Investigating the Human Brain's Integration of Internal and External Reference Frames: The Role of the Alpha and Beta Bands in a Modified Temporal Order Judgment Task
Funding: This work was supported by GuangDong Basic and Applied Basic Research Foundation (Grant No. 2023A1515012929), Science and Technology Planning Project of Guangdong Province, China (Grant No. 2023A0505050162), Natural Science Foundation of Jilin Province (Grant No. 20210101413JC), and Shenzhen Basic Research Program (Grant No. 20210324101402008).
Xianhao Wei and Jian Zhang contributed equally to this study.
ABSTRACT
The integration of the internal and external reference frames of the human brain is crucial for achieving accurate tactile spatial localization. However, the mechanisms underlying this integration have yet to be fully elucidated. This study adopted a modified temporal order judgment paradigm with an advanced weighted phase lag index method to investigate brain network interactions when the internal and external reference frames were integrated. We found that when the brain integrated internal and external reference frames, alpha oscillations decreased, beta oscillations increased, and inter-hemispheric connectivity increased. Specifically, compared with the match condition: first, the alpha band oscillation predominantly contributed to processing the internal reference frame mismatch; second, the alpha and late beta band oscillation predominantly contributed to processing the external reference frame mismatch; third, the early alpha and late beta band oscillation predominantly contributed to processing the internal and external reference frame mismatch. These findings suggest that the neural oscillation of the alpha and beta bands plays an essential role in tactile spatial localization.
1 Introduction
Humans, as well as several nonhuman species, are able to localize a touch in space. This ability often requires the integration of internal and external reference frames. The internal reference frame primarily comprises somatotopic representations where the tactile stimulus occurs on the skin (Haggard et al. 2003; Penfield and Boldrey 1937; Roux et al. 2018; Serino and Haggard 2010). In contrast, the external reference frame is composed primarily of spatiotopic representations where the position of the stimulated limbs is in the external space (Azañón et al. 2015; Heed et al. 2015; Yamamoto and Kitazawa 2001). To perceive the location of the stimulus and take action, the brain must not only identify which part of the body is in contact with tactile stimuli but also locate these stimuli in the surrounding environment (Azañón et al. 2015; Heed and Azañon 2014; Heed et al. 2015; Overvliet et al. 2011).
Temporal order judgment (TOJ) is typically used to explore how the brain integrates internal and external reference frames to localize tactile stimuli in space (Heed and Azañon 2014; Sambo et al. 2013). In the TOJ task, participants receive two rapid and sequential stimuli on both the left and right hands and are asked to immediately judge the order of the stimuli. With uncrossed hands (default posture), participants can discriminate stimulus order accurately even at very short time intervals (~30–70 ms). However, when the hands are crossed over the body midline, performance becomes significantly impaired, and a larger time interval is required between stimuli for correct performance (∼120–300 ms) (Sambo et al. 2013; Schicke and Röder 2006; Shore et al. 2002; Yamamoto and Kitazawa 2001). These findings suggest that the somatotopic representation mismatches the spatiotemporal representation (e.g., a stimulus applied on the left hand of the internal reference frame coming from the right part of the external reference frame, and vice versa), thereby impairing the ability to correctly judge the localization of tactile stimuli in space. Moreover, the crossing effect is reliable and stable, as it persists even when the two tactile stimuli differ in frequency or duration (Heed and Azañon 2014; Sambo et al. 2013; Shore et al. 2002; Yamamoto and Kitazawa 2001). However, previous studies have focused only on the mismatch between internal and external reference frames, while the mismatches within internal and external reference frames to localize tactile stimuli in space have yet to be fully elucidated.
