Surgical management of the recurrent laryngeal nerve in thyroidectomy: American Head and Neck Society Consensus Statement
Abstract
“I have noticed in operations of this kind, which I have seen performed by others upon the living, and in a number of excisions, which I have myself performed on the dead body, that most of the difficulty in the separation of the tumor has occurred in the region of these ligaments…. This difficulty, I believe, to be a very frequent source of that accident, which so commonly occurs in removal of goiter, I mean division of the recurrent laryngeal nerve.” Sir James Berry (1887)
1 INTRODUCTION
Injury to the recurrent laryngeal nerve (RLN) and/or external branch of the superior laryngeal nerve (SLN) resulting in postoperative vocal disability is one of the most feared complications after thyroid and parathyroid surgery. Subjective postthyroidectomy voice complaints occur in 30%-87% of patients and traditionally quoted rates of RLN injury (3%-5%) significantly underestimate the true incidence, which is likely closer to 10%.1, 2 Similarly, although the actual incidence of external branch of the SLN injury remains unknown, it may be as high as 58%.3, 4
Inconsistencies in reported injury rates stem from a lack of standardization of postoperative laryngeal examination practices, reporting biases from large thyroid centers in which complication rates are low, and the subtlety and variability of nerve paralysis symptoms.5-8 The patient may experience dyspnea from air escape, dysphonia (hoarseness, vocal fatigue, and breathy voice), and dysphagia with potential aspiration. Symptoms may be severe enough to warrant change of vocation. Bilateral injury to RLNs may lead to stridor, respiratory distress, and airway compromise due to obstruction. Clinical symptoms of external branch of the SLN injury include voice fatigue and changes in vocal range and pitch, all of which can have a significant impact on quality of life, particularly if the patient is a professional voice user.9
Risk factors for RLN injury during thyroidectomy include revision procedures, those for malignancy, Graves’ disease, recurrent goiter, substernal goiter, hematoma exploration, and surgeon volume.10 Lower volume surgeons have higher complication rates and longer hospital stays. In one series, the odds of a complication were 87% higher if the surgeon performed 1 case/year, 68% for 2–5 cases/year, 42% for 6–10 cases/year, 22% for 11–15 cases/year, 10% for 16–20 cases/year, and 3% for 21–25 cases/year. This is a major health issue within the United States; 51% of surgeons in this series performed 1 thyroidectomy per year.11 Racial disparity may also be a risk factor for intraoperative RLN injury. African Americans and Hispanics experienced higher complication rates and they were more likely to have operations with low volume surgeons. In this study, high volume surgeons (>100 thyroidectomies per year) performed <5% of the thyroidectomies.12
As surgical volumes, preferences, and techniques vary, the purpose of this American Head and Neck Society (AHNS) consensus document is to provide a concise review of RLN anatomy, surgical approaches emphasizing proper identification of the RLN in every case, technical advances in RLN monitoring, and postoperative clinical implications to encourage safe and successful outcomes.
The document includes anatomic and embryologic background, surgical maneuvers, and technical information as well as a summary of state-of-the-art neural monitoring recommendations. The recommendations of this AHNS multidisciplinary consensus panel of the American Head and Neck Society, the largest surgical organization in the United States focused on thyroid cancer treatment, are intended to help guide surgeons in the clinical decision-making process when managing the RLN at thyroidectomy. It is our hope to improve the quality and reduce variation in management of the RLN at thyroidectomy and facilitate further communication and research for thyroid surgical patients. In our opinion, this monograph represents best contemporary surgical care for this patient population.
The consensus panel is comprised of members of the AHNS, including the Endocrine Section of the Society, with representation from medical endocrinology and both national and international surgical representation drawn from general/endocrine surgery and otolaryngology/head and neck surgery. Authors provided expertise for their respective sections, and consensus recommendations were created regarding RLN management at thyroidectomy. Evidence-based literature support is drawn from thyroid surgical publications as well as recent existing American Academy of Otolaryngology – Head and Neck Surgery, AHNS, and American Thyroid Association guidelines. The manuscript was then distributed to members of the AHNS author panel and governing council for further feedback.
