the original side by a lateral dissection (between straps and sternocleidomastoid) to avoid bleeding from peeling the scarred strap muscles off the thyroid.64
3.3.2 Lateral approach
The lateral approach is ideal for missed superior parathyroid glands in patients who have previously undergone a standard midline approach (see Figure 2).65 One of the most commonly missed parathyroid adenomas is the superior adenoma posterior to the recurrent laryngeal nerve within the tracheoesophageal groove or retroesophageal region. With this approach, a plane is developed between the sternocleidomastoid and the strap muscles on the side in question. The contents of the carotid sheath are then retracted laterally, thus exposing the prevertebral fascia and the tracheoesophageal groove. Typically, the previously undisturbed recurrent laryngeal nerve (RLN) can be easily identified via this approach in a previously undissected plane. The lateral approach may be more difficult after prior low anterior cervical spine surgery as its plane of dissection is similar; however, this alone should not contraindicate the lateral approach.
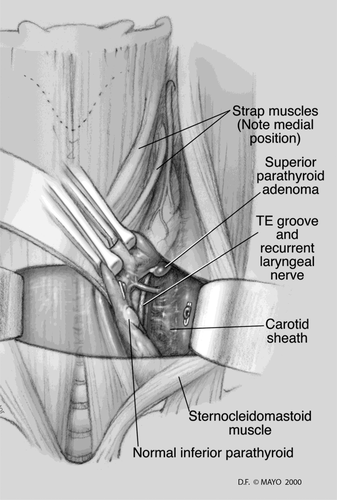
3.3.3 Superior approach
If preoperative localization identifies an undescended inferior parathyroid gland at the level of the carotid bifurcation, an ultrasound scan can be utilized to determine the most direct route to this missing gland (see Figure 3).65 A small incision can be made in a skin crease directly overlying the gland, and, using a focused approach through previously nondissected planes, the adenoma can be easily identified and removed. Care must be taken to avoid injury to the nearby vagus, superior laryngeal, and hypoglossal nerves.
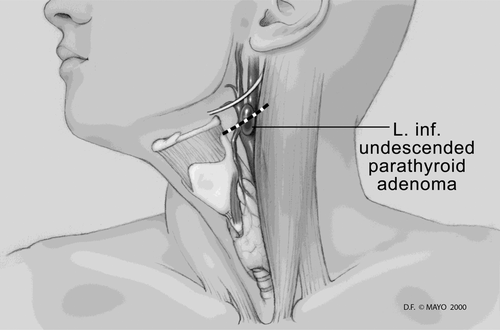
3.3.4 Inferior approach
An inferior adenoma within the upper portion of the thymus and/or superior mediastinum can be approached through a previously nondissected plane once the original incision is reopened (see Figure 4).65 There is no need to mobilize the thyroid gland on either side. The lower thyroid pole can be exposed, along with the thyrothymic ligament. The thyrothymic ligament can then be separated from the inferior pole and gently retracted upward, often exposing the missed inferior parathyroid adenoma. A map of typical locations for parathyroid discovered at reoperation is presented in Figure 5.8
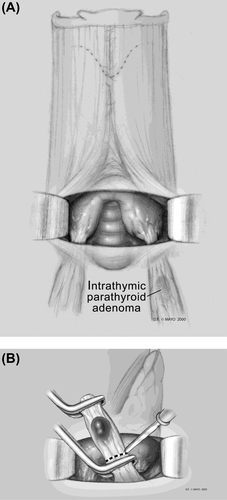
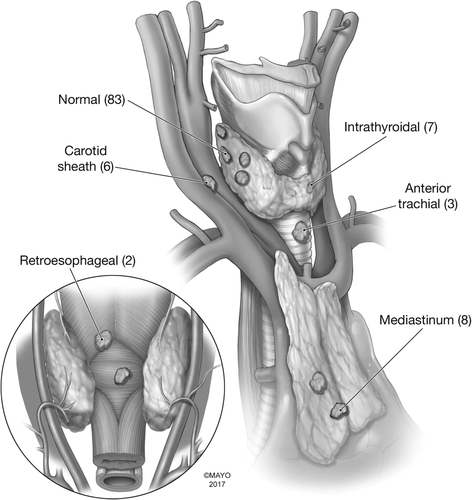
3.4 Recommendation 9
Intraoperative adjuncts, such as neural monitoring and PTH assay, should be used during reoperative parathyroid surgery. Radioguidance, visible tracers, or ultrasound can be used based on surgeon experience and availability. Level of evidence: 3, level of recommendation: moderate.
3.4.1 Intraoperative nerve monitoring
There is consensus supporting the routine use of neural monitoring during complex and reoperative thyroid and parathyroid surgery.20, 66-68 Intraoperative nerve monitoring can be very valuable in reoperative parathyroid surgery, particularly when the missed gland is within a heavily scarred area. With neural monitoring, the vagus can be positively stimulated in the carotid sheath then the RLN can be electrically mapped through the scarred paratracheal region as in a reoperative central neck dissection for recurrent thyroid cancer. Turning up the stimulus intensity to 2 mA can help localize the nerve if scarring prevents direct visualization.69 Once the nerve is localized, turning the stimulus intensity back to about 1 mA will reduce false-positive stimulation of surrounding structures. The RLN is generally posterior and deep to inferior parathyroid glands and anterior and superficial to superior glands, excising the latter putting the nerve at greater risk of injury during reoperative parathyroid surgery.67, 70
3.4.2 Intraoperative parathyroid hormone monitoring
When the surgery is directed at preoperatively localized glands, PTH confirmation of success is very accurate.61-63 Intraoperative PTH (IOPTH) assay is recommended in reoperative parathyroidectomy to confirm successful excision of hyperfunctioning tissue, with a >50% drop from baseline and into the normal range being the standard.8, 11, 71 This approach can assist in the confirmation of a biochemical cure while avoiding the risks associated with prolonged and unnecessary exploration.