In this study, we employed a modified TOJ paradigm involving mismatches within internal and external reference frames to elucidate the neural mechanisms underlying tactile spatial processing. The participants received two rapid and sequential stimuli on both the hand and the foot and were asked to judge the order of the stimuli from the hand to the foot or vice versa. In the mismatch of the internal reference frame condition, two tactile stimuli were delivered to different body sides, such as the left hand with the right foot or the right hand with the left foot combinations. The participants needed to integrate skin-based tactile information across different body sides for TOJ. Compared with the limbs on the opposite sides of the body, the limbs on the same side are more likely to be part of a unified internal system. As demonstrated by behavioral data, a touch is more likely to be misattributed to the same side of the body than to the opposite side of the body (Badde et al. 2019). In terms of the mismatch of the external reference frame condition, two stimuli are delivered to different sides of the external space, such as the left hand and the right foot combination if the hands and foot are placed on their default (uncross) side of the external place or the right hand crossed over the body midline and the right foot combination if the hands and foot are placed on the crossed side of the external place. This setup requires participants to integrate tactile information across the body midline to make a temporal judgment. Compared with stimuli presented on the same side of the external space, stimuli on opposite sides are less likely to be perceived as part of a unified external spatial system, as processing tactile information across different sides of the external space can lead to increased errors and thresholds (Ritterband-Rosenbaum et al. 2014; Tamè et al. 2019). This modified TOJ paradigm, which involves tactile stimuli presented across different sides of the body and external space, allows us to assess mismatches separately within internal and external reference frames. By identifying the distinct contributions and challenges associated with each reference frame, this approach can enhance our understanding of tactile spatial processing.
Electroencephalography (EEG) has long been considered to have excellent temporal resolution for studying brain activity (Hassan and Wendling 2018; O'Neill et al. 2018). Rhythmic brain activity in the alpha (8–15 Hz) and beta (17–25 Hz) frequency bands is influenced by sensorimotor tasks (Cheyne et al. 2003). Researchers have reported that oscillations in the alpha and beta bands are related to tactile localization in the integration of internal and external reference frames, with these reference frames being self-centered (Buchholz et al. 2011; Heed et al. 2015). Recent studies have suggested that the integration of internal and external reference frames involves dynamic functional connectivity of alpha and beta regions in brain networks (Moharramipour et al. 2023). To capture the complexity of brain networks, the weighted phase lag index (WPLI) is utilized for functional connectivity analysis (Imperatori et al. 2019; Lau et al. 2012; Xie et al. 2022). Specifically, the WPLI is employed to construct phase synchronization networks from EEG signals, which are then converted into functional connectivity networks for analyzing brain network interactions. The WPLI cannot overestimate the phase lag values because of the volume conduction effects of uncorrelated noise sources. Therefore, the WPLI is reported to be less sensitive to noise than the traditional PLI, and even under conditions of a high signal-to-noise ratio, it has a more reliable relationship with true phase consistency (Stam et al. 2007). This characteristic makes the WPLI a suitable tool for exploring phase synchronization relationships across the brain network in different frequency bands, leading to more reliable insights into the neural mechanisms underlying the integration of internal and external reference frames.
In this study, we adopted a modified TOJ paradigm with an advanced WPLI method to investigate the neural oscillation of the brain network when the internal and external reference frames are mismatched and what in these networks contributes to localizing tactile stimuli in space. We delivered rapid, sequential electrical stimulation to the hand and foot and asked participants to judge the order of the stimuli, with the hand and foot stimulation positions following these conditions: (1) different versus same body sides to examine brain network interactions associated with mismatches within the internal reference frames, (2) different versus same sides of the external space to explore network interactions related to mismatches within external reference frames, and (3) hand crossing the body midline versus default position to investigate brain network interactions during the integration of mismatches between internal and external reference frames.
2 Materials and Methods
2.1 Participants
Twenty-nine right-handed adults (12 males and 17 females, ranging in age from 21 to 33 years, mean ± SD: 25.2 ± 3.0 years) participated in our study. Handedness was determined on the basis of the Edinburgh Handedness Inventory (Oldfield 1971). The sample size was determined a priori using G*Power v.3.1 to estimate the number of participants needed to detect significant effects with 80% statistical power (Faul et al. 2007). With the assumption of a matched samples t-test, an effect size of dz = 0.6, a type I error probability of α = 0.05, and an analytical power of (1 − β) = 0.80, we needed a minimum sample of 24 participants. Our sample size was greater than the estimated sample size needed. All participants were physically healthy without any motor or sensory impairments. All participants signed an informed consent form in advance, and the entire procedure of the experiment was clearly explained to each participant before EEG data collection. The experiment was approved by the ethics committee of the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences.
2.2 Experimental Paradigm
Before the experiment, a Digitimer DS7A Constant Current Stimulator (Digitimer Ltd., Welwyn Garden City, UK) was used to determine the stimulation intensity at four electrical stimulation sites for each participant: the second joint of the left middle finger, the second joint of the right middle finger, the area above the left ankle joint, and the area above the right ankle joint. The stimulation intensity at the four sites was set at 1.5-fold the minimal intensity perceived at each body site (Alouit et al. 2024; Williamson et al. 2022). The minimal intensity perceived was defined as the lowest intensity detected by the participant in all 10 out of 10 consecutive stimuli, with the intensity increasing from 1 in 0.1-mA intervals (Rocchi et al. 2016). The stimulation current used in the experiment consisted of a single square wave pulse with a duration of 0.2 ms (Williamson et al. 2022).