2 RECOMMENDATIONS
2.1 Comprehensive understanding of the embryology and anatomy of the recurrent laryngeal nerve, larynx, and neck base is essential for optimal management of the recurrent laryngeal nerve during thyroidectomy
2.1.1 Embryology
The thyroid originates from the fusion of the medial thyroid anlage (derived from the primitive pharynx) and the lateral thyroid anlage (derived from the neural crest). The tubercle of Zuckerkandl represents this fusion site, a posterior lateral projection from the thyroid.13, 14 The superior parathyroid gland originates from the fourth branchial pouch of the primitive pharynx.15 It has a close association with the tubercle of Zuckerkandl, thus, proper management of the tubercle and its close neighbor, the RLN is very important in the preservation of not only nerve function but superior parathyroid function.
The RLN arises from the vagus nerve and carries motor, sensory, and parasympathetic fibers. During development, as the heart descends and the neck elongates, both the RLNs are dragged down by respective aortic arches. Hence, the left RLN recurs around the ligamentum arteriosum (VI arch); but in the absence of the ligamentum arteriosum, the right RLN recurs around the right subclavian artery (IV arch) and, thus, ascends in a more anterior, oblique, and lateral path in its paratracheal position. The left RLN loops around the aortic arch and ascends more vertically and deeper in the left tracheoesophageal groove (Figure 1).16 Both nerves may cross the inferior thyroid artery superficially or run deep to it or between its branches. Both nerves enter the larynx below the cricothyroid articulation under the inferior most fibers of the inferior constrictor muscles of the pharynx. The nerve divides into an anterior and posterior branch. The anterior branch supplies motor function to 4 intrinsic laryngeal muscles. The cricothyroid muscle is not innervated by the RLN but receives its innervation by the SLN. The posterior branch provides sensation to the vocal folds and subglottic region, extending to a robust posterior laryngeal sensory anastomosis, which is termed the nerve of Galen. The RLN provides branches to the inferior constrictor and cricopharyngeus muscles before entering the larynx. Nonrecurrence of the RLN occurs on the right side in just 0.5%-1% of cases. This is due to failure of development of the fourth right aortic arch, creating an aberrant subclavian artery (which normally draws the RLN down). The nonrecurrent laryngeal nerve (NRLN) has a direct medial course from the right vagus nerve, usually at the level of the inferior thyroid artery (ITA) and then ascends in the tracheoesophageal groove (Figure 2).17 An NRLN is thus seen in association with a retroesophageal right subclavian artery. The presence of an NRLN on the left side is exceedingly rare and associated with situs inversus and dextrocardia.

Recurrent laryngeal nerve anatomy. Randolph et al. Surgical Anatomy and Monitoring of the Recurrent Laryngeal Nerve. Surgery of the Thyroid and Parathyroid Glands, Second Edition. Philadelphia, PA, Elsevier, 2013:309. With permission, © Gregory Randolph
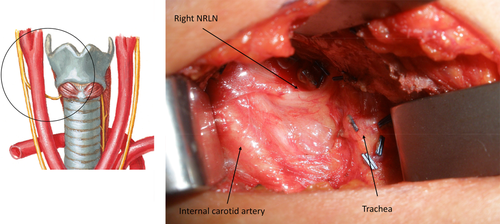
Abnormal embryologic variant; nonrecurrent right laryngeal nerve (right NRLN). Illustration by Netter, photograph courtesy of Nathan Hales [Color figure can be viewed at wileyonlinelibrary.com]
2.2 Knowledge of the anatomically complex Ligament of Berry is essential for safe thyroid and parathyroid surgery
2.2.1 Ligament of Berry
The Ligament of Berry is a suspensory ligament that anchors the thyroid gland to the laryngotracheal complex. It is a dense consolidation of pretracheal vascular fascia, which was first described by Berry in 1888. The distal 2 cm of the extralaryngeal course of the RLN is intimately related to the Ligament of Berry. Neural monitoring mapping studies show that this is the most common site of surgical injury to the RLN.18, 19 At this level, the RLN is enveloped by 2 layers (in the past, it has been loosely described as the Ligament of Berry). However, the more superficial and lateral layer is a superficial vascular fascial layer that covers the RLN and contains the tertiary branches of the inferior thyroid artery and vein, it also usually contains the superior parathyroid gland, and the tubercle of Zuckerkandl.20 Deep to this layer, medial to the RLN, is a dense fibrous layer—the true Ligament of Berry. The true Ligament of Berry may itself consist of 2 fascial layers with thyroid tissue between them, but the RLN is typically lateral (Figure 3). The true Ligament of Berry typically extends over 11 mm (from the cricoid and trachea to the thyroid) and is of variable thickness, ranging from 2–7 mm.