3.4.3 Radioguidance
Radioguidance can be helpful in MIBI-positive single gland persistent or recurrent disease. If this approach is adopted, the ex vivo examination of parathyroid tissue, which is hyperfunctional, should exceed 20% of a patient background measurement.72, 73
3.5 Recommendation 10
During reoperative parathyroid surgery when postoperative hypoparathyroidism is a concern, primary autotransplantation should be considered after frozen section biopsy confirmation. Parathyroid cryopreservation can also be considered if available. Level of evidence: 3-5, level of recommendation: moderate.
There are no data to support the use of IOPTH as a determinant in deciding when to autotransplant. Primary (immediate) autotransplantation should be undertaken with caution owing to the possibility of supernumerary gland(s) in reoperative parathyroid cases.74, 75 This should be optimally reserved for circumstances when it is known that 3 normal glands have previously been excised.76
Parathyroid cryopreservation is the process of freezing and storage of parathyroid tissue with a view to later autotransplantation. It is used when it is envisaged that there may be a need to restore or improve parathyroid function in patients rendered hypoparathyroid after parathyroid surgery. At reoperative surgery, the role for cryopreservation/secondary autotransplantation is controversial owing to the limited success rates for survival of auto grafts (30%-50%).8, 12 The European consensus statement proposes judicious autograft use.21, 22 There is a view that cryopreservation should be restricted to centers that have a large experience in this technique owing to a high failure graft rate (up to 90%).74, 77-79
4 SECTION 3: ALTERNATIVES TO REOPERATIVE SURGERY
4.1 Recommendation 11
Nutritional and pharmacologic optimization should be performed in patients refusing or are not candidates for reoperative parathyroid surgery. Treatable metabolic abnormalities should also be addressed in operative patients. Level of evidence: 1-5, level of recommendation: strong.
There are situations in which the patient who was an initial candidate for parathyroid surgery is no longer suitable in the context of recurrent or persistent parathyroid disease. In these situations, medical approaches can be considered as an alternative to surgery.
4.1.1 Nutritional
Nutritional guidelines include sufficient intake of calcium and vitamin D. Calcium intake should adhere to the Institute of Medicine guidelines for adults without parathyroid disease.80 Intake of between 1000 and 1200 mg from all sources is recommended. The advice to ensure adequate calcium intake is based on the concern that diets low in calcium could conceivably stimulate parathyroids to secrete additional parathyroid hormone.81
Vitamin D sufficiency is important because vitamin D deficiency in primary hyperparathyroidism is associated with greater manifestations of biochemical abnormalities and target organ compromise.82-85 Because vitamin D deficiency is associated with more severe manifestations of hyperparathyroidism, it seems prudent to aim for levels >30 ng/mL.1 In patients who are vitamin D deficient, cautious replacement with judicious amounts of cholecalciferol (vitamin D3) starting with 1000 IU/day is wise.
4.1.2 Pharmacological
In patients whose hypercalcemia is within 1 mg/dL above the upper limits of normal, most experts would not recommend any specific measures to reduce the serum calcium86 because mild hypercalcemia is virtually never associated with symptoms. In patients whose serum calcium is above this threshold, reduction of the serum calcium is a reasonable goal. The few studies with the selective estrogen receptor modulator, raloxifene, have shown modest reductions in the serum calcium.87, 88 More impressively, the calcimimetic agent, cinacalcet, has been shown in several clinical trials to reduce the serum calcium promptly and persistently.89-91 Cinacalcet increases the sensitivity of the calcium-sensing receptor to extracellular calcium. Although cinacalcet is very effective in reducing the serum calcium, circulating levels of PTH fall only modestly. Higher amounts of cinacalcet (>30 mg q.d. or b.i.d.) can be used but gastrointestinal and central nervous system systems can be limiting.
In patients with reduced bone mineral density, cinacalcet is not of benefit because studies extending over 5 years have not shown beneficial bone density changes, despite reductions in the serum calcium.91 Studies with the bisphosphonate, alendronate, have shown that it increases bone mineral density of the lumbar spine within a year of administration, with further increases seen during the second year.92 However, bone density of the hip and distal one-third of the radius is not appreciably changed by alendronate in primary hyperparathyroidism.
In patients who present with both high levels of the serum calcium and reduced bone mineral density that need attention, there have been a few studies using cinacalcet and alendronate together. Dual therapy leads to reductions in the serum calcium and increases in the bone mineral density.93, 94
The situation in patients who have had kidney stones and are not candidates for parathyroid surgery is particularly difficult. The etiology of kidney stones, even in those with hypercalciuria, is likely to be multifactorial.3 The use of thiazide diuretics is problematic because serum calcium levels could rise even higher and should be avoided.
| First-time PTX | Reoperative PTX | |
|---|---|---|
| Vocal cord palsy | 0.8% | 5% |
| Bleeding | 0.5% | 1.25% |
| Recommendation for neural monitoring | No recommendation | Yes |
| Recommendation for IOPTH | No recommendation | Yes |
- Abbreviations: IOPTH, intraoperative parathyroid hormone; PTX, parathyroidectomy.