The participants subsequently performed a tactile TOJ task involving internal and external reference frames while their EEG data were recorded. Figure 1A illustrates the experimental paradigm. At the beginning of the experiment, the participants received a randomly ordered sequence of the eight electrical stimulation postures shown in Figure 1B, which they adopted sequentially according to the assigned order. In each posture, the participants fixated on a white cross on the screen. After an intertrial interval of 1200–3000 ms, the participants received two sequential electrical stimuli with 15 random interstimulus interval (ISI) situations (ISI ranging from −350 ms to +350 ms in 50-ms steps). An ISI of 0 ms indicated that the two stimuli were presented simultaneously. The “+” indicates the hand before the foot, and the “−” indicates the opposite. The participants completed 120 trials in each posture, consisting of eight sets, each with 15 different randomized ISI conditions. This approach ensured that each ISI was randomly presented eight times within each posture. When the second stimulus of each trial appeared, participants had a 2000-ms window to make a forced-choice response and to judge whether the sequence of events was “hand first, foot second” or “foot first, hand second.” The “1” and “3” keys on the keyboard were designated response keys, and the key mapping was balanced across participants. After making a judgment, the participants immediately proceeded to the next trial. If participants did not respond within the 2000-ms window, the next trial began automatically. Each participant ultimately completed the TOJ task across eight postures (blocks), totaling 960 trials. A 5-min break was provided between each block.
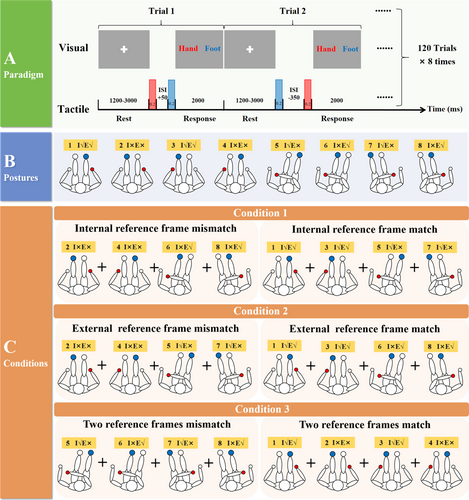
The overall experiment and the three experimental conditions are shown in Figure 1. The participants were required to complete the modified TOJ experiment shown in Figure 1A, following the eight different postures in a pseudorandom order displayed in Figure 1B. We subsequently analyzed the task-related EEG data on the basis of the three conditions in Figure 1C.
2.3 EEG Data Collection and Analysis
2.3.1 Data Collection and Preprocessing
We processed the 32-channel EEG data related to the task (using the Grael 4K EEG Amplifier, standard Ag/AgCl electrodes placed on the scalp according to the international 10-20 frame, with impedance reduced to 10 kΩ) by applying a notch filter at 50 Hz to remove power line interference, followed by bandpass from 0.1 to 40 Hz. We downsampled the 1024-Hz EEG data to 200 Hz. Independent component analysis was employed to eliminate electrooculograms, electrocardiograms, electromyograms, and other artifacts. We then rereferenced the data via the M1 and M2 electrodes. Finally, we segmented the data according to the eight different electrical stimulation postures (excluding bad segments) and performed baseline correction. All neurophysiological data analyses were conducted using the MNE toolbox (Gramfort et al. 2013).
2.3.2 Functional Connectivity Analysis
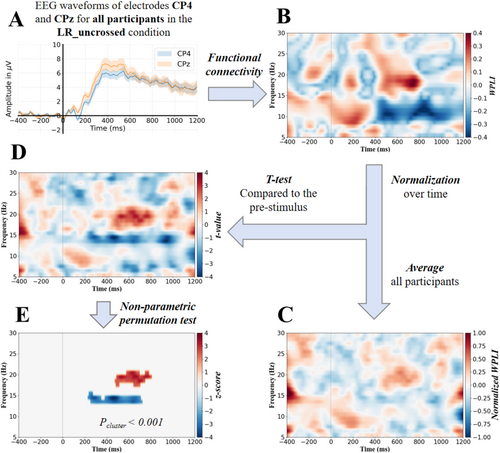
We standardized the WPLI values at the individual participant level at each frequency row via z-score normalization (mean = 0, SD = 1). The normalization was applied independently on each frequency, as the distribution of the WPLI value usually varied across frequencies. The normalization can help better detect the couplings' evolution during the task across the participants. Thus, it would improve the identification of task-related couplings. Figure 2C shows an example of the normalized WPLI (NWPLI) at each time–frequency bin averaged across the participants.