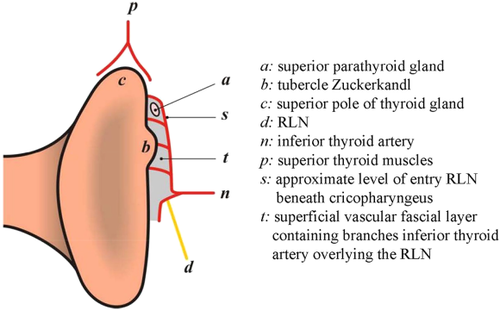
Tubercle of Zuckerkandl and superior parathyroid gland in the superficial vascular fascial layer. RLN, recurrent laryngeal nerve. Republished as adapted from Serpell JW. New operative surgical concept of two fascial layers enveloping the recurrent laryngeal nerve. Ann Surg Oncol. 2010;17:1628–1636, with permission of Springer [Color figure can be viewed at wileyonlinelibrary.com]
During medial rotation of the thyroid during thyroidectomy, the RLN is typically pulled anterior between 2 fixed points, the attachment of loose fascia into an artificial genu, lateral to the true Ligament of Berry and the subclavian artery or the aorta inferiorly. The apex of the genu becomes the site of greatest tension or tensile force on the RLN due to traction, the most common cause of postoperative RLN palsy (Figure 4). The small caliber RLN fibers may be at particular risk of traction injury. Upon division of the true Ligament of Berry, and when the RLN is released backward, tension is lost, and the nerve becomes relaxed, assuming a more serpiginous disposition.21
Genu and common point of injury of recurrent laryngeal nerve seen with ventral retraction. Recurrent Laryngeal Nerve Branching. The Recurrent and Superior Laryngeal Nerves, First Edition. Switzerland, Springer, 2016:83–93. With permission, © Gregory Randolph [Color figure can be viewed at wileyonlinelibrary.com]
The inferior laryngeal artery, a posterior branch of the inferior thyroid artery (ITA), may course within the superior portion of the Ligament of Berry and requires careful control during thyroidectomy. Managing inadvertent bleeding at this site increases the risk of thermal or mechanical trauma to the adjacent RLN. Superior to the ITA, both RLNs course in the tracheoesophageal groove and enter the larynx at the cricothyroid articulation, deep to the lowest most fibers of the inferior constrictor or cricopharyngeus muscle (Figures 5 and 6).
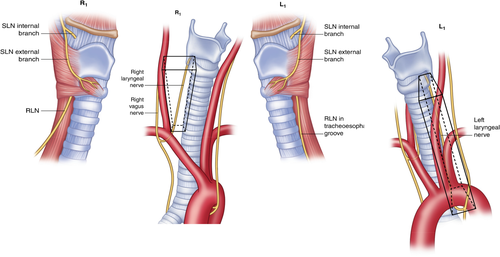
R1 = Normal pathway of the right recurrent laryngeal nerve (RLN) through the right paratracheal region. L1 = Normal pathway of the left RLN through the left paratracheal region. Recurrent Laryngeal Nerve Branching. The Recurrent and Superior Laryngeal Nerves, First Edition. Switzerland, Springer, 2016:125–138. With permission, © Gregory Randolph [Color figure can be viewed at wileyonlinelibrary.com]
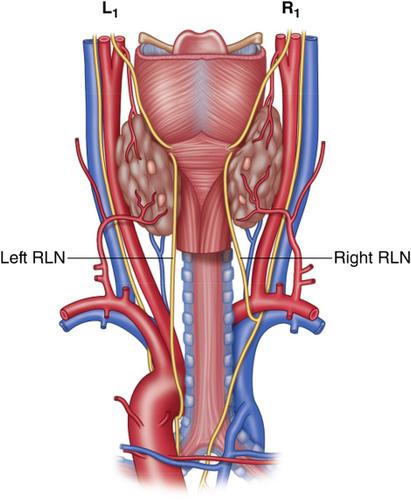
Posterior view of the right and left recurrent laryngeal nerve (RLN). Recurrent Laryngeal Nerve Branching. The Recurrent and Superior Laryngeal Nerves, First Edition. Switzerland, Springer, 2016:125–138. With permission, © Gregory Randolph [Color figure can be viewed at wileyonlinelibrary.com]
A right NRLN demands careful consideration during thyroidectomy, as the RLN will not be found inferior to the ITA; these findings should alert the surgeon to the possibility of an NRLN.
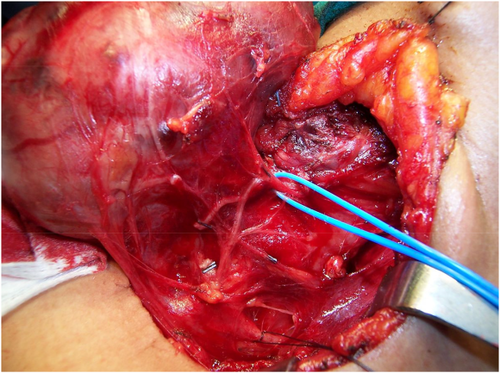
The RLN will branch in up to 90% of cases. The majority of these are posterior sensory divisions to the trachea, esophagus, and hypopharynx and do not enter the larynx. However, true motor branches enter into the larynx underneath the cricopharyngeus muscle in 30%-76% of cases, usually separating within the last 2 cm of the extralaryngeal course of the RLN (Figure 7). Most RLNs branch immediately before laryngeal entry. Therefore, if only the RLN divisions occurring at a distance greater than 5 mm from laryngeal entry point were considered as branches, the incidence reduces to about 34%.22 The branching typically occurs at a median distance of 18 mm on the right and 13 mm on the left. Several studies have shown the rate of bifurcation is greater on the right than the left side. Studies using intraoperative nerve monitoring have shown that the majority of motor fibers to the larynx for both adductors and abductors are located in the anterior branch.23 The anterior branch is thinner than the main trunk of the RLN, and, therefore, the anterior branch is subjected to a greater tensile stress for an equivalent traction force, leading to an increased risk of traction-induced neuropraxic RLN injury.24-26
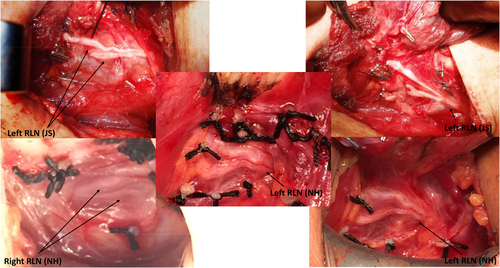
Operative photos of recurrent laryngeal nerve (RLN) branching patterns; courtesy of Jon Serpell (JS) and Nathan Hales (NH) [Color figure can be viewed at wileyonlinelibrary.com]
2.3 Surgeons should be familiar and adept at applying the four surgical approaches to the recurrent laryngeal nerve (lateral, inferior, superior, and medial)
2.3.1 Approaches to the recurrent laryngeal nerve at the Ligament of Berry
The approaches to identifying the RLN during thyroid surgery vary according to the circumstances and surgeon preference. They may be divided into lateral, inferior, superior, and medial approaches, with particular advantages and limitations. The specific dissection tactics for an individual patient may blend these discrete approaches, but distinguishing between the individual methods is helpful to understand the optimal and varied RLN dissection techniques as currently described.
2.3.2 The lateral approach
This is the most common approach for routine thyroidectomy. The RLN is identified relatively high in the neck at the midpolar level after releasing the superior and inferior thyroid poles, and retracting the gland over the trachea (Figure 8). The inferior parathyroid gland will often be found near the inferior pole, before encountering the nerve. Advantages include preserving the inferior parathyroid blood supply and limiting exposure of the RLN. Dense scar tissue in the thyroid bed and large thyroid/tubercle of Zuckerkandl are 2 situations in which the lateral approach may be frustrating. In addition, the RLN may be identified at a level where extralaryngeal branching may have already occurred; thus, an unrecognized anterior branch of the nerve may be inadvertently injured.