2.3.3 Statistical Analysis
To identify the significant time–frequency clusters on the t-statistic maps (e.g., Figure 2D), we applied a nonparametric permutation test (Maris and Oostenveld 2007). In each permutation, the t-statistic was calculated after randomly permuting the NWPLI values and the median of the NWPLI during the prestimulus period (i.e., X ↔ XB) across participants. The null distribution of the t-statistic was then empirically derived using all the t-statistics that were calculated by the large number of permutation sets (99,200) (Moharramipour et al. 2023). For each permutation set, the time–frequency clusters whose t-statistic was smaller than the 2.5th or greater than the 97.5th of the null distribution were identified. Subsequently, the clusters with the overall maximum positive and minimum negative t statistics were identified. This process was repeated for all the permutation sets, and the histogram of the maximum/minimum cluster t-values was acquired. This histogram corresponds to the null hypothesis in which there should be no time–frequency cluster of connectivity significantly altered by the stimuli. Finally, the threshold for a significant cluster t-value was determined by using this histogram and a specified alpha threshold (Pc) (i.e., the probability of falsely detecting a cluster as significant). Moreover, we accounted for another secondary multiple comparisons problem at the network level by applying the Bonferroni correction. In this correction, since there were 496 connectivity pairs, the alpha threshold needed to be divided by 496. The alpha thresholds of 0.05/496 (Bonferroni correction), 0.001, and 0.05 were employed in the present study.
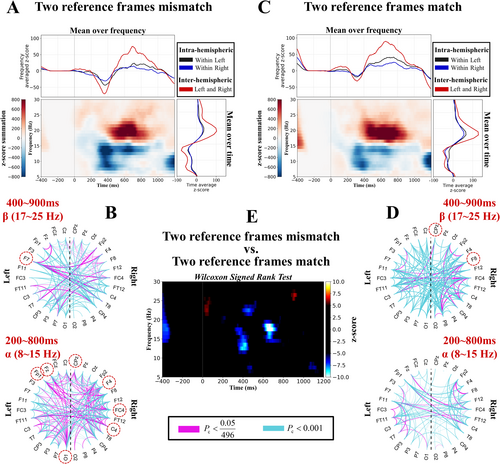
To show the overall behavior (i.e., density) of the brain network over time and frequency (e.g., Figure 3A), we summed the task-related z-scores (e.g., Figure 2E) of all 496 connectivity pairs (Pc < 0.05). Furthermore, we statistically compared the network density difference between the experimental conditions across time and frequency via the Wilcoxon signed-rank test (Hollander et al. 2013) (e.g., Figure 3E). At each time–frequency point, the Wilcoxon signed-rank test (p < 0.05) was applied to the z-scores of 496 connectivity pairs of two different conditions. Then, the significant time–frequency clusters were identified via the nonparametric permutation test (Maris and Oostenveld 2007), similar to what was explained previously (Pc < 0.05). Next, we plotted the brain networks associated with the time window of 200–800 ms and the frequency range of 8–15 Hz for the alpha band, and the brain networks associated with the time window of 400–900 ms and the frequency range of 17–25 Hz for the beta band, as shown in Figure 3B and Figure 3D, respectively. Magenta and cyan colors indicate the connections that pass the test with a Pc smaller than 0.05/496 (Bonferroni correction) and 0.001, respectively.
Moreover, we selected the connections passing the test (Pc < 0.05/496 [Bonferroni correction]) and applied the Wilcoxon signed-rank test (p < 0.05) separately to each node of the network to identify those nodes with significant density differences between the two experimental conditions. For each electrode, the test was applied to the z-scores of its 31 connectivity pairs with the other electrodes across conditions, and the significant electrodes are marked with red dashed circles.