Approaches to the recurrent laryngeal nerve (RLN). A, Inferior approach. B, Superior approach. C, Lateral approach. Randolph et al. Surgical Approaches to the RLN. Surgery of the Thyroid and Parathyroid Glands, Second Edition. Philadelphia, PA, Elsevier, 2013:309. With permission, © Gregory Randolph
2.3.3 The inferior approach
The surgeon identifies the RLN at the thoracic inlet and follows the nerve cephalad. The nerve is typically found more laterally on the right as it courses diagonally and more vertically in the tracheoesophageal groove on the left (Figure 8). This method is particularly useful in revision surgery, in which the nerve may be identified outside of the scarred surgical bed. It may be preferable for goiters when the lower pole can be mobilized. The nerve is identified below potential extralaryngeal branching; however, a longer segment of the RLN is dissected with potential risk of devascularizing the inferior parathyroid gland. The technique is not suitable for identification of a nonrecurrent RLN.
2.3.4 The superior approach
This technique starts with the transection of the upper pedicle and mobilization of the superior thyroid pole. The inferior constrictor muscle serves as the critical landmark; the nerve courses deep to this muscle. The RLN may be gently exposed at the lower border of the inferior constrictor muscle.27 Once the proximal RLN, running dorsally, is demonstrated, the Ligament of Berry may be safely divided (Figure 8).
A superior approach may be advisable for a large goiter (particularly one with a significant substernal component) in which mobilization of the lobe sufficient to identify the RLN through a lateral or inferior approach is more challenging. Dividing the Ligament of Berry early in the procedure allows detachment of the thyroid from the trachea and facilitates delivery of the thyroid, which may decrease the risk of RLN traction injury. The superior approach may help when a search for the nerve in a more proximal location has failed. For some remote access surgical approaches (eg, retroauricular or facelift thyroidectomy), the vector of the procedure (from superior to inferior) favors superior methods. It is noteworthy that the nerve in this approach is identified between the larynx and the laterally reflected superior pole, somewhat limiting the exposure. In some cases, neural stimulation can help provide discrete nerve location information despite limitation in exposure.
2.3.5 The medial approach
The medial approach to the RLN begins with division of the isthmus. The medial aspect of the thyroid lobe is mobilized laterally in an avascular plane between the gland and the anterolateral trachea. Dissection is generally best initiated near the middle part of the gland, performing the middle and inferior dissection first. The inferior border of the cricothyroid muscle is identified, following the avascular plane between the medial superior thyroid lobe and the cricothyroid muscle fascia. This is a safe plane to dissect because the RLN resides deep to the inferior pharyngeal constrictor located immediately below. This plane can then be followed cephalad until the superior pole is identified and ligated, preserving the parathyroid gland. The focus of dissection then returns to the medial suspensory ligament, just inferior to the cricoid, continuing the dorsal dissection, as previously performed on the mid/inferior portions. Once the fibers are divided, the surgeon encounters the tubercle of Zuckerkandl. With both poles released, the only point of adherence of the gland is the tubercle. Most commonly, the RLN will be identified deep to the tubercle (Figure 9).
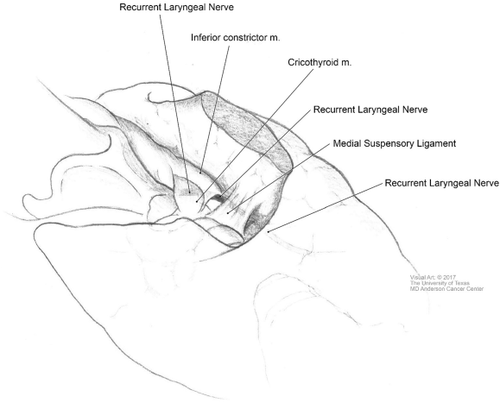
Medial approach to the recurrent laryngeal nerve. Permission granted by The University of Texas M.D. Anderson Cancer Center
This method first releases nearly all attachments of the thyroid gland, limiting traction on the RLN, and may be helpful in cases of small incisions. It can be useful for large goiters, because once the tracheal attachments are released the bulk of the gland can be mobilized out of the paratracheal space.
2.4 Instruments/technology/intraoperative nerve monitoring - loss of signal
2.4.1 Physiology of nerve monitoring
Systems that rely on the endotracheal tube-based surface electrodes have proliferated and represent the most common monitoring equipment format to date.28 The endotracheal tube-based systems record electromyographic (EMG) data from the vocal fold (ie, thyroarytenoid or vocalis muscle). During intraoperative nerve monitoring (IONM), a handheld stimulation probe is needed to depolarize the vagus or RLN nerve to evoke the laryngeal EMG response. The EMG response quantification typically includes both amplitude and latency of the signal after nerve stimulation. The EMG magnitude may be correlated with the number of muscle fibers participating in the depolarization, which in turn relates to RLN function.29, 30 The monitor should be set for an appropriate event threshold at 100 μV, and a stimulator probe should be set on a value of 1–2 mA. Pulsatile stimulus with 100-ms duration at 4 Hz can be applied through a flexible stimulator probe, which may be monopolar or bipolar, and may also be configured as dissecting instruments.