We analyzed the EEG data of all the participants following the aforementioned procedures, and the processed data were summarized under the following three conditions: internally reference frame mismatch versus internally reference frame match; externally reference frame mismatch versus externally reference frame match; two reference frames mismatch versus two reference frames match (Figures 1-3).
3 Results
We calculated the WPLI values in pairwise nondirectional coupling between the 32 EEG channels over time as well as the frequency for each participant. The WPLI was normalized at each frequency row (Figure 2B, normalized WPLI). For each time–frequency bin, we subsequently computed the t statistic against the prestimulus median at the same frequency for each participant (p < 0.05) (Figure 2D and Equation 3). Significant time–frequency clusters were then extracted via a nonparametric permutation test (Pc < 0.05/496, 0.001, and 0.05) (Figure 2E), thereby yielding the task-related time–frequency profile of the neural oscillation.
3.1 Condition 1: Internal Reference Frame Mismatch Versus Internal Reference Frame Match
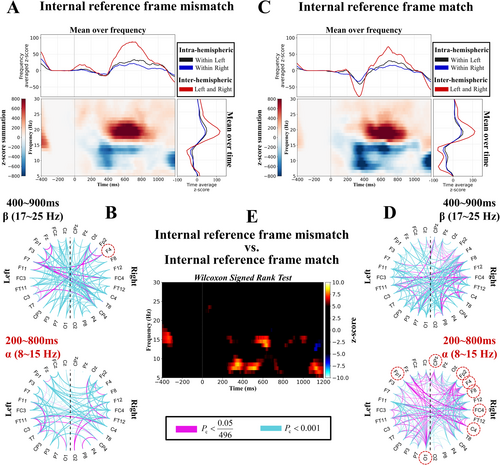
As shown in Figure 3A,C, the neural oscillation across the whole-brain network revealed that the alpha band (8–15 Hz) significantly decreased at approximately 400 ms and that the beta band (17–25 Hz) significantly increased at approximately 600 ms, regardless of whether the internal reference frames were mismatched or matched (Pc < 0.05). Compared with the internal reference frame match condition, the alpha band was significantly greater under the internal reference frame mismatch condition (Pc < 0.05) (Figure 3E). This result indicated that the alpha band played a more important role when the internal reference frame was mismatched than when it was matched.
Next, we investigated the network connectivity density across the whole-brain network, as shown in Figure 3B,D. In the alpha band, the connectivity density was greater under the internal reference frame match condition than under the internal reference frame mismatch condition (bottom of Figure 3B,D). We also explored the task-related connectivity patterns across the whole-brain networks, and we observed that the inter-hemispheric connectivity ratios (left and right) were greater than the intra-hemispheric connectivity ratios (within left and right) in the upper and right parts of Figure 3A,C. This result suggested that the human brain utilized inter-hemispheric connectivity to process internal reference frames.
Finally, we investigated whether the connectivity density of each electrode differed significantly between the two conditions. We found that in the alpha band, electrodes FP1, FP2, F4, F8, FC4, C4, CPz, and O1 exhibited greater connectivity density in the brain network under the internal reference frame match condition than under the mismatch condition (p < 0.05) (red dashed circles in Figure 3D). In the beta band, electrode F4 presented greater connectivity density than the other electrodes did under the mismatch condition compared with the match condition (p < 0.05) (red dashed circles in Figure 3B). The majority of these electrodes were located in the right hemisphere. Overall, these results suggest that when the brain integrates the internal reference frame, inter-hemispheric connectivity increases, and compared with the internal reference frame match condition, the brain predominantly relies on modulating alpha band oscillations to process the internal reference frame mismatch.
3.2 Condition 2: External Reference Frame Mismatch Versus External Reference Frame Match
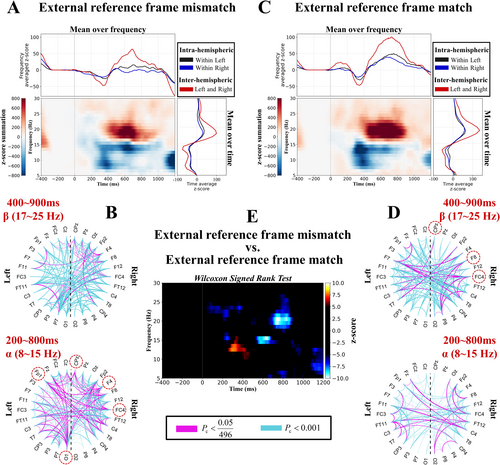
As shown in Figure 4A,C, the neural oscillation across the whole-brain network revealed that the alpha band (8–15 Hz) significantly decreased at approximately 400 ms and that the beta band (17–25 Hz) significantly increased at approximately 600 ms, regardless of whether the external reference frame was a mismatch or a match (Pc < 0.05). In the alpha band, the initial decrease in neural oscillation was greater under the match condition (approximately 300 ms), but later (approximately 600 ms), the decrease was more pronounced under the mismatch condition (Pc < 0.05) (Figure 4E). In the beta band, the neural oscillation showed a stronger increase under the match condition (Pc < 0.05) (Figure 4E). This result indicated that the alpha band and the late beta band played a more important role when the external reference frame was mismatched than when it was matched.