2.4.2 Current options: intermittent intraoperative neuromonitoring - handheld probe versus continuous intraoperative neuromonitoring – temporary implantable vagus electrode
Although international standards guidelines28 for conventional intermittent-IONM have been formulated to promote best practices, the drawback of using the handheld stimulation probe is due to exposing the RLN to the risk of injury between intermittent stimulations. The continuous intraoperative neuromonitoring (C-IONM) overcomes this limitation of intermittent-IONM by offering real-time RLN monitoring using temporary vagus electrode stimuli.16, 31-33 The C-IONM may be more accurately described as repeated pulsed stimulation that is contemporaneous with the surgical maneuvers.34
2.5 Intraoperative neural monitoring can provide more information than sight alone during thyroidectomy
This information includes: (1) neural mapping information before visual nerve identification; (2) prognostic information regarding nerve function that is unavailable through visual inspection alone and, therefore, has utility in all cases of bilateral thyroidectomy; (3) information as to where along the nerve a specific injury/neuropraxic segment is located; (4) better management of the SLN by improving rates of identification over visual inspection alone; and (5) information regarding the likelihood and duration of subsequent vocal cord paralysis.
2.5.1 Clinical implications
The use of IONM during thyroid surgery has given surgeons a tool for better understanding of the possible mechanisms of RLN injury.16, 18, 28-30, 35-37 By enabling early detection and elucidating the mechanism of RLN injury, IONM can help clinicians learn and plan better intraoperative and postoperative management.16, 28, 37, 38 The advantage of a C-IONM format is that it has the potential to monitor the vagus and RLN functional integrity in real-time throughout surgery and may identify EMG signals associated with early-impending injury states.31, 34, 39-43 This can alert the surgeon to stop a maneuver causing stretching or pressure on the RLN, with better recovery of nerve function.31
2.5.2 Decision making based on intraoperative neuromonitoring in case of loss of signal
Guidelines from the International Neural Monitoring Study Group call for initial vagal nerve stimulation to confirm intact RLN function and electrode position, followed by visual identification and direct stimulation of the RLN during the course of thyroid lobectomy, and final reconfirmation of intact RLN function with vagal nerve stimulation after completion of thyroid lobectomy.28
Three primary mechanisms of RLN injury are: first and most common, traction (neuropraxia) at points of relative nerve fixation; second, compression from a ligature, clip, surgical instrument, or crossing taut blood vessel; and third, direct sharp or thermal trauma.18, 44 Generally, only the latter RLN injury is potentially permanent. Traction RLN injury is not a sudden complete loss of signal to stimulation, but a gradual, reversible process as more and more motor nerve fibers close to the point of fixation become compromised.45 The trajectory of EMG amplitude and latency changes in response to injury relate to the type and time course of injury. The physiologic result is a gradual decrease in the amplitude of vocal fold contraction and an increase in the latency to nerve stimulation that is observed as more and more motor fibers lose function. The process is reversible if recognized early, but becomes established with prolonged traction as a point of loss of signal to stimulation. As long as the RLN is anatomically intact, full recovery of RLN function may be anticipated in many patients. If IONM reveals an irreversible loss of signal from traction or compression RLN injury during the first lobectomy of a planned bilateral thyroidectomy, then this information is critical with regard to considering postponing contralateral thyroidectomy until the complete return of RLN function. This avoids a potential bilateral RLN injury, which may lead to the need for a tracheostomy.46
2.5.3 Likelihood and duration of subsequent vocal cord paralysis
With loss of signal type I (focal nerve injury) rates of postoperative vocal cord paralysis 2 days after surgery are 95%. For loss of signal to be determined with surety, the surgeon must rule out equipment problems with the IONM loss of signal troubleshooting algorithms.28 Most series document a negative predictive value (ie, signal maintenance with preservation of RLN function) of 95% or greater.43, 47, 48 Cases of nontransection neural injury with loss of signal (clip/ligature, thermal, compression, and stretch) that do not improve intraoperatively can initially be managed with a “wait and see” approach. Data suggest there may be a benefit from the addition of calcium channel blockers for these types of RLN injury49-51; whereas the use of corticosteroids seems to have a slight positive effect,52 and decreased edema may improve the recovery time in temporary paralysis.53, 54 In segmental transection, nerve transfer (ansa cervicalis-to-RLN anastomosis) with epineural microsuturing is advocated if tension-free primary anastomosis cannot be obtained. With complete transection, recovery of tone but not vocal fold motion is expected with repair.55 In cases in which grafting/repair cannot be performed, laryngeal medialization/reinnervation procedures are often performed secondarily, as spontaneous recovery of an acceptable level of voice/swallowing function can be seen in some patients.