Next, we investigated the network connectivity density across the whole-brain network, as shown in Figure 4B,D. In the alpha band, the connectivity density was greater under the external reference frame mismatch condition than under the external reference frame match condition (bottom of Figure 4B,D). To explore the task-related connectivity patterns across the whole-brain networks, we also observed that the inter-hemispheric connectivity ratios (left and right) were greater than the intra-hemispheric ratios (within left and right) in the upper and right parts of Figure 4A,C. This result suggested that the human brain utilizes inter-hemispheric connectivity to process external reference frames.
Finally, we investigated whether the connectivity density of each electrode differed significantly between the two conditions. We found that in the alpha band, electrodes FP1, F4, FC4, CPz, and O1 exhibited greater connectivity density in the brain network under the mismatch condition than under the match condition (p < 0.05) (red dashed circles in Figure 4B). In the beta band, electrodes FC4, F8, and CPz presented greater connectivity density under the match condition than under the mismatch condition (p < 0.05) (red dashed circles in Figure 4D). The majority of these electrodes were located in the right hemisphere. Overall, these results indicate that when the brain integrates the external reference frame, inter-hemispheric connectivity increases, and compared with the external reference frame match condition, the brain predominantly relies on modulating alpha and late beta band oscillations to process the external reference frame mismatch.
3.3 Condition 3: Two Reference Frames Mismatch Versus Two Reference Frames Match
As shown in Figure 5A,C, the neural oscillation across the whole-brain network revealed that the alpha band (8–15 Hz) significantly decreased at approximately 400 ms and that the beta band (17–25 Hz) significantly increased at approximately 600 ms, regardless of whether the internal or external reference frame was mismatched or matched (Pc < 0.05). In the alpha band, the neural oscillation decreased more under the mismatch condition (approximately 400 ms), whereas in the beta band, it increased more under the match condition (approximately 700 ms) (Pc < 0.05) (Figure 5E). This result indicated that the early alpha and late beta bands played a more important role when the two reference frames were mismatched than when they were matched.
Next, we investigated the connectivity density across the whole-brain network, as shown in Figure 5B,D. In the alpha band, the network connectivity density was greater under the internal and external reference frame mismatch condition than under the match condition, whereas in the beta band, the network connectivity density was lower under the internal and external reference frame mismatch condition than under the match condition. Additionally, to explore the task-related connectivity patterns across the whole-brain networks, we also observed that the inter-hemispheric connectivity ratios (left and right) were greater than the intra-hemispheric ratios (within left and right) in the upper and right parts of Figure 5A,C. This result suggested that the human brain utilized inter-hemispheric connectivity to process the internal and external reference frames.
Finally, we investigated whether the connectivity density of each electrode differed significantly between the two conditions. We found that in the alpha band, electrodes FP1, F4, Fz, FC4, C4, CPz, and O1 exhibited greater connectivity density in the brain network under the mismatch condition than under the match condition (p < 0.05) (red dashed circles at the bottom of Figure 5B). In the beta band, electrode F7 showed greater connectivity density under the mismatch condition, whereas electrodes F8 and CPz showed greater connectivity density under the match condition (p < 0.05) (red dashed circles at the top of Figure 5B,D). The majority of these electrodes were located in the right hemisphere. Overall, these results indicate that when the brain integrates the internal and external reference frame, inter-hemispheric connectivity increases, and compared with the internal and external reference frame match condition, the brain predominantly relies on modulating early alpha and late beta band oscillations to process the internal and external reference frame mismatch.