2.6 Optimal management of the recurrent laryngeal nerve that is adherent to or invaded by cancer requires knowledge of preoperative glottic function through preoperative laryngeal examination as well as intraoperative monitoring electromyography signal
In disease with superficial epineural invasion, shave or partial nerve sheath excision can allow for macroscopically clean margins with a functionally intact RLN.56 With more extensive invasion, resection of the RLN is recommended if the nerve is nonfunctioning. Functional status is determined by 2 elements: (1) preoperative laryngeal examination; and (2) intraoperative EMG signal.57 With an invaded functioning nerve,58 neural preservation should always be attempted due to limited evidence showing survival benefit with complete resection versus leaving a small remnant with adjuvant therapy.57, 59, 60 Complete resection should be considered in cases of expected improvement in disease-free survival or overall survival (eg, aggressive histopathologic subtypes, iodine-refractory disease/recurrence, previous external beam radiation, and lack of distant metastases) and when morbidity is acceptably low.57
2.7 In cases of loss of signal without electromyography recovery, the surgeon should consider staging the contralateral procedure to limit risk of bilateral cord paralysis and tracheotomy
In cases of unintentional nerve injury with loss of signal without EMG recovery, staged surgery should be considered. Possible false positive loss of signal61 or concern for complete cancer resection should be weighed against risk of bilateral vocal cord paralysis.62-66 Completion surgery can be performed if vocal fold mobility recovers postoperatively or based on multidisciplinary discussion and patient counseling.
2.8 Management of the recurrent laryngeal nerve and Ligament of Berry intraoperatively have substantial implications for postoperative radioactive iodine thyroid bed scintillographic uptake and, therefore, significant implications for endocrinologists
2.8.1 Ligament of Berry, postsurgical consequences - Bed uptake and postoperative adjuvant therapy
Despite meticulous attempts to remove all visible normal thyroid tissue during total thyroidectomy by experienced surgeons, high-resolution postoperative radioactive iodine scanning can detect very small foci of uptake in the thyroid bed and/or pyramidal lobe in >95% of patients.67-70 The radioactive iodine uptake in these small foci is usually very low, being <2% in >90% of the patients in a multicenter randomized phase III trial and demonstrating a median of only 0.32% in a single high-volume institution.69, 71
When evaluated using posttherapy high-resolution single photon emission computerized tomography-CT, discrete foci can be localized in the region of Berry's ligament in 87%, superior thyroid poles in 79%, paratracheal lobar regions in 67%, the isthmus region in 54%, and the pyramidal lobe in 46% of the patients69 (Figure 10). Thus, even after total thyroidectomy, discrete areas of radioactive iodine uptake can be expected in anatomically identifiable areas.
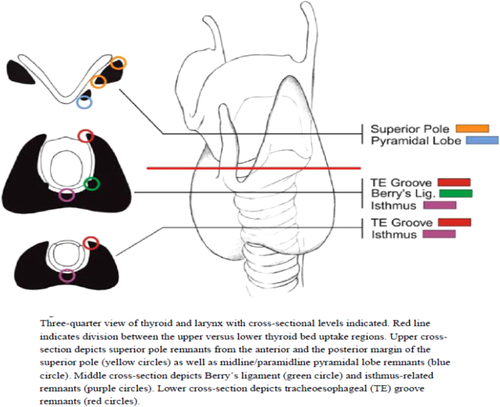
Identifiable areas of uptake as seen on single photon emission computerized tomography-CT post total thyroidectomy. Republished with permission from Zeuren R, Biagini A, Grewal RK, et al. RAI thyroid bed uptake after total thyroidectomy: a novel SPECT-CT anatomic classification system. Laryngoscope. 2015;125(10):2417–2424, with permission of Wiley [Color figure can be viewed at wileyonlinelibrary.com]
Despite the identification of these sites of minimal residual thyroid tissue after total thyroidectomy, postoperative serum thyroglobulin levels are usually very low,71-74 and often decline further over time even without radioactive iodine ablation.73 After total thyroidectomy, serum thyroglobulin values have been reported to be <0.2 ng/mL in 60% of the patients within the first year and by the 5-year evaluation point,79% of patients achieve this low thyroglobulin level even in the absence of radioactive iodine ablation.75 When analyzed in multicenter randomized radioactive iodine remnant ablation trials several weeks after surgery, the postoperative thyroglobulin is <1–2 ng/mL in 20%-30% of the patients.71, 74 However, when the analysis is confined to very low-risk patients, 93% demonstrate a thyroglobulin <1 ng per mL shortly after surgery.72 Therefore, in the absence of residual metastatic disease, a total thyroidectomy will commonly achieve a nonstimulated thyroglobulin in the 1–2 ng/mL or lower range, depending on the completeness of resection. Postoperative thyroglobulin levels may be higher in situations in which thyroid tissue or gross disease is either unintentionally left behind, a remnant at the Ligament of Berry is intentionally left behind in an effort to “protect the nerve,” or when RLN was not appropriately identified. It is not recommended to leave gross disease and the RLN should be identified in all cases. Postoperative radioactive iodine therapy is not intended to ablate gross gland/tumor intentionally left behind or to supplant meticulous surgical technique. Recent evidence suggests that the BRAFV600E mutation significantly reduces sodium-iodine symporter expression and radioactive iodine uptake.75 Thus, postoperative I-131 may prove less effective in BRAF-positive tumors, further emphasizing the importance of meticulous surgical technique and complete thyroidectomy.
Although endorsing the concept that postoperative serum thyroglobulin values should be considered when deciding whether or not additional therapy is required, the American Thyroid Association guidelines note that, presently, the optimal cutoff value is not defined.76 Nonetheless, the guidelines suggest that a postoperative thyroglobulin levels obtained more than 3–4 weeks after total thyroidectomy and >5–10 ng/mL may lead to selection of radioactive iodine ablation in low-or-intermediate risk patients who may not otherwise have required therapy. Recently, response to therapy definitions have been published for patients treated with either lobectomy or total thyroidectomy without radioactive iodine remnant ablation. These authors consider a nonstimulated thyroglobulin level of <5 ng/mL after total thyroidectomy, or <30 ng/mL after thyroid lobectomy, as acceptable outcomes that would allow for continued monitoring without additional initial therapy.77 Serum thyroglobulin levels above these cutoffs should lead clinicians to reevaluate the completeness of the total thyroidectomy and to consider additional imaging studies to identify possible locoregional or distant metastases. A confirmed incomplete surgical thyroidectomy should be managed with options, including observation, thyroid-stimulating hormone suppressive therapy, revision surgery, or radioactive iodine ablation of the remnant tissue based on the risk to individual patient and thorough multidisciplinary team discussion.
Serum thyroglobulin values <5 ng/mL are expected and acceptable after a complete extracapsular total thyroidectomy. Aggressive attempts to remove “all” thyroid tissue must be balanced with expected meaningful postoperative benefit. Small remnants of normal thyroid tissue extending into the joint or around the RLN near the Ligament of Berry should be excised with caution to avoid unnecessary risk to the nerve. Lapses in technique and inadequate resection (such as preserving the tubercle of Zuckerkandl) are not recommended. The surgeon should use common sense and ethical risk/benefit strategies when dissecting near the Ligament of Berry.
3 CONCLUSION
Injury to the RLN and/or external branch of the SLN resulting in postoperative vocal disability after thyroid and parathyroid surgery is largely preventable with appropriate understanding of the embryology and anatomy of the RLN, surgical experience, an understanding and attention to surgical detail at the Ligament of Berry and tubercle of Zuckerkandl, and understanding of the applications of RLN monitoring. In this manner, a safe, complete, and thorough thyroidectomy can be reliably performed with subsequent coordination, in a multidisciplinary (endocrinology and surgery) approach, of postoperative adjuvant therapy, thus providing the highest of surgical and medical outcomes in the treatment of patients requiring thyroid surgery.