3.4 Correlation Analysis
Finally, Pearson correlation analysis was used to explore the relationships between the significant differences in the alpha and beta bands and the differences in accuracy. For accuracy (mean ± SD), the internal reference frame mismatch was 0.87 ± 0.07, and the internal reference frame match was 0.81 ± 0.08; the external reference frame mismatch was 0.84 ± 0.07, and the external reference frame match was 0.84 ± 0.08; and the two reference frames mismatch were 0.84 ± 0.07, and the two reference frames match were 0.84 ± 0.08.
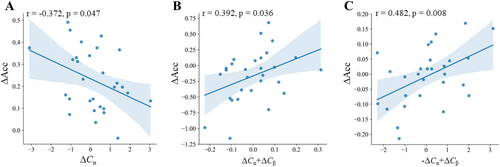
Under each condition, we explored the relationship between the significant differences in the alpha and beta bands and the differences in accuracy via Pearson correlation analysis. Figure 6A shows the correlation results under Condition 1: ΔCα was negatively correlated with ΔAcc (r[29] = −0.372, p = 0.047), meaning that in the alpha frequency band, as the difference in total coupling values between the internal reference frame mismatch and match increased, the accuracy difference between the two conditions decreased. Figure 6B shows the correlation results under Condition 2: ΔCα + ΔCβ was positively correlated with ΔAcc (r[29] = 0.392, p = 0.036), meaning that in the alpha and late beta frequency bands, as the difference in total coupling values between the external reference frame mismatch and match increased, the accuracy difference between the two conditions increased. Figure 6C shows the correlation results under Condition 3: −ΔCα + ΔCβ was positively correlated with ΔAcc (r[29] = 0.482, p = 0.008), meaning that in the early alpha and late beta frequency bands, as the difference in total coupling values between internal and external reference frame mismatch and match increased, the accuracy difference between the two conditions increased.
These results suggest that during the regulation of different reference frame mismatch, the brain relies on oscillatory activity in different frequency bands. In the case of internal reference frame mismatch, the brain predominantly relies on modulation of the alpha band; in external reference frame mismatch, both alpha and late beta band oscillations are involved; and when processing the mismatch between internal and external reference frames, the synergistic modulation of early alpha and late beta band oscillations plays a crucial role.
4 Discussion
In this study, we observed that the alpha band oscillation significantly decreased and the beta band oscillation significantly increased under the internal reference frame mismatch and match conditions, the external reference frame mismatch and match conditions, and the internal and external reference frame mismatch and match conditions (Figures 3A,C, 4A,C, and 5A,C, respectively). Correlation analysis suggested that, during internal reference frame mismatch, the brain predominantly relied on modulation of the alpha band; during external reference frame mismatch, both alpha and late beta band activities were involved; and when processing the mismatch between internal and external reference frames, synergistic modulation of early alpha and late beta band oscillations was required (Figure 6). Differences in coupling predict behavioral performance, and the two are closely related. These results suggest that the alpha and beta bands play important roles in regulating the brain's integration of internal and external reference frames. On the one hand, the decrease in alpha band oscillation is closely related to cognitive control over irrelevant information suppression. This decrease helps humans optimize the allocation of cognitive resources, thereby enabling the brain to focus more on task-relevant information processing (Jensen and Mazaheri 2010; Klimesch 2012). On the other hand, the increase in beta band oscillation is closely related to sensorimotor integration and attention maintenance (Engel and Fries 2010; Kilavik et al. 2013). Moreover, the increase in beta band activity helps us promote communication between cortical regions (Kilavik et al. 2013). Recent studies have shown that when hands are crossed, beta band communication is crucial for making accurate judgments (Moharramipour et al. 2023). Rueda-Delgado et al. also reported that an increase in task difficulty during a bimanual coordination task is correlated with greater beta band power and stronger inter-hemispheric connectivity (Rueda-Delgado et al. 2017). This correlation might explain why we observed increased beta band coupling under each condition and why the brain mainly utilized inter-hemispheric connectivity to process the modified TOJ task.
By comparing the significant connectivity densities across electrode sites under each pair of conditions, we found that these electrodes were predominantly located in the right hemisphere, which may be related to the spatial processing advantages of this hemisphere (Hugdahl 2013; Shulman et al. 2010) In summary, the decrease in interactions in the alpha band and the increase in interactions in the beta band complement each other, thus enabling the brain to suppress irrelevant information while enhancing the processing of task-relevant information, thereby achieving precise tactile spatial localization.
When investigating internal reference frame mismatch, we found that the brain relies more heavily on a decrease in alpha band oscillation when processing the internal reference frame. The decrease in alpha band oscillation reflects decreased inhibitory control over internal states, which enables the brain to release more neural resources for integrating and processing reference frame information (Foxe and Snyder 2011). At the beginning of the experimental design, we aimed to study brain network interactions under internal reference frame mismatch. However, an interesting phenomenon emerged in the results: compared with the internal reference frame mismatch condition, the internal reference frame match condition resulted in a more significant decrease in alpha band coupling and greater network connectivity (Figure 3B,D,E). These findings may be attributed to the fact that coordination of the different side limbs is more frequent in daily life, leading to increased processing complexity under the match (same side) condition. This increased complexity is consistent with the findings of Nakagawa et al., who reported that ipsilateral limb coordination activates the auxiliary motor areas more strongly than contralateral coordination does (Nakagawa et al. 2016).
When investigating external reference frame mismatch, we found that the alpha and beta bands played important roles in processing external reference frames. This result is similar to how the brain integrates internal and external reference frames, supporting the concept of tactile remapping: the brain maps internal reference frame information to the external reference frame, allowing for precise tactile spatial localization. We also found that a greater increase in beta band oscillation was observed under the external reference frame match conditions than under the external reference frame mismatch conditions. This difference may be due to the fact that the stimulated hand and foot are on the same side, with a smaller spatial distance, which increases the need for sustained attention to the target area, thereby promoting an increase in beta band activity (Heed and Roeder 2010).
When investigating internal and external reference frame mismatch, we found that, compared with the match condition, early alpha oscillations and late beta oscillations were reduced (Figure 5E). Schicke and Röder suggested that when internal and external reference frames conflict, task complexity increases, leading to a greater cognitive load and the depletion of attentional resources (Schicke and Röder 2006). The reduction in early alpha oscillations further supports the idea that diminished alpha band activity plays a crucial role in enhancing task processing under conditions of high cognitive load (Jensen and Mazaheri 2010). Moreover, the increase in cognitive load may also prevent the brain from maintaining sustained cognitive states during the later stages of task performance, as reflected by the decrease in beta oscillations in the later task phases (Engel and Fries 2010; Yu et al. 2021). Specifically, when the brain can maintain cognitive stability while processing internal and external reference frame mismatch, more attentional resources are required to regulate the conflict between internal and external information. However, when the cognitive load becomes too high, the brain is unable to sustain its original neural synchronization state, leading to a reduction in beta band oscillations.
In our experimental design, the inclusion of a different numbers of males and females may have influenced the results. Future studies should include a larger equal number of males and females to verify the results. Second, the spatial resolution of the task-related EEG data we collected was limited. Future studies should employ functional magnetic resonance imaging to gain deeper insights into the brain mechanisms involved in the integration of internal and external reference frames. Finally, we stimulated relatively distal sites on the body (the second joint of the middle finger and above the ankle). Future research should investigate whether the effects observed are also evident at other body sites, such as more proximal regions of the limbs or even nonlimb areas.
5 Conclusion
In summary, this study explored neural oscillation when the human brain integrated internal and external reference frames for accurate tactile spatial localization. The alpha and beta band oscillations with inter-hemispheric connectivity contributed to the processing of the internal and external reference frames. These findings could provide insights into the effective use of neural oscillation-based assessments for neurorehabilitation.
Author Contributions
Xianhao Wei: study and design of concept, analysis and interpretation of data, and writing of the manuscript. Jian Zhang: study and design of concept, interpretation of data, and revision of manuscript for critical intellectual content. Jinyan Zhang: interpretation of data and revision of manuscript for critical intellectual content. Zimo Li: acquisition of data and revision of manuscript for critical intellectual content. Jingjing Yang: revision of manuscript for critical intellectual content. Jinglong Wu: revision of manuscript for critical intellectual content. Qi Li: study and design of concept, study supervision and interpretation of data, and revision of manuscript for critical intellectual content. Zhilin Zhang: study and design of concept, study supervision and interpretation of data, and revision of manuscript for critical intellectual content.
Acknowledgments
This study was supported by the Science and Technology Planning Project of Guangdong Province, China (2023A0505050162), the GuangDong Basic and Applied Basic Research Foundation (2023A1515012929), the Shenzhen Basic Research Program (20210324101402008), and the Natural Science Foundation of Jilin Province of China (20210101413JC).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